Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Definitions
2.3. Biomarkers
2.4. Outcome
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Patients with Low and High mNUTRIC Scores
3.2. Baseline Characteristics of Patients in the Different BMI Groups
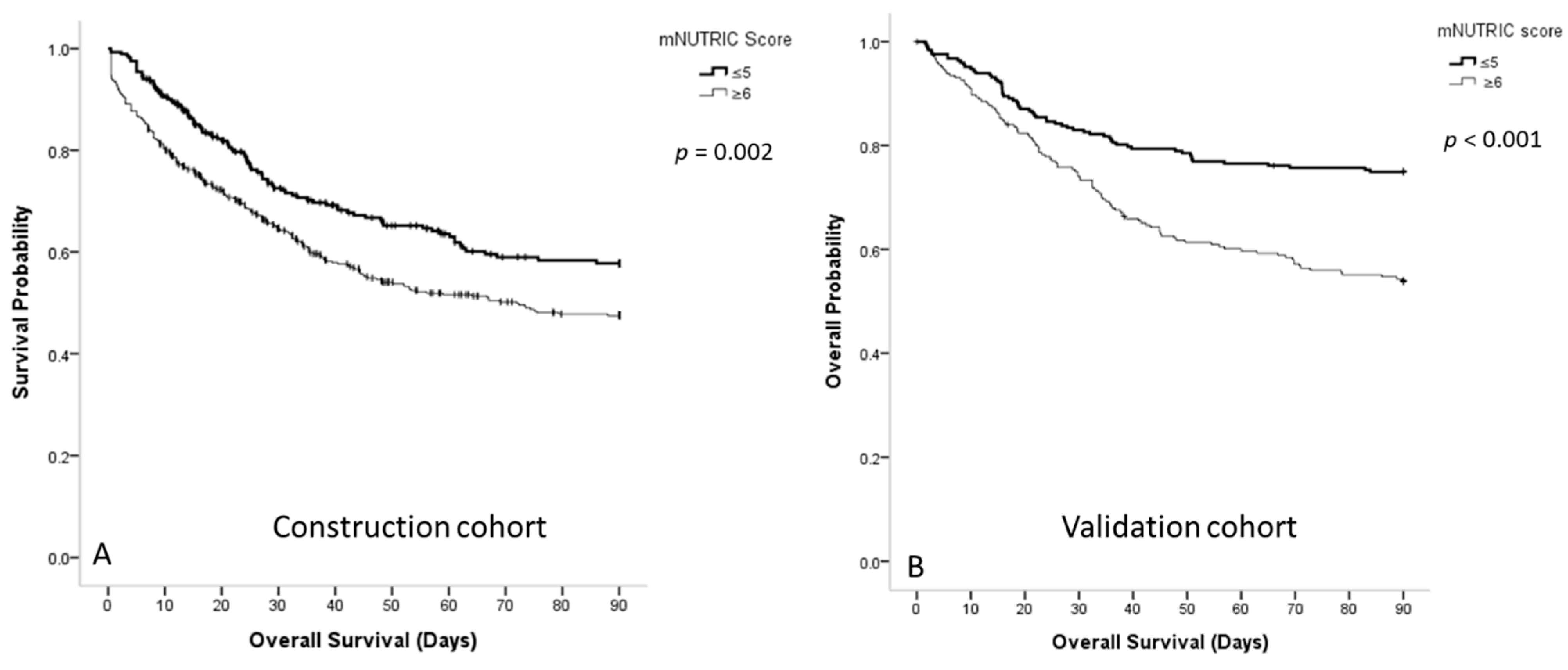
3.3. Survival Curve of Patients with Sepsis Classified According to the mNUTRIC Scores
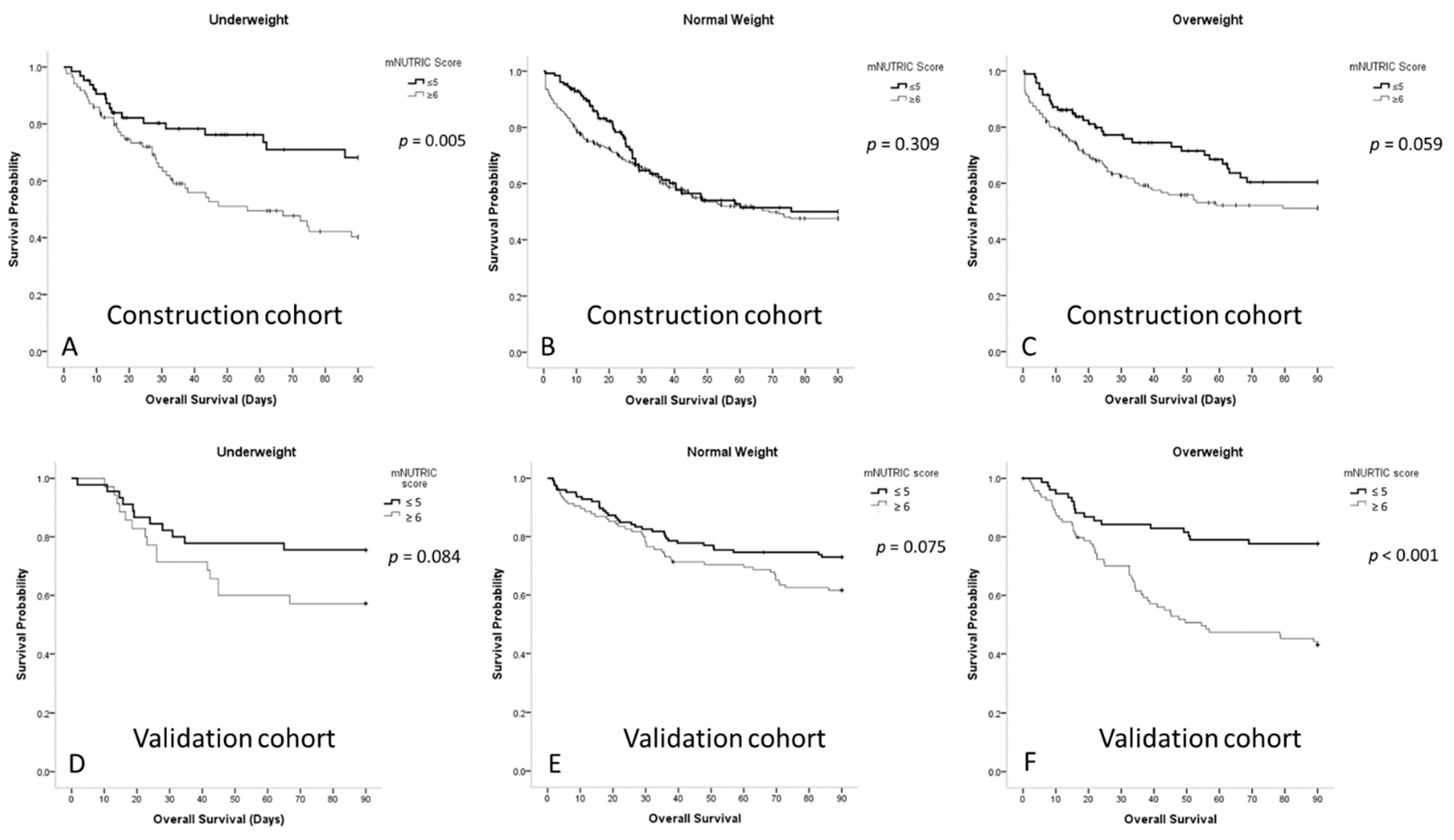
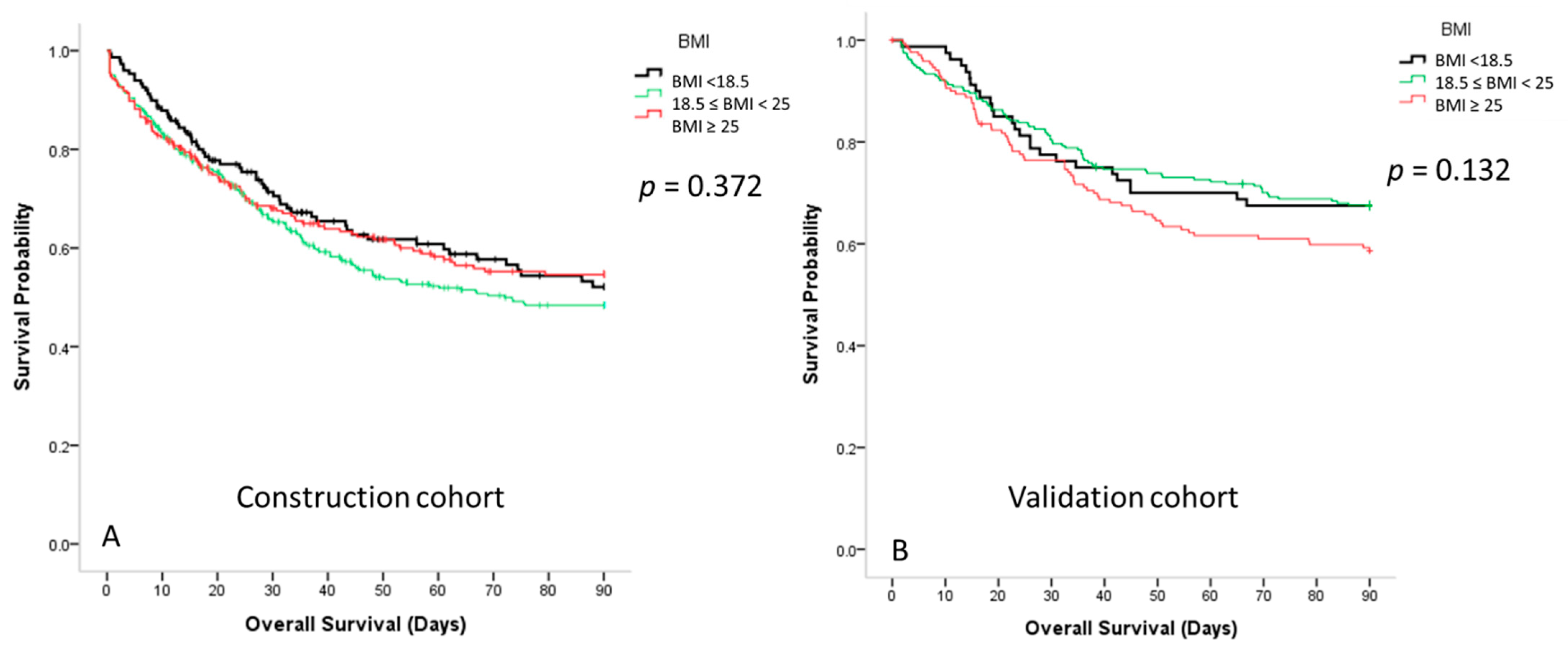
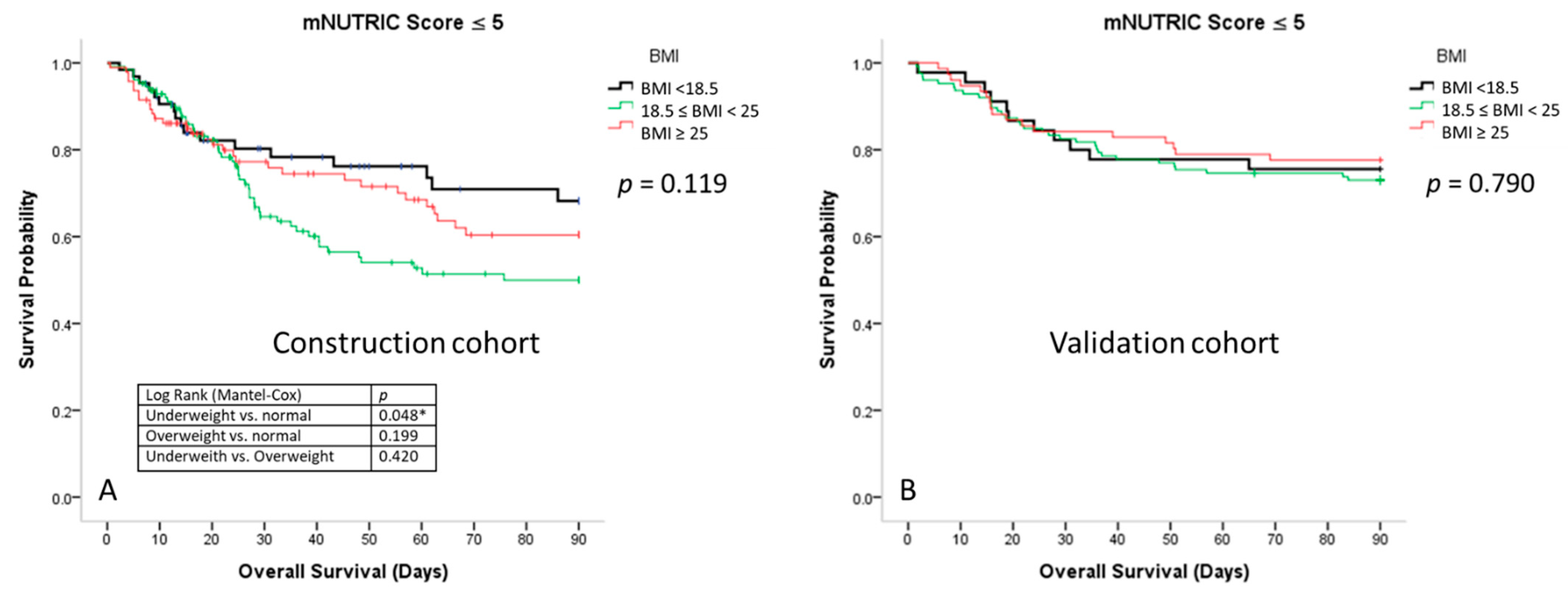
3.4. Survival Curves for Patients with Sepsis According to the Different BMI Groups
3.5. Biomarkers of Patients with Sepsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Koh, Y.; Du, B.; Tang, Y.-Q.; Divatia, J.V.; Tan, C.C.; Gomersall, C.D.; Faruq, M.O.; Shrestha, B.R.; Binh, N.G. Management of severe sepsis in patients admitted to Asian intensive care units: Prospective cohort study. BMJ 2011, 342, d3245. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-F.; Chen, Y.-M.; Lin, C.-Y.; Huang, K.-T.; Kao, H.-C.; Fang, Y.-T.; Huang, C.-H.; Chang, Y.-T.; Wang, Y.-H.; Wang, C.-C. Immune profiles and clinical outcomes between sepsis patients with or without active cancer requiring admission to intensive care units. PLoS ONE 2017, 12, e0179749. [Google Scholar]
- Yang, Y.; Yang, K.S.; Hsann, Y.M.; Lim, V.; Ong, B.C. The effect of comorbidity and age on hospital mortality and length of stay in patients with sepsis. J. Crit. Care 2010, 25, 398–405. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Chen, Y.-M.; Wang, C.-C.; Wang, Y.-H.; Lin, C.-Y.; Chang, Y.-T.; Huang, K.-T.; Lin, M.-C.; Fang, W.-F. Insufficient nutrition and mortality risk in septic patients admitted to ICU with a focus on immune dysfunction. Nutrients 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Brascher, J.M.M.; Peres, W.A.F.; Padilha, P.C. Use of the modified "Nutrition Risk in the critically ill" score and its association with the death of critically ill patients. Clin. Nutr. ESPEN 2020, 35, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-F.; Chen, Y.-M.; Wang, Y.-H.; Huang, C.-H.; Hung, K.-Y.; Fang, Y.-T.; Chang, Y.-C.; Lin, C.-Y.; Chang, Y.-T.; Chen, H.-C. Incorporation of dynamic segmented neutrophil-to-monocyte ratio with leukocyte count for sepsis risk stratification. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Fang, W.-F.; Fang, Y.-T.; Huang, C.-H.; Chen, Y.-M.; Chang, Y.-C.; Lin, C.-Y.; Hung, K.-Y.; Chang, Y.-T.; Chen, H.-C.; Huang, K.-T. Risk factors and associated outcomes of ventilator-associated events developed in 28 days among sepsis patients admitted to intensive care unit. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Fang, W.-F.; Douglas, I.S.; Chen, Y.-M.; Lin, C.-Y.; Kao, H.-C.; Fang, Y.-T.; Huang, C.-H.; Chang, Y.-T.; Huang, K.-T.; Wang, Y.-H. Development and validation of immune dysfunction score to predict 28-day mortality of sepsis patients. PLoS ONE 2017, 12, e0187088. [Google Scholar] [CrossRef]
- Grimble, R.F. Basics in clinical nutrition: Main cytokines and their effect during injury and sepsis. Clin. Nutr. ESPEN 2008, 6, e289–e292. [Google Scholar] [CrossRef][Green Version]
- Scicluna, B.P.; Van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Henry, J.; Ong, V.; Leong, C.S.-F.; Teh, A.L.; van Dam, R.M.; Kowitlawakul, Y. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin. Nutr. 2017, 36, 1143–1148. [Google Scholar] [CrossRef]
