Assessment of Body Composition and Dietary Intake in Nursing-Home Residents: Could Lessons Learned from the COVID-19 Pandemic Be Used to Prevent Future Casualties in Older Individuals?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Data Collection
2.2. Anthropometric and Bioimpedance Measurements
2.3. Dietary Intake
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization; National Institute of Aging; National Institute of Health. Global Health and Aging; World Health Organisation: Geneva, Switzerland, 2011; ISBN 1830-7906. [Google Scholar]
- European Commission. Active Ageing and Solidarity between Generations: A Statistical Portrait of the European Union 2012; Eurostata, European Commission: Bruxelles, Belgium, 2012; ISBN 978-92-79-21507-0. [Google Scholar]
- Croatian Bureau of Statistics. Statistical Reports: Census of Population, Households and Dwellings 2011, Population by Sex and Age; Croatian Bureau of Statistics: Zagreb, Croatia, 2013. [Google Scholar]
- Centers for Disease Control and Prevention. Older Adults. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed on 10 March 2021).
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res. Rev. 2014, 15, 51–60. [Google Scholar] [CrossRef] [PubMed]
- JafariNasabian, P.; Inglis, J.E.; Reilly, W.; Kelly, O.J.; Ilich, J.Z. Aging human body: Changes in bone, muscle and body fat with consequent changes in nutrient intake. J. Endocrinol. 2017, 234, R37–R51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh. Hig. Rada Toksikol. 2014, 65, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E. Osteosarcopenic obesity syndrome: What is it and how can it be identified and diagnosed? Curr. Gerontol. Geriatr. Res. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilich, J.Z.; Gilman, J.C.; Cvijetic, S.; Boschiero, D. Chronic stress contributes to osteosarcopenic adiposity via inflammation and immune modulation: The case for more precise nutritional investigation. Nutrients 2020, 12, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilich, J.Z. Nutritional and behavioral approaches to body composition and low-grade chronic inflammation management for older adults in the ordinary and COVID-19 times. Nutrients 2020, 12, 3898. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z. Osteosarcopenic adiposity syndrome update and the role of associated minerals and vitamins. Proc. Nutr. Soc. 2021, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kelly, O.; Gilman, J.; Boschiero, D.; Ilich, J. Osteosarcopenic obesity: Current knowledge, revised identification criteria and treatment principles. Nutrients 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hita-Contreras, F.; Martínez-Amat, A.; Cruz-Díaz, D.; Pérez-López, F.R. Osteosarcopenic obesity and fall prevention strategies. Maturitas 2015, 80, 126–132. [Google Scholar] [CrossRef]
- Tanaka, S.; Ando, K.; Kobayashi, K.; Nakashima, H.; Seki, T.; Ishizuka, S.; Machino, M.; Morozumi, M.; Kanbara, S.; Ito, S.; et al. Higher extracellular water-to-total body water ratio more strongly reflects the locomotive syndrome risk and frailty than sarcopenia. Arch. Gerontol. Geriatr. 2020, 88, 104042. [Google Scholar] [CrossRef]
- Yoshida, M.; Asagiri, K.; Fukahori, S.; Tanaka, Y.; Hashizume, N.; Ishii, S.; Saikusa, N.; Higashidate, N.; Masui, D.; Komatsuzaki, N.; et al. The utility of a phase angle analysis in patients with severe motor and intellectual disabilities. Brain Dev. 2017, 39, 557–563. [Google Scholar] [CrossRef]
- Wang, J.; Pierson, R.N. Disparate hydration of adipose and lean tissue require a new model for body water distribution in man. J. Nutr. 1976, 106, 1687–1693. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Fogelholm, M.; Lichtenbelt, M.; Van, W.D.; Fo, M. Increased extracellular water compartment, relative to intracellular water compartment, after weight reduction. J. Appl. Physiol. 1999, 87, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef] [Green Version]
- McMurray, R.G.; Hackney, A.C. Interactions of metabolic hormones, adipose tissue and exercise. Sports Med. 2005, 35, 393–412. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, O.; Shin, C.S.; Lee, S.M. Use of bioelectrical impedance analysis for the assessment of nutritional status in critically ill patients. Clin. Nutr. Res. 2015, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, M.; Munoz, N. Position of the Academy of Nutrition and Dietetics: Food and nutrition for older adults: Promoting health and wellness. J. Acad. Nutr. Diet. 2012, 112, 1255–1277. [Google Scholar] [CrossRef]