- Correia, M.I.T.; Campos, A.C.L.; Study, E.C. Prevalence of hospital malnutrition in Latin America: The multicenter ELAN study. Nutrition 2003, 19, 823–825. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Caiaffa, W.T.; Correia, M.I.T. Hospital malnutrition: The Brazilian national survey (IBRANUTRI): A study of 4000 patients. Nutrition 2001, 17, 573–580. [Google Scholar] [CrossRef]
- Kamath, S.K.; Lawler, M.; Smith, A.E.; Kalat, T.; Olson, R. Hospital malnutrition: A 33-hospital screening study. J. Am. Diet. Assoc. 1986, 86, 203. [Google Scholar]
- O’Flynn, J.; Peake, H.; Hickson, M.; Foster, D.; Frost, G. The prevalence of malnutrition in hospitals can be reduced: Results from three consecutive cross-sectional studies. Clin. Nutr. 2005, 24, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yu, J.; Higashikuchi, T.; Compher, C. Does Low Body Mass Index Predict Mortality in Asian Hospitalized Patients? J. Parenter. Enter. Nutr. 2020, 44, 722–728. [Google Scholar] [CrossRef]
- Lee, S.; Kang, J.; Jo, Y.; Kim, K.; Lee, J.; Lee, J.; Rim, K. Underweight is associated with mortality in patients with severe sepsis and septic shock. Intensive Care Med. Exp. 2015, 3, 1. [Google Scholar] [CrossRef]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007, 298, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; He, J.; Duan, X.; Reynolds, K.; Wu, X.; Chen, J.; Huang, G.; Chen, C.-S.; Whelton, P.K. Body weight and mortality among men and women in China. JAMA 2006, 295, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005, 293, 1861–1867. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Li, S.; Zhang, J.; Ma, Q.; Ding, Y.; Ge, H.; Shen, N.; Zheng, Y.; Sun, Y. Impact of body mass index on survival of medical patients with sepsis: A prospective cohort study in a university hospital in China. BMJ Open 2018, 8, e021979. [Google Scholar] [CrossRef]
- Weng, L.; Fan, J.; Yu, C.; Guo, Y.; Bian, Z.; Wei, Y.; Yang, L.; Chen, Y.; Du, H.; Chang, L.; et al. Body-mass index and long-term risk of sepsis-related mortality: A population-based cohort study of 0.5 million Chinese adults. Crit. Care 2020, 24, 534. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Hecht, M.; Flavin, K.E.; Khorana, A.; Gould, M.K. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest 2004, 125, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Pepper, D.J.; Demirkale, C.Y.; Sun, J.; Rhee, C.; Fram, D.; Eichacker, P.; Klompas, M.; Suffredini, A.F.; Kadri, S.S. Does Obesity Protect Against Death in Sepsis? A Retrospective Cohort Study of 55,038 Adult Patients. Crit. Care Med. 2019, 47, 643–650. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Kowitlawakul, Y.; Henry, J.; Ong, V.; Leong, C.S.-F.; Tai, B.C. Higher BMI is associated with reduced mortality but longer hospital stays following ICU discharge in critically ill Asian patients. Clin. Nutr. ESPEN 2018, 28, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Chen, Q.; Liu, C.; Huang, C.; Fang, X. The role of increased body mass index in outcomes of sepsis: A systematic review and meta-analysis. BMC Anesthesiol. 2017, 17, 118. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Welsh, J.; Cui, X.; Suffredini, A.F.; Eichacker, P.Q. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2016, 20, 181. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Tsai, C.L.; Hwang, L.Y.; Lai, D.; Markham, C.; Patel, B. Obesity and Mortality, Length of Stay and Hospital Cost among Patients with Sepsis: A Nationwide Inpatient Retrospective Cohort Study. PLoS ONE 2016, 11, e0154599. [Google Scholar] [CrossRef]
- Sakr, Y.; Alhussami, I.; Nanchal, R.; Wunderink, R.G.; Pellis, T.; Wittebole, X.; Martin-Loeches, I.; Francois, B.; Leone, M.; Vincent, J.L.; et al. Being Overweight Is Associated With Greater Survival in ICU Patients: Results From the Intensive Care Over Nations Audit. Crit. Care Med. 2015, 43, 2623–2632. [Google Scholar] [CrossRef]
- Prescott, H.C.; Chang, V.W.; O’Brien, J.M., Jr.; Langa, K.M.; Iwashyna, T.J. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit. Care Med. 2014, 42, 1766–1774. [Google Scholar] [CrossRef]
- Ng, P.Y.; Eikermann, M. The obesity conundrum in sepsis. BMC Anesthesiol. 2017, 17, 147. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Aretha, D.; Zotou, A.; Koutsileou, K.; Zbouki, A.; Lefkaditi, A.; Sklavou, C.; Marangos, M.; Fligou, F. The Role of Obesity in Sepsis Outcome among Critically Ill Patients: A Retrospective Cohort Analysis. Biomed Res. Int. 2016, 2016, 5941279. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Verschuren, W.M.; Van Leer, E.M.; Kromhout, D. Overweight, underweight, and mortality: A prospective study of 48287 men and women. Arch. Intern. Med. 1996, 156, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Dara, S.I.; Tamim, H.M.; Rishu, A.H.; Bouchama, A.; Khedr, M.K.; Feinstein, D.; Parrillo, J.E.; Wood, K.E.; Keenan, S.P.; et al. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: An international multicenter cohort study. Crit. Care 2013, 17, R72. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, T.G.; MacNabb, C.M.; Mikkelsen, M.E.; Agarwal, A.K.; Sante, S.C.; Shah, C.V.; Gaieski, D.F. A retrospective cohort study examining the association between body mass index and mortality in severe sepsis. Intern. Emerg. Med. 2015, 10, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, E.F.; Showalter, J.W.; Lehman, E.B.; Leib, A.E.; Kraschnewski, J.L. The impact of obesity on sepsis mortality: A retrospective review. BMC Infect. Dis. 2013, 13, 377. [Google Scholar] [CrossRef]
- Martino, J.L.; Stapleton, R.D.; Wang, M.; Day, A.G.; Cahill, N.E.; Dixon, A.E.; Suratt, B.T.; Heyland, D.K. Extreme obesity and outcomes in critically ill patients. Chest 2011, 140, 1198–1206. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Wurzinger, B.; Dunser, M.W.; Wohlmuth, C.; Deutinger, M.C.; Ulmer, H.; Torgersen, C.; Schmittinger, C.A.; Grander, W.; Hasibeder, W.R. The association between body-mass index and patient outcome in septic shock: A retrospective cohort study. Wien. Klin. Wochenschr. 2010, 122, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Madl, C.; Filipescu, D.; Moreno, R.; Groeneveld, J.; Artigas, A.; Reinhart, K.; Vincent, J.-L. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008, 34, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, R.; Marques, J.; Kobylka, K.; Zeidan, M.; Kabisch, B.; Brunkhorst, F.; Reinhart, K.; Sakr, Y. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011, 37, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.F.; Rumgay, H.; Dunlop, C.; Ryan, M.; Quartly, F.; Cox, A.; Deas, A.; Elliss-Brookes, L.; Gavin, A.; Hounsome, L. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 2018, 118, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R. The Obesity Paradox in Cancer—Moving Beyond BMI. Cancer Epidemiol Biomarkers Prev. 2017, 26, 13–16. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Martin, G.S. The effect of diabetes mellitus on organ dysfunction with sepsis: An epidemiological study. Crit. Care 2009, 13, R18. [Google Scholar] [CrossRef]
- Kolyva, A.S.; Zolota, V.; Mpatsoulis, D.; Skroubis, G.; Solomou, E.E.; Habeos, I.G.; Assimakopoulos, S.F.; Goutzourelas, N.; Kouretas, D.; Gogos, C.A. The role of obesity in the immune response during sepsis. Nutr. Diabetes 2014, 4, e137. [Google Scholar] [CrossRef]
| Variable | Range | Points |
|---|---|---|
| Age | <50 | 0 |
| ≥50 and <75 | 1 | |
| ≥75 | 2 | |
| APACHE II | <15 | 0 |
| ≥15 and <20 | 1 | |
| ≥20 and <28 | 2 | |
| ≥28 | 3 | |
| SOFA | <6 | 0 |
| ≥6 and <10 | 1 | |
| ≥10 | 2 | |
| Number of comorbidities | 0–1 | 0 |
| ≥2 | 1 | |
| Days from hospital to ICU admission | ≥0 and <1 | 0 |
| ≥1 | 1 |
| mNUTRIC Score on the First Day | All (n = 799) | mNUTRIC Score ≥ 6 (n = 513) | mNUTRIC Score ≤ 5 (n = 286) | p | Under-Weight (n = 149) | Normal-Weight (n = 405) | Overweight (n = 245) | p |
|---|---|---|---|---|---|---|---|---|
| Demographic characteristics, mean ± SD | ||||||||
| Age (years) | 67.1 ± 14.9 | 71.1 ± 12.5 | 60.0 ± 16.1 | <0.001 *** | 68.5 ± 15.2 | 68.0 ± 14.4 | 64.7 ± 15.2 | 0.013 * |
| BMI, kg/m2 | 22.8 ± 4.9 | 22.9 ± 4.7 | 22.7 ± 5.2 | 0.464 | 16.5 ± 1.5 | 21.7 ± 1.8 | 28.8 ± 3.5 | <0.001 *** |
| Sex (female), n (%) | 323 (40.5) | 215 (41.9) | 108 (37.9) | 0.268 | 45 (30.2) | 154 (38.0) | 124 (50.6) | <0.001 *** |
| Score, mean ± SD | ||||||||
| APACHE II | 24.7 ± 8.4 | 28.5 ± 6.9 | 18.1 ± 6.4 | <0.001 *** | 23.8 ± 7.5 | 25.3 ± 8.3 | 24.4 ± 9.0 | 0.094 |
| Charlson comorbidity index | 2.6 ± 2.0 | 2.8 ± 1.9 | 2.3 ± 2.1 | <0.001 *** | 2.7 ± 2.1 | 2.7 ± 2.0 | 2.4 ± 1.9 | 0.315 |
| SOFA | 9.0 ± 3.8 | 10.2 ± 3.4 | 6.8 ± 3.5 | <0.001 *** | 8.2 ± 3.5 | 8.9 ± 3.7 | 9.7 ± 4.1 | 0.001 ** |
| mNUTRIC | 6.0 ± 1.8 | 7.1 ± 1.0 | 4.1 ± 1.1 | <0.001 *** | 5.8 ± 1.8 | 6.1 ± 1.7 | 5.9 ± 1.8 | 0.127 |
| Comorbidities (number) | 1.7 ± 1.2 | 2.0 ± 1.1 | 1.1 ± 1.0 | <0.001 *** | 1.5 ± 1.2 | 1.8 ± 1.2 | 1.6 ± 1.2 | 0.014 * |
| Comorbidities, n (%) | ||||||||
| Coronary artery disease | 204 (25.5) | 164 (32.0) | 40 (14.0) | <0.001 *** | 26 (17.4) | 117 (28.9) | 61 (24.9) | 0.023 * |
| History of stroke | 147 (18.4) | 119 (23.2) | 28 (9.8) | <0.001 *** | 35 (23.5) | 79 (19.5) | 33 (13.5) | 0.032 * |
| Hypertension | 444 (55.6) | 318 (62.0) | 126 (44.4) | <0.001 *** | 73 (49.0) | 225 (55.7) | 146 (59.6) | 0.121 |
| COPD | 117 (14.6) | 82 (16.0) | 34 (11.9) | 0.119 | 24 (16.1) | 72 (17.8) | 21 (8.6) | 0.005 ** |
| Cancer | 190 (23.8) | 127 (25.0) | 62 (21.8) | 0.309 | 39 (26.4) | 112 (28.0) | 39 (16.0) | 0.002 ** |
| CKD | 239 (29.9) | 197 (38.4) | 42 (14.7) | <0.001 *** | 32 (21.5) | 125 (30.9) | 82 (33.5) | 0.035 * |
| Liver cirrhosis | 69 (8.6) | 50 (9.7) | 19 (6.7) | 0.138 | 9 (6.0) | 33 (8.1) | 27 (11.0) | 0.206 |