- Assis, B.S.; Jairza, J.M.B.-M.; Lopes, J.A.; Roriz, A.K.C.; Melo, A.L.; Previdell, A.; Aquino, R.D.C.; Ramos, L.B. Micronutrient intake in elderly living in nursing homes. Nutr. Hosp. 2018, 35, 59. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Alva, M.C.; Irigoyen-Camacho, M.E.; Cabrer-Rosales, M.F.; Lazarevich, I.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; Zepeda-Zepeda, M.A. Prevalence of malnutrition and depression in older adults living in nursing homes in Mexico City. Nutrients 2020, 12, 2429. [Google Scholar] [CrossRef]
- Salva, A.; Coll-Planas, L.; Bruce, S.; Groot, L.; Andrieu, S.; Abellan, G.; Vellas, B. Nutritional assessment of residents in Long-Term Care Facilities (LTCFS): Recommendations of the task force on nutrition and ageing of the IAGG European Region and the IANA. J. Nutr. Healh Aging 2009, 13, 475–483. [Google Scholar] [CrossRef]
- Lengyel, C.O.; Whiting, S.J.; Zello, G.A. Nutrient inadequacies among elderly residents of long-term care facilities. Can. J. Diet. Pract. Res. 2008, 69, 82–88. [Google Scholar] [CrossRef]
- de Boer, A.; Ter Horst, G.J.; Lorist, M.M. Physiological and psychosocial age-related changes associated with reduced food intake in older persons. Ageing Res. Rev. 2013, 12, 316–328. [Google Scholar] [CrossRef]
- Inglis, J.E.; Ilich, J.Z. The Microbiome and Osteosarcopenic Obesity in Older Individuals in Long-Term Care Facilities. Curr. Osteoporos. Rep. 2015, 13, 358–362. [Google Scholar] [CrossRef]
- Jongenelis, K.; Pot, A.M.; Eisses, A.M.H.; Beekman, A.T.F.; Kluiter, H.; Ribbe, M.W. Prevalence and risk indicators of depression in elderly nursing home patients: The AGED study. J. Affect. Disord. 2004, 83, 135–142. [Google Scholar] [CrossRef]
- Morley, J.E.; Kraenzle, D. Causes of weight loss in a community nursing home. J. Am. Geriatr. Soc. 1994, 42, 583–585. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Micronutrient intake in the etiology, prevention and treatment of osteosarcopenic obesity. Curr. Aging Sci. 2016, 9, 260–278. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Macronutrient intake and distribution in the etiology, prevention and treatment of osteosarcopenic obesity. Curr. Aging Sci. 2017, 10, 83–105. [Google Scholar] [CrossRef]
- Belmin, J.; Georges, S.; Franke, F.; Daniau, C.; Cochet, A.; Durand, C.; Noury, U.; do Espirito Santo, M.E.G.; Fonteneau, L.; Pariel, S.; et al. Coronavirus disease 2019 in French residential care facilities: A nationwide study. J. Am. Med. Dir. Assoc. 2021. [Google Scholar] [CrossRef]
- Levere, M.; Rowan, P.; Wysocki, A. The adverse effects of the COVID-19 pandemic on nursing home resident well-being. J. Am. Med. Dir. Assoc. 2021. [Google Scholar] [CrossRef]
- Perrotta, F.; Corbi, G.; Mazzeo, G.; Boccia, M.; Aronne, L.; D’Agnano, V.; Komici, K.; Mazzarella, G.; Parrella, R.; Bianco, A. COVID-19 and the elderly: Insights into pathogenesis and clinical decision-making. Aging Clin. Exp. Res. 2020, 32, 1599–1608. [Google Scholar] [CrossRef]
- Maha Hossam Eldin, I.; Abeer Awad, A. Elderly involvement in the era of COVID-19 infection. Int. Arch. Intern. Med. 2020, 4. [Google Scholar] [CrossRef]
- Morrison, A.; Fan, T.; Sen, S.; Weisenfluh, L. Epidemiology of falls and osteoporotic fractures: A systematic review. Clin. Outcomes Res. 2012, 5, 9. [Google Scholar]
- Steijaert, M.; Deurenberg, P.; Van Gaal, L.; De Leeuw, I. The use of multi-frequency impedance to determine total body water and extracellular water in obese and lean female individuals. Int. J. Obes. 1997, 21, 930–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartorio, A.; Malavolti, M.; Agosti, F.; Marinone, P.G.; Caiti, O.; Battistini, N.; Bedogni, G. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2005, 59, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Senta, A.; Pucarin-Cvetković, J.; Doko Jelinić, J. Kvantitavni Modeli Namirnica i Obroka, 1st ed.; Medicinska Naklada: Zagreb, Croatia, 2004; ISBN 953-176-252-6. [Google Scholar]
- U.S. Department of Agriculture. Agricultural Research Service. FoodData Central. Available online: Fdc.nal.usda.gov./ (accessed on 12 February 2021).