| Diabetes mellitus | 356 (44.6) | 271 (52.8) | 85 (29.8) | <0.001 *** | 48 (32.1) | 176 (43.5) | 132 (53.9) | <0.001 *** |
| Site of suspected infection, n (%) | ||||||||
| Lung | 515 (64.5) | 316 (61.6) | 198 (69.5) | 0.026 * | 119 (79.9) | 271 (66.9) | 125 (51.0) | <0.001 *** |
| UTI | 169 (21.2) | 113 (22.0) | 56 (19.6) | 0.431 | 13 (22.1) | 79 (19.5) | 57 (23.3) | 0.496 |
| Intraabdominal infection | 57 (7.1) | 40 (7.8) | 17 (6.0) | 0.336 | 5 (3.4) | 33 (8.1) | 19 (7.8) | 0.137 |
| Soft tissue infection | 40 (5.0) | 18 (3.5) | 22 (7.7) | 0.009 ** | 8 (5.4) | 14 (3.5) | 18 (7.3) | 0.086 † |
| Bacteraemia | 61 (7.6) | 44 (8.6) | 17 (6.0) | 0.183 | 6 (4.0) | 27 (6.7) | 28 (11.4) | 0.016 * |
| Baseline glucose and HbA1c, mean ± SD | ||||||||
| HbA1c (%) ** | 7.3 ± 2.2 | 7.3 ± 2.3 | 7.2 ± 2.1 | 0.453 | 6.9 ± 2.0 | 7.1 ± 2.1 | 7.6 ± 2.4 | 0.014 * |
| Glucose (mg/dL) | 205.7 ± 117.6 | 213.8 ± 106.2 | 189.5 ± 136.7 | <0.001 *** | 172.1 ± 73.7 | 210.7 ± 135.9 | 216.9 ± 102.2 | <0.001 *** |
| Admission days, mean ± SD | ||||||||
| ICU days | 12.5 ± 9.7 | 12.3 ± 9.5 | 12.9 ± 10.1 | 0.411 | 14.0 ± 10.0 | 12.0 ± 9.2 | 12.3 ± 10.3 | 0.035 * |
| LOS | 30.1 ± 28.4 | 30.5 ± 31.0 | 29.5 ± 23.0 | 0.364 | 35.6 ± 32.3 | 28.6 ± 28.1 | 29.3 ± 26.1 | 0.070 † |
| Mortality, n (%) | ||||||||
| 7-day mortality | 102 (12.8) | 83 (16.2) | 18 (6.3) | <0.001 *** | 13 (8.7) | 54 (13.3) | 35 (14.3) | 0.245 |
| 14-day mortality | 152 (19) | 117 (22.8) | 34 (11.9) | <0.001 *** | 23 (15.4) | 81 (20.0) | 48 (19.6) | 0.461 |
| 28-day mortality | 227 (28.4) | 164 (32.0) | 62 (21.8) | 0.002 ** | 35 (23.5) | 120 (29.6) | 72 (29.4) | 0.335 |
| 90-day mortality | 339 (42.4) | 240 (46.8) | 98 (34.4) | 0.001 ** | 60 (40.3) | 181 (44.7) | 98 (40.0) | 0.422 |
| mNUTRIC Score ≤ 5 (n = 286) | Underweight (n = 64) | Normal-Weight (n = 128) | Overweight (n = 94) | p | |
|---|---|---|---|---|---|
| Demographic characteristics, mean ± SD | |||||
| Age (years) | 60.0 ± 16.1 | 61.8 ± 15.4 | 60.4 ± 15.9 | 58.2 ± 16.7 | 0.283 |
| BMI, kg/m2 | 22.7 ± 5.2 | 16.4 ± 1.6 | 21.5 ± 1.9 | 28.6 ± 3.5 | <0.001 *** |
| Sex (female), n (%) | 108 (37.9) | 19 (29.7) | 44 (34.6) | 45 (47.9) | 0.041 * |
| Score, mean ± SD | |||||
| APACHE II | 18.1 ± 6.4 | 18.8 ± 6.4 | 18.1 ± 6.5 | 17.4 ± 6.3 | 0.276 |
| Charlson comorbidity index | 2.3 ± 2.1 | 2.6 ± 2.3 | 2.5 ± 2.3 | 2.0 ± 1.9 | 0.354 |
| SOFA on day 1 | 6.8 ± 3.5 | 6.1 ± 2.9 | 6.6 ± 3.1 | 7.6 ± 4.2 | 0.039 * |
| SOFA on day 3 | 6.5 ± 3.1 | 5.5 ± 2.2 | 6.5 ± 3.1 | 7.2 ± 3.5 | 0.006 ** |
| SOFA on day 7 | 5.9 ± 3.3 | 4.5 ± 2.3 | 6.4 ± 3.5 | 6.2 ± 3.4 | 0.001 ** |
| mNUTRIC | 4.1 ± 1.1 | 4.1 ± 1.1 | 4.1 ± 1.0 | 4.0 ± 1.1 | 0.550 |
| Comorbidities (number) | 1.1 ± 1.0 | 1.0 ± 0.9 | 1.2 ± 1.1 | 1.0 ± 1.1 | 0.112 |
| Comorbidities, n (%) | |||||
| Coronary artery disease | 40 (14.0) | 7 (10.9) | 19 (15) | 14 (14.9) | 0.720 |
| History of stroke | 28 (9.8) | 8 (12.5) | 17 (13.4) | 3 (3.2) | 0.030 * |
| Hypertension | 126 (44.4) | 23 (35.9) | 57 (45.2) | 46 (48.9) | 0.262 |
| COPD | 34 (11.9) | 7 (10.9) | 23 (18.1) | 4 (4.3) | 0.007 ** |
| Cancer | 62 (21.8) | 16 (25.0) | 35 (27.8) | 11 (11.7) | 0.013 * |
| CKD | 42 (14.7) | 5 (7.8) | 21 (16.5) | 16 (17.0) | 0.206 |
| Liver cirrhosis | 19 (6.7) | 4 (6.3) | 7 (5.5) | 8 (8.5) | 0.669 |
| Diabetes mellitus | 85 (29.8) | 13 (20.3) | 34 (26.8) | 38 (40.4) | 0.015 * |
| Site of suspected infection, n (%) | |||||
| Lung | 198 (69.5) | 50 (78.1) | 94 (74.0) | 54 (54.4) | 0.007 ** |
| UTI | 56 (19.6) | 12 (18.8) | 24 (18.9) | 20 (21.3) | 0.889 |
| Intraabdominal infection | 17 (6.0) | 1 (1.6) | 12 (9.4) | 4 (4.3) | 0.066 † |
| Soft tissue infection | 22 (7.7) | 5 (7.8) | 7 (5.5) | 10 (10.6) | 0369 |
| Bacteraemia | 17 (6.0) | 3 (4.7) | 7 (5.5) | 7 (7.4) | 0.741 |
| Baseline glucose and HbA1c, mean ± SD | |||||
| HbA1c (%) ** | 7.2 ± 2.1 | 7.0 ± 2.0 | 6.7 ± 1.7 | 7.7 ± 2.4 | 0.027 * |
| Glucose (mg/dL) | 189.5 ± 136.7 | 165.8 ± 72.4 | 197.8 ± 184.2 | 194.5 ± 86.6 | 0.069 † |
| Admission days, mean ± SD | |||||
| ICU days | 12.9 ± 10.1 | 14.3 ± 11.2 | 12.3 ± 8.2 | 12.9 ± 11.7 | 0.399 |
| LOS | 29.5 ± 23.0 | 32.7 ± 27.3 | 26.5 ± 18.3 | 31.5 ± 25.2 | 0.630 |
| Mortality, n (%) | |||||
| 7-day mortality | 18 (6.3) | 3 (4.7) | 7 (5.5) | 8 (8.5) | 0.551 |
| 14-day mortality | 34 (11.9) | 8 (12.5) | 13 (10.5) | 13 (13.5) | 0.708 |
| 28-day mortality | 62 (21.8) | 12 (18.8) | 30 (23.6) | 20 (21.3) | 0.736 |
| 90-day mortality | 98 (34.4) | 17 (26.6) | 50 (39.4) | 31 (33.0) | 0.200 a |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Intercept | 0.598 | 0.330 | 0.437 | 0.651 | 1.578 | 1.433 |
| Underweight | 0.557 † | 0.571 | 0.529 † | 0.560 † | 0.577 | 0.594 |
| Overweight | 0.756 | 0.712 | 0.926 | 0.735 | 0.853 | 0.905 |
| Age | 1.002 | 1.005 | 1.002 | 1.003 | 1.011 | 1.012 |
| Sex (female), n (%) | 0.950 | 0.924 | 0.936 | 0.951 | 0.661 | 0.544 |
| Score | ||||||
| SOFA on day 1 | 1.062 | 1.002 | ||||
| Comorbidities | ||||||
| Coronary artery disease | 0.785 | 0.593 | ||||
| COPD | 0.948 | 1.927 | ||||
| Cancer | 3.921 *** | 3.833 * | ||||
| DM | 0.821 | 0.758 | ||||
| Site of suspected infection | ||||||
| Lung | 0.841 | 0.717 | ||||
| Baseline glucose and HbA1c | ||||||
| HbA1c (%) | 0.792 † | 0.819 | ||||
| Goodness-of-fit | ||||||
| p | 0.498 | 0.315 | 0.001 ** | 0.586 | 0.206 | 0.242 |
| r2 | 0.012 | 0.021 | 0.086 | 0.013 | 0.054 | 0.10 |
| mNUTRIC Score ≥ 6 (n = 513) | Underweight (n = 85) | Normal-Weight (n = 277) | Overweight (n = 151) | p | |
|---|---|---|---|---|---|
| Demographic characteristics, mean ± SD | |||||
| Age (years) | 71.1 ± 12.5 | 73.6 ± 12.9 | 71.6 ± 12.2 | 68.8 ± 12.5 | 0.006 ** |
| BMI, kg/m2 | 22.9 ± 4.7 | 16.5 ± 1.5 | 21.8 ± 1.8 | 28.5 ± 3.5 | <0.001 *** |
| Sex (female), n (%) | 215 (41.9) | 26 (30.6) | 110 (39.7) | 79 (52.3) | 0.003 ** |
| Score, mean ± SD | |||||
| APACHE II | 24.7 ± 8.4 | 27.4 ± 5.9 | 28.6 ± 6.8 | 28.8 ± 7.5 | 0.445 |
| Charlson comorbidity index | 2.6 ± 2.0 | 2.7 ± 2.0 | 2.8 ± 1.9 | 2.6 ± 1.8 | 0.633 |
| SOFA on day 1 | 9.0 ± 3.8 | 9.8 ± 3.0 | 10.0 ± 3.5 | 11.0 ± 3.6 | 0.007 ** |
| SOFA on day 3 | 8.4 ± 3.6 | 7.9 ± 3.0 | 8.3 ± 3.8 | 8.8 ± 3.6 | 0.118 |
| SOFA on day 7 | 7.3 ± 3.8 | 6.9 ± 3.0 | 7.2 ± 3.9 | 7.9 ± 4.2 | 0.346 |
| mNUTRIC | 7.1 ± 1.0 | 7.1 ± 0.9 | 7.1 ± 1.0 | 7.1 ± 1.0 | 0.796 |
| Comorbidities (number) | 2.0 ± 1.1 | 1.9 ± 1.2 | 2.0 ± 1.1 | 2.0 ± 1.1 | 0.272 |
| Comorbidities, n (%) | |||||
| Coronary artery disease | 164 (32.0) | 19 (22.4) | 98 (35.4) | 47 (31.1) | 0.327 |
| History of stroke | 119 (23.2) | 27 (31.8) | 62 (22.4) | 30 (19.9) | 0.103 |
| Hypertension | 318 (62.0) | 50 (58.8) | 168 (60.6) | 100 (66.2) | 0.423 |