- Kolbaşı, E.N.; Demirdağ, F. Prevalence of osteosarcopenic obesity in community-dwelling older adults: A cross-sectional retrospective study. Arch. Osteoporos. 2020, 15, 166. [Google Scholar] [CrossRef]
- Hooper, L.; Bunn, D.; Jimoh, F.O.; Fairweather-Tait, S.J. Water-loss dehydration and aging. Mech. Ageing Dev. 2014, 136–137, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Delbono, O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr. Aging Sci. 2011, 4, 248–259. [Google Scholar] [CrossRef]
- Ribeiro, S.M.L.; Kehayias, J.J. Sarcopenia and the analysis of body composition. Adv. Nutr. 2014, 5, 260–267. [Google Scholar] [CrossRef]
- Bian, A.-L.; Hu, H.-Y.; Rong, Y.-D.; Wang, J.; Wang, J.-X.; Zhou, X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [Green Version]
- Hara, N.; Iwasa, M.; Sugimoto, R.; Mifuji-Moroka, R.; Yoshikawa, K.; Terasaka, E.; Hattori, A.; Ishidome, M.; Kobayashi, Y.; Hasegawa, H.; et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern. Med. 2016, 55, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Waki, M.; Kral, J.G.; Mazariegos, M.; Wang, J.; Pierson, R.N.; Heymsfield, S.B. Relative expansion of extracellular fluid in obese vs. nonobese women. Am. J. Physiol. Metab. 1991, 261, E199–E203. [Google Scholar] [CrossRef]
- Pencharz, P.B.; Azcue, M. Use of bioelectrical impedance analysis measurements in the clinical management of malnutrition. Am. J. Clin. Nutr. 1996, 64, 485S–488S. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.J. Fruit intake and osteosarcopenic obesity in Korean postmenopausal women aged 50–64 years. Maturitas 2020, 134, 41–46. [Google Scholar] [CrossRef]
- Park, S.; Na, W.; Sohn, C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal Korean women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J. Clin. Biochem. Nutr. 2018, 63, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, J.H.; Chon, D.; Lee, K.E. Diet quality and osteosarcopenic obesity in community-dwelling adults 50 years and older. Maturitas 2017, 104, 73–79. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Lee, O. Association of serum vitamin D with osteosarcopenic obesity: Korea National Health and Nutrition Examination Survey 2008–2010. J. Cachexia Sarcopenia Muscle 2017, 8, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.K.; Bae, Y.J. Protein intake and osteosarcopenic adiposity in Korean adults aged 50 years and older. Osteoporos. Int. 2020, 31, 2363–2372. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Brownbill, R.A.; Tamborini, L. Bone and nutrition in elderly women: Protein, energy, and calcium as main determinants of bone mineral density. Eur. J. Clin. Nutr. 2003, 57, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-15742-1. [Google Scholar]
- European Food Safety Authority. Nutrient Recommendations. Available online: https://efsa.gitlab.io/multimedia/drvs/index.htm (accessed on 12 February 2021).
- Massoulard, A.; Bonnabau, H.; Gindre-Poulvelarie, L.; Baptistev, A.; Preux, P.M.; Villemonteix, C.; Javerliat, V.; Fraysse, J.L.; Desport, J.C. Analysis of the food consumption of 87 elderly nursing home residents, depending on food texture. J. Nutr. Health Aging 2011, 15, 192–195. [Google Scholar] [CrossRef]
- Pohlhausen, S.; Uhlig, K.; Kiesswetter, E.; Diekmann, R.; Heseker, H.; Volkert, D.; Stehle, P.; Lesser, S. Energy and protein intake, anthropometrics, and disease burden in elderly home-care receivers—A cross-sectional study in Germany (ErnSIPP study). J. Nutr. Health Aging 2016, 20, 361–368. [Google Scholar] [CrossRef]
- Buckinx, F.; Allepaerts, S.; Paquot, N.; Reginster, J.Y.; de Cock, C.; Petermans, J.; Bruyère, O. Energy and nutrient content of food served and consumed by nursing home residents. J. Nutr. Health Aging 2017, 21, 727–732. [Google Scholar] [CrossRef]
- Grieger, J.A.; Nowson, C.A. Nutrient intake and plate waste from an Australian residential care facility. Eur. J. Clin. Nutr. 2007, 61, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suominen, M.H.; Kivisto, S.M.; Pitkala, K.H. The effects of nutrition education on professionals’ practice and on the nutrition of aged residents in dementia wards. Eur. J. Clin. Nutr. 2007, 61, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Bouchiche, C.; Soulier, K.; Lesourd, B. Dietary intakes in nursing home residents. Clin. Nutr. 2008, 3, 58. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kerstetter, J.E. Nutrition in bone health revisited: A story beyond calcium. J. Am. Coll. Nutr. 2000, 19, 715–737. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E.; Insogna, K.L.; Rodriguez, N.R.; Kerstetter, J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 2009, 57, 1073–1079. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paperfFrom the PROT-AGE study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr. Res. 2013, 33, 521–533. [Google Scholar] [CrossRef]
- de Andrade, F.B.; de França Caldas, A., Jr.; Kitoko, P.M.; Zandonade, E. The relationship between nutrient intake, dental status and family cohesion among older Brazilians. Cad. Saude Publica 2011, 27, 113–122. [Google Scholar] [CrossRef] [Green Version]
- de Lima Costa, B.V. Nutritional Status and Associated Factors in Institutionalized Elderly. J. Nutr. Disord. Ther. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Keller, H.H.; Lengyel, C.; Carrier, N.; Slaughter, S.E.; Morrison, J.; Duncan, A.M.; Steele, C.M.; Duizer, L.; Brown, K.S.; Chaudhury, H.; et al. Prevalence of inadequate micronutrient intakes of Canadian long-term care residents. Br. J. Nutr. 2018, 119, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Ilich, J.Z.; Brownbill, R.; Furr, H.; Craft, N. Dietary Vitamin A is Negatively Related to Bone Mineral Density in Postmenopausal Women. In Nutritional Aspects of Osteoporosis; Burckhardt, P., Dawson-Huges, B., Heaney, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2004; pp. 93–108. ISBN 9780080551104. [Google Scholar]