| COPD | 82 (16.0) | 17 (20.2) | 48 (17.3) | 17 (11.3) | 0.142 |
| Cancer | 127 (25.0) | 23 (27.4) | 76 (27.8) | 28 (17.7) | 0.099 |
| CKD | 197 (38.4) | 27 (31.8) | 104 (37.5) | 66 (43.7) | 0.177 |
| Liver cirrhosis | 50 (9.7) | 5 (5.9) | 26 (9.4) | 19 (12.6) | 0.239 |
| Diabetes mellitus | 271 (52.8) | 35 (41.2) | 142 (51.3) | 94 (62.3) | 0.006 ** |
| Site of suspected infection, n (%) | |||||
| Lung | 316 (61.6) | 69 (81.2) | 176 (63.5) | 71 (47.0) | <0.001 *** |
| UTI | 113 (22.0) | 21 (24.7) | 55 (19.9) | 37 (24.5) | 0.437 |
| Intraabdominal infection | 40 (7.8) | 4 (4.7) | 21 (7.6) | 15 (9.9) | 0.349 |
| Soft tissue infection | 18 (3.5) | 3 (3.5) | 7 (2.5) | 8 (5.3) | 0.330 |
| Bacteremia | 44 (8.6) | 3 (3.5) | 20 (7.2) | 21 (13.9) | 0.012 * |
| Baseline glucose and HbA1c, mean ± SD | |||||
| HbA1c (%) ** | 7.3 ± 2.3 | 6.7 ± 2.1 | 7.3 ± 2.2 | 7.6 ± 2.5 | 0.078 † |
| Glucose (mg/dL) | 213.8 ± 106.2 | 176.5 ± 74.8 | 216.2 ± 110.6 | 228.9 ± 108.1 | 0.001 ** |
| Admission days, mean ± SD | |||||
| ICU days | 12.3 ± 9.5 | 13.8 ± 9.0 | 12.0 ± 9.6 | 11.9 ± 9.4 | 0.083 † |
| LOS | 30.5 ± 31.0 | 37.7 ± 35.6 | 29.7 ± 31.6 | 28.0 ± 26.6 | 0.077 † |
| Mortality, n (%) | |||||
| 7-day mortality | 83 (16.2) | 10 (11.8) | 46 (16.6) | 27 (17.9) | 0.454 |
| 14-day mortality | 117 (22.8) | 15 (17.6) | 67 (24.2) | 35 (23.2) | 0.450 |
| 28-day mortality | 164 (32.0) | 23 (27.1) | 89 (32.1) | 52 (34.4) | 0.504 |
| 90-day mortality | 240 (46.8) | 43 (50.6) | 130 (46.9) | 67 (44.4) | 0.654 |
| mNUTRIC Score ≤ 5 (n = 53) | Underweight (n = 10) | Normal-Weight (n = 18) | Overweight (n = 25) | p | |
|---|---|---|---|---|---|
| IL-6 on day 1 (10/18/25) | 110.6 ± 239.9 | 174.3 ± 253.4 | 83.7 ± 164.4 | 104.5 ± 281.4 | 0.076 † |
| IL-6 on day 3 (10/17/24) | 108.8 ± 376.3 | 188.1 ± 346.2 | 32.9 ± 47.7 | 129.7 ± 501.9 | 0.072 † |
| IL-6 on day 7 (10/15/21) | 75.5 ± 241.4 | 51.5 ± 58.4 | 75.4 ± 155.9 | 87.0 ± 344.9 | 0.274 |
| IL-1RA on day 1 | 160.4 ± 288.2 | 151.7 ± 123.4 | 49.5 ± 50.7 | 243.7 ± 394.9 | 0.085 † |
| IL-1RA on day 3 | 78.6 ± 126.6 | 147.3 ± 167.1 | 39.6 ± 49.8 | 77.6 ± 137.9 | 0.061 † |
| IL-1RA on day 7 | 84.9 ± 142.2 | 108.5 ± 133.1 | 104.4 ± 166.2 | 58.9 ± 130.3 | 0.351 |
| IL-10 on day 1 | 30.3 ± 41.4 | 41.0 ± 53.2 | 21.7 ± 24.6 | 32.3 ± 46.0 | 0.826 |
| IL-10 on day 3 | 35.7 ± 36.8 | 36.3 ± 51.2 | 17.3 ± 18.6 | 27.1 ± 39.7 | 0.784 |
| IL-10 on day 7 | 32.0 ± 74.9 | 40.3 ± 81.9 | 42.9 ± 109.0 | 20.3 ± 32.0 | 0.792 |
| IL-17 on day 1 | 10.6 ± 17.8 | 19.6 ± 29.3 | 6.9 ± 14.0 | 9.7 ± 13.6 | 0.124 |
| IL-17 on day 3 | 12.5 ± 19.5 | 23.7 ± 30.4 | 7.8 ± 13.4 | 11.1 ± 16.3 | 0.099 |
| IL-17 on day 7 | 9.5 ± 13.4 | 8.9 ± 8.1 | 9.8 ± 18.9 | 9.6 ± 11.0 | 0.901 |
| TNF-α on day 1 | 33.5 ± 31.8 | 45.0 ± 29.8 | 20.7 ± 11.7 | 38.1 ± 39.4 | 0.159 |
| TNF-α on day 3 | 29.8 ± 23.7 | 34.8 ± 26.8 | 30.4 ± 20.7 | 27.2 ± 25 | 0.276 |
| TNF-α on day 7 | 23.1 ± 15.6 | 22.7 ± 18.6 | 22.9 ± 15.0 | 23.4 ± 15.3 | 0.963 |
| IFN-R on day 1 | 19.4 ± 38.6 | 55.0 ± 77.3 | 9.3 ± 11.3 | 12.5 ± 15.5 | 0.068 † |
| IFN-R on day 3 | 23.1 ± 43.7 | 64.1 ± 83.9 | 12.2 ± 13.2 | 13.7 ± 19.2 | 0.600 |
| IFN-R on day 7 | 21.0 ± 34.6 | 42.1 ± 60.8 | 16.1 ± 17.6 | 14.4 ± 23.0 | 0.677 |
| HLA-DR on day 1 | 90.1 ± 13.5 | 94.3 ± 8.5 | 82.1 ± 19.0 | 94.3 ± 6.0 | 0.007 ** |