- Keser, I.; Ilich, J.Z.; Vrkić, N.; Giljević, Z.; Colić Barić, I. Folic acid and vitamin B12 supplementation lowers plasma homocysteine but has no effect on serum bone turnover markers in elderly women: A randomized, double-blind, placebo-controlled trial. Nutr. Res. 2013, 33, 211–219. [Google Scholar] [CrossRef]
- Porter, K.; Hoey, L.; Hughes, C.; Ward, M.; McNulty, H. Causes, consequences and public health implications of low B-vitamin status in ageing. Nutrients 2016, 8, 725. [Google Scholar] [CrossRef] [Green Version]
- Lewis, L.N.; Hayhoe, R.P.G.; Mulligan, A.A.; Luben, R.N.; Khaw, K.-T.; Welch, A.A. Lower dietary and circulating vitamin C in middle- and older-aged men and women are associated with lower estimated skeletal muscle mass. J. Nutr. 2020, 150, 2789–2798. [Google Scholar] [CrossRef]
- Post, L.J.; Ilich, J.Z. Controversies in Vitamin D recommendations and its possible roles in nonskeletal health issues. J. Nutr. Food Sci. 2013, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Welch, A.A.; Jennings, A.; Kelaiditi, E.; Skinner, J.; Steves, C.J. Cross-sectional associations between dietary antioxidant vitamins C, E and carotenoid intakes and sarcopenic indices in women aged 18–79 years. Calcif. Tissue Int. 2020, 106, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Inoue, S. Multiple modes of vitamin K actions in aging-related musculoskeletal disorders. Int. J. Mol. Sci. 2019, 20, 2844. [Google Scholar] [CrossRef] [Green Version]
- Ilich, J.Z.; Kelly, O.J.; Liu, P.-Y.; Shin, H.; Kim, Y.; Chi, Y.; Wickrama, K.K.A.S.; Colic-Baric, I. Role of calcium and low-fat dairy foods in weight-loss outcomes revisited: Results from the randomized trial of effects on bone and body composition in overweight/obese postmenopausal women. Nutrients 2019, 11, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvijetić, S.; Bashota, L.; Šatalić, Z. Characteristic of calcium intake in nursing home residents in Zagreb. Mljekarstvo 2020, 70, 85–92. [Google Scholar] [CrossRef]
- Kim, H.-N.; Song, S.-W.; Choi, W.-S. Association between serum zinc level and body composition: The Korean National Health and Nutrition Examination Survey. Nutrition 2016, 32, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Posso, M.; Comas, M.; Román, M.; Domingo, L.; Louro, J.; González, C.; Sala, M.; Anglès, A.; Cirera, I.; Cots, F.; et al. Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a University Hospital in Spain. Arch. Bronconeumol. 2020, 56, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Cvijetic, S.; Kovacic, J. Association between quantitative bone ultrasound and self-reported physical activity in nursing homes residents. Eur. Geriatr. Med. 2019, 10, 659–666. [Google Scholar] [CrossRef]
- Laddu, D.R.; Ozemek, C.; Sabbahi, A.; Severin, R.; Phillips, S.A.; Arena, R. Prioritizing movement to address the frailty phenotype in heart failure. Prog. Cardiovasc. Dis. 2021. [Google Scholar] [CrossRef]
- Ghram, A.; Briki, W.; Mansoor, H.; Al-Mohannadi, A.S.; Lavie, C.J.; Chamari, K. Home-based exercise can be beneficial for counteracting sedentary behavior and physical inactivity during the COVID-19 pandemic in older adults. Postgrad. Med. 2020, 1–12. [Google Scholar] [CrossRef]


| Parameters | Women (n = 69) | Men (n = 15) | p Value * |
|---|---|---|---|
| Age (years) | 80.7 ± 6.8 | 86.3 ± 3.5 | <0.001 |
| Years in residency (years) | 4.5 ± 3.7 | 3.7 ± 3.5 | 0.499 |
| Osteoporotic fractures (n) | 21 (30.4%) | 1 (6.7%) | 0.058 |
| Limited mobility (n) | 8 (11.6%) | 0 | / |
| Smoking (n) | 5 (7.2%) | 2 (2.8%) | 0.528 |
| Medications for: | |||
| Hypertension (n) | 57 (82.0%) | 10 (66.6%) | 0.193 |
| Hyperlipidemia (n) | 9 (13.0%) | 2 (13.3%) | 0.975 |
| Diabetes (n) | 2 (2.8%) | 0 | / |
| Stroke prevention (n) | 5 (7.2%) | 2 (13.3%) | 0.437 |
| Osteoporosis (n) | 2 (2.8%) | 0 | / |
| Hypothyroidism (n) | 4 (5.7%) | 0 | / |
| Medication index | 3.1 ± 4.8 | 2.7 ± 2.9 | 0.757 |
| Body Composition | |||
| Height (cm) | 156.1 ± 6.1 | 170.6 ± 6.7 | <0.001 |
| Weight (kg) | 72.3 ± 14.2 | 79.3 ± 11.2 | 0.078 |
| Body mass index (kg/m2) | 29.6 ± 5.2 | 27.2 ± 3.2 | 0.027 |
| Fat free mass (% body weight) | 57.8 ± 5.9 | 63.3 ± 5.4 | 0.001 |
| Total bone (kg) | 2.7 ± 0.6 | 3.8 ± 0.6 | <0.001 |
| T-score | −1.4 ± 0.9 | −1.0 ± 0.6 | 0.099 |
| Muscle mass (% body weight) | 14.9 ± 2.1 | 21.8 ± 2.1 | <0.001 |
| S-score | −1.2 ± 1.5 | −1.3 ± 0.9 | 0.853 |
| Fat mass (% body weight) | 42.2 ± 5.9 | 36.7 ± 5.4 | 0.001 |
| Intramuscular adipose tissue (% body weight) | 3.0 ± 0.3 | 2.9 ± 0.3 | 0.268 |
| Total body water (% body weight) | 36.4 ± 3.2 | 45.1 ± 5.0 | <0.001 |
| Extracellular water (% TBW) | 54.7 ± 5.3 | 49.1 ± 2.7 | <0.001 |
| Intracellular water (% TBW) | 45.3 ± 5.3 | 50.9 ± 2.7 | <0.001 |
| Parameters | Women (n = 69) | Men (n = 15) | p Value * |
|---|---|---|---|
| Energy (kcal) | 1363.3 ± 330.0 | 1364.8 ± 425.9 | 0.987 |
| Total protein (g) | 50.5 ± 14.4 | 55.1 ± 20.3 | 0.304 |
| Total protein (g/kg) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.594 |
| Total protein (% kcal) | 15.0 ± 3.3 | 16.3 ± 4.1 | 0.208 |
| Total fat (g) | 54.8 ± 21.6 | 48.3 ± 17.1 | 0.277 |
| Total fat (% kcal) | 35.3 ± 9.1 | 32.0 ± 6.8 | 0.189 |
| Saturated fatty acids (g) | 23.1 ± 10.3 | 20.6 ± 6.8 | 0.307 |
| Saturated fatty acids (% kcal) | 14.8 ± 4.8 | 13.8 ± 3.1 | 0.301 |
| Omega–3 fatty acids (g) | 0.1 ± 0.6 | 0.1 ± 0.1 | 0.944 |
| Cholesterol (mg) | 135.8 ± 99.6 | 149.5 ± 55.2 | 0.187 |
| Carbohydrate (g) | 172.7 ± 43.1 | 185.4 ± 65.2 | 0.351 |
| Carbohydrate (% kcal) | 51.4 ± 9.6 | 54.1 ± 7.5 | 0.316 |
| Dietary fiber (g) | 15.9 ± 5.8 | 17.5 ± 7.2 | 0.335 |
| Parameters | Women (n = 69) | Men (n = 15) | p Value * | ||