| HLA-DR on day 3 | 92.2 ± 9.3 | 91.8 ± 14.0 | 91.0 ± 5.0 | 93.2 ± 9.3 | 0.047 * |
| HLA-DR on day 7 | 93.3 ± 7.7 | 95.0 ± 3.8 | 90.0 ± 8.5 | 94.0 ± 8.4 | 0.361 |
| mNUTRIC Score ≥ 6 (n = 112) | Underweight (n = 20) | Normal-Weight (n = 61) | Overweight (n = 31) | p | |
|---|---|---|---|---|---|
| IL-6 on day 1 (20/61/31) | 623.2 ± 2281.8 | 926.2 ± 3544.1 | 354.8 ± 1061.3 | 955.8 ± 2955.5 | 0.621 |
| IL-6 on day 3 (20/54/26) | 109.9 ± 320.5 | 61.8 ± 65.1 | 106.1 ± 200.1 | 154.8 ± 545.0 | 0.831 |
| IL6 on day 7 (18/46/23) | 50.1 ± 106.1 | 71.0 ± 195.9 | 40.5 ± 42.3 | 52.7 ± 100.8 | 0.875 |
| IL-1RA on day 1 | 854.7 ± 2526.7 | 548.1 ± 1285.0 | 983.5 ± 3087.2 | 799.1 ± 1847.9 | 0.854 |
| IL-1RA on day 3 | 361.7 ± 1474.9 | 748.2 ± 2951.2 | 279.3 ± 832.1 | 235.4 ± 613.7 | 0.936 |
| IL-1RA on day 7 | 302.0 ± 1648.7 | 912.9 ± 3608.9 | 108.2 ± 165.1 | 211.6 ± 366.4 | 0.504 |
| IL-10 on day 1 | 177.5 ± 446.8 | 130.2 ± 256.6 | 170.1 ± 448.4 | 222.5 ± 539.5 | 0.298 |
| IL-10 on day 3 | 72.2 ± 222.2 | 47.5 ± 57.1 | 98.8 ± 297.1 | 36.0 ± 49.2 | 0.549 |
| IL-10 on day 7 | 47.8 ± 168.7 | 45.9 ± 73.1 | 55.2 ± 225.8 | 34.5 ± 51.0 | 0.705 |
| IL-17 on day 1 | 16.5 ± 34.2 | 29.4 ± 56.2 | 12.0 ± 18.2 | 17.1 ± 38.3 | 0.166 |
| IL-17 on day 3 | 18.9 ± 40.0 | 40.1 ± 71.1 | 11.4 ± 22.0 | 18.3 ± 31.6 | 0.247 |
| IL-17 on day 7 | 24.8 ± 98.7 | 66.2 ± 211.4 | 14.2 ± 25.3 | 13.7 ± 21.3 | 0.599 |
| TNF-α on day 1 | 83.0 ± 143.3 | 53.8 ± 35.3 | 79.3 ± 108.1 | 109.3 ± 224.7 | 0.775 |
| TNF-α on day 3 | 58.2 ± 87.0 | 35.6 ± 24.3 | 58.5 ± 87.5 | 74.6 ± 112.2 | 0.351 |
| TNF-α on day 7 | 44.9 ± 38.3 | 37.5 ± 25.8 | 41.8 ± 30.6 | 56.8 ± 55.6 | 0.946 |
| IFN-R on day 1 | 36.8 ± 88.9 | 64.1 ± 147.6 | 29.0 ± 72.4 | 34.4 ± 65.8 | 0.540 |
| IFN-R on day 3 | 40.7 ± 142.9 | 122.9 ± 299.7 | 21.0 ± 47.3 | 18.4 ± 33.2 | 0.256 |
| IFN-R on day 7 | 70.1 ± 389.5 | 233.3 ± 839.4 | 36.1 ± 100.0 | 10.1 ± 17.0 | 0.062 † |
| HLA-DR on day 1 | 84.3 ± 16.5 | 77.3 ± 17.1 | 84.7 ± 17.3 | 88.0 ± 13.4 | 0.031 * |
| HLA-DR on day 3 | 86.5 ± 15.4 | 78.1 ± 20.2 | 87.4 ± 14.7 | 90.7 ± 10.0 | 0.060 † |
| HLA-DR on day 7 | 92.8 ± 11.3 | 89.7 ± 14.5 | 92.0 ± 12.3 | 96.7 ± 3.3 | 0.218 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-H.; Lin, C.-Y.; Chen, Y.-M.; Chang, Y.-P.; Hung, K.-Y.; Chang, Y.-C.; Chen, H.-C.; Huang, K.-T.; Chen, Y.-C.; Wang, Y.-H.; et al. Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores. Nutrients 2021, 13, 1873. https://doi.org/10.3390/nu13061873
Tsai Y-H, Lin C-Y, Chen Y-M, Chang Y-P, Hung K-Y, Chang Y-C, Chen H-C, Huang K-T, Chen Y-C, Wang Y-H, et al. Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores. Nutrients. 2021; 13(6):1873. https://doi.org/10.3390/nu13061873
Chicago/Turabian StyleTsai, Yi-Hsuan, Chiung-Yu Lin, Yu-Mu Chen, Yu-Ping Chang, Kai-Yin Hung, Ya-Chun Chang, Hung-Cheng Chen, Kuo-Tung Huang, Yung-Che Chen, Yi-Hsi Wang, and et al. 2021. "Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores" Nutrients 13, no. 6: 1873. https://doi.org/10.3390/nu13061873
APA StyleTsai, Y.-H., Lin, C.-Y., Chen, Y.-M., Chang, Y.-P., Hung, K.-Y., Chang, Y.-C., Chen, H.-C., Huang, K.-T., Chen, Y.-C., Wang, Y.-H., Wang, C.-C., Lin, M.-C., & Fang, W.-F. (2021). Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores. Nutrients, 13(6), 1873. https://doi.org/10.3390/nu13061873