|---|---|---|---|---|---|
| Intake /Day | EFSA | Intake/Day | EFSA | ||
| Vitamins | |||||
| Vitamin A (µg RE) 2 | 421.2 ± 269.6 | 650 | 419.7 ± 254.4 | 750 | 0.986 |
| Vitamin B1 (mg/MJ) 3 | 0.5 ± 1.4 | 0.1 | 0.1 ± 0.1 | 0.1 | 0.875 |
| Vitamin B2 (mg) | 3.3 ± 8.8 | 1.6 | 0.8 ± 0.3 | 1.6 | 0.939 |
| Vitamin B3 (mg NE/MJ) 4 | 2.5 ± 2.0 | 1.6 | 2.2 ± 0.8 | 1.6 | 0.362 |
| Vitamin B6 (mg) | 3.5 ± 8.0 | 1.6 | 1.0 ± 0.5 | 1.7 | 0.158 |
| Vitamin C (mg) | 167.6 ± 178.8 | 95 | 82.3 ± 57.7 | 110 | 0.038 |
| Vitamin D (µg) | 1.1 ± 0.7 | 15 | 0.1 ± 0.4 | 15 | 0.730 |
| Vitamin E (mg) | 5.4 ± 2.1 | 11 | 4.5 ± 2.4 | 13 | 0.172 |
| Vitamin K (µg) | 58.9 ± 46.4 | 70 | 58.0 ± 53.9 | 70 | 0.766 |
| Minerals | |||||
| Calcium (mg) | 576.3 ± 285.0 | 950 | 638.4 ± 397.4 | 950 | 0.949 |
| Copper (mg) | 1.0 ± 0.4 | 1.3 | 1.0 ± 0.6 | 1.6 | 0.856 |
| Iron (mg) | 7.4 ± 2.4 | 11 | 7.4 ± 2.1 | 11 | 0.977 |
| Magnesium (mg) | 275.8 ± 206.8 | 300 | 135.3 ± 58.5 | 350 | 0.083 |
| Phosphorus (mg) | 776.4 ± 229.5 | 550 | 841.4 ± 289.1 | 550 | 0.378 |
| Potassium (mg) | 1941.9 ± 553.7 | 3500 | 1967.5 ± 614.4 | 3500 | 0.866 |
| Selenium (µg) | 72.3 ± 20.8 | 70 | 80.7 ± 32.3 | 70 | 0.204 |
| Sodium (mg) | 1587.1 ± 676.4 | 2000 | 1526.5 ± 688.2 | 2000 | 0.875 |
| Zinc (mg) | 5.6 ± 2.6 | 7.5 | 6.5 ± 2.9 | 9.4 | 0.185 |
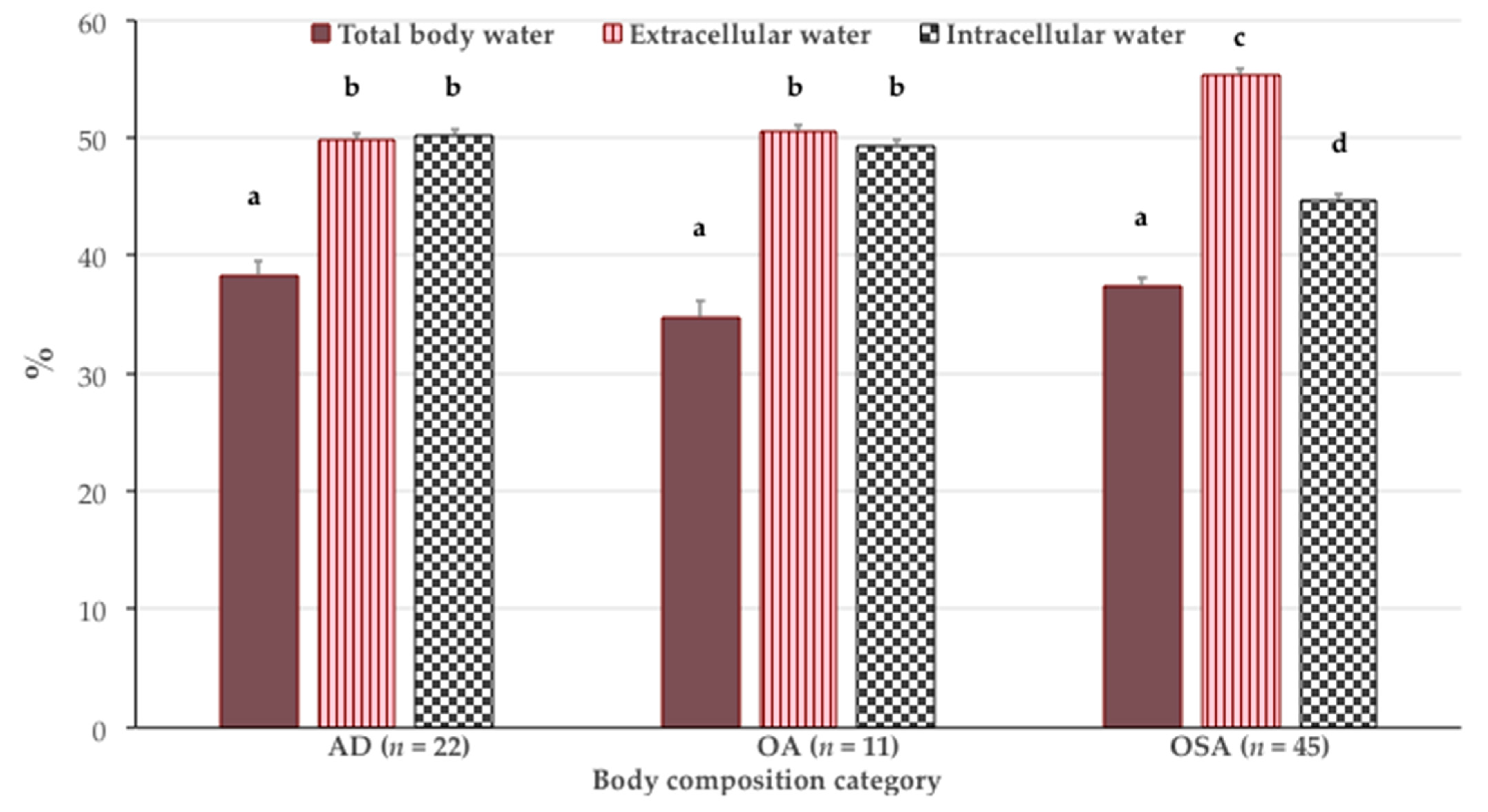
| Parameters | AD (n = 22) | OA (n = 11) | OSA (n = 45) | p Value * |
|---|---|---|---|---|
| Age (years) | 81.7 ± 6.3 | 78.0 ± 7.8 | 82.4 ± 6.7 | 0.165 |
| Height (cm) | 161.6 ± 6.9 | 155.6 ± 4.9 | 158.1 ± 8.4 | 0.079 |
| Weight (kg) | 88.0 ± 1.6 a | 79.8 ± 7.5 b | 67.5 ± 9.2 c | <0.001 |
| Body mass index (kg/m2) | 33.8 ± 4.0 a | 33.0 ± 3.4 a | 27.0 ± 2.8 b | <0.001 |
| Fat free mass (% body weight) | 55.8 ± 4.8 a | 53.6 ± 5.1 a | 59.8 ± 4.3 b | <0.001 |
| Total bone (kg) | 3.7 ± 0.6 a | 3.0 ± 0.3 b | 2.6 ± 0.5 b | <0.001 |
| T-score | −0.2 ± 0.6 a | −1.2 ± 0.1 b | −1.8 ± 0.5 c | <0.001 |
| Muscle (% body weight) | 18.4 ± 2.9 a | 15.9 ± 2.4 b | 15.3 ± 2.8 c | <0.001 |
| S-score | 0.5 ± 1.0 a | −0.5 ± 0.4 b | −2.0 ± 0.7 c | <0.001 |
| Fat mass (% body weight) | 44.2 ± 4.8 a | 46.4 ± 5.1 a | 40.2 ± 4.3 b | <0.001 |
| Intramuscular adipose tissue (% body weight) | 3.0 ± 0.2 a | 3.1 ± 0.3 b | 2.9 ± 0.2 c | 0.023 |
| Parameters | AD (n = 22) | OA (n = 11) | OSA (n = 45) | p Value * |
|---|---|---|---|---|
| Energy (kcal) | 1353.1 ± 311.5 | 1443.5 ± 440.4 | 1376.9 ± 337.9 | 0.777 |
| Total protein (g) | 57.0 ± 15.5 | 52.3 ± 14.9 | 49.7 ± 14.7 | 0.177 |
| Total protein (g/kg) | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.172 |
| Total protein (% kcal) | 17.0 ± 3.6 a | 14.7 ± 3.0 b | 14.6 ± 3.3 b | 0.021 |
| Total fat (g) | 53.4 ± 20.0 | 59.8 ± 27.3 | 53.5 ± 20.4 | 0.661 |
| Total fat (% kcal) | 35.1 ± 9.1 | 36.0 ± 7.0 | 34.2 ± 9.0 | 0.803 |
| Saturated fatty acids (g) | 22.1 ± 8.5 | 25.0 ± 11.3 | 22.8 ± 10.2 | 0.716 |
| Saturated fatty acids (% kcal) | 14.4 ± 3.8 | 15.3 ± 3.7 | 14.5 ± 5.0 | 0.833 |
| Omega—3 fatty acids (g) | 0.1 ± 0.03 | 0.1 ± 0.1 | 0.2 ± 0.7 | 0.555 |
| Cholesterol (mg) | 124.5 ± 108.6 | 171.9 ± 145.5 | 136.3 ± 69.8 | 0.397 |
| Carbohydrate (g) | 166.7 ± 43.6 | 178.1 ± 44.8 | 181.0 ± 49.4 | 0.507 |
| Carbohydrate (% kcal) | 49.6 ± 9.0 | 50.5 ± 6.8 | 53.2 ± 9.4 | 0.265 |
| Dietary fiber (g) | 16.2 ± 6.4 | 14.3 ± 6.0 | 16.8 ± 6.1 | 0.499 |
| Parameters | AD (n = 22) | OA (n = 11) | OSA (n = 45) | p Value * |
|---|---|---|---|---|
| Vitamins | ||||
| Vitamin A (µg RE) 2 | 331.5 ± 264.9 | 429.0 ± 173.6 | 458.1 ± 285.2 | 0.195 |
| Vitamin B1 (mg/MJ) 3 | 0.3 ± 0.8 | 0.5 ± 1.0 | 0.6 ± 1.5 | 0.722 |
| Vitamin B2 (mg) | 1.9 ± 5.4 | 3.3 ± 7.6 | 3.6 ± 9.6 | 0.739 |
| Vitamin B3 (mg NE/MJ) 4 | 2.3 ± 1.8 | 2.4 ± 1.6 | 2.5 ± 2.1 | 0.926 |
| Vitamin B6 (mg) | 2.2 ± 4.3 | 3.2 ± 6.0 | 3.7 ± 9.1 | 0.753 |
| Vitamin C (mg) | 151.0 ± 188.4 | 166.3 ± 146.6 | 155.8 ± 171.5 | 0.972 |
| Vitamin D (µg) | 1.0 ± 0.4 | 1.4 ± 0.9 | 1.1 ± 0.6 | 0.191 |
| Vitamin E (mg) | 6.4 ± 2.1 | 6.6 ± 2.6 | 5.0 ± 2.1 | 0.099 |
| Vitamin K (µg) | 54.8 ± 45.4 | 69.5 ± 48.2 | 59.1 ± 48.8 | 0.708 |
| Minerals | ||||
| Calcium (mg) | 534.9 ± 305.2 | 744.9 ± 417.7 | 569.5 ± 262.1 | 0.151 |
| Copper (mg) | 0.9 ± 0.4 | 0.9 ± 0.3 | 1.0 ± 0.5 | 0.357 |
| Iron (mg) | 7.9 ± 2.4 | 7.6 ± 2.3 | 7.4 ± 2.3 | 0.767 |
| Magnesium (mg) | 257.1 ± 195.8 | 272.0 ± 182.3 | 245.0 ± 204.9 | 0.914 |
| Phosphorus (mg) | 832.4 ± 236.1 | 879.8 ± 256.0 | 759.4 ± 235.1 | 0.237 |
| Potassium (mg) | 1947.0 ± 597.7 | 2052.0 ± 464.2 | 1939.9 ± 596.6 | 0.844 |
| Selenium (µg) | 84.6 ± 20.9 a | 69.9 ± 15.3 b | 71.1 ± 23.4 b | 0.048 |
| Sodium (mg) | 1575.2 ± 522.5 | 1769.2 ± 687.7 | 1546.8 ± 695.4 | 0.596 |
| Zinc (mg) | 5.6 ± 2.7 | 5.8 ± 2.3 | 5.9 ± 2.9 | 0.925 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keser, I.; Cvijetić, S.; Ilić, A.; Colić Barić, I.; Boschiero, D.; Ilich, J.Z. Assessment of Body Composition and Dietary Intake in Nursing-Home Residents: Could Lessons Learned from the COVID-19 Pandemic Be Used to Prevent Future Casualties in Older Individuals? Nutrients 2021, 13, 1510. https://doi.org/10.3390/nu13051510
Keser I, Cvijetić S, Ilić A, Colić Barić I, Boschiero D, Ilich JZ. Assessment of Body Composition and Dietary Intake in Nursing-Home Residents: Could Lessons Learned from the COVID-19 Pandemic Be Used to Prevent Future Casualties in Older Individuals? Nutrients. 2021; 13(5):1510. https://doi.org/10.3390/nu13051510
Chicago/Turabian StyleKeser, Irena, Selma Cvijetić, Ana Ilić, Irena Colić Barić, Dario Boschiero, and Jasminka Z. Ilich. 2021. "Assessment of Body Composition and Dietary Intake in Nursing-Home Residents: Could Lessons Learned from the COVID-19 Pandemic Be Used to Prevent Future Casualties in Older Individuals?" Nutrients 13, no. 5: 1510. https://doi.org/10.3390/nu13051510






