A Randomized Study of Nutritional Supplementation in Patients with Unilateral Wet Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Interventions
2.3. Evaluations and Outcomes
2.4. Biochemical Analyses
2.4.1. Determination and Analysis of Lutein and Zeaxanthin
2.4.2. Multiplex Cytokine Analysis of IL-1b, -6, -8, -9, -10, -12p70, IFN-γ, MCP1 and TNF-α
2.4.3. MMP-10 Analysis by ELISA
2.4.4. VEGF Measurement by Western Blot
2.4.5. Fatty Acid Profile Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Disposition and Baseline Characteristics
3.2. Best Corrected Visual Acuity
3.3. Carotenoids and Polyunsaturated Fatty Acids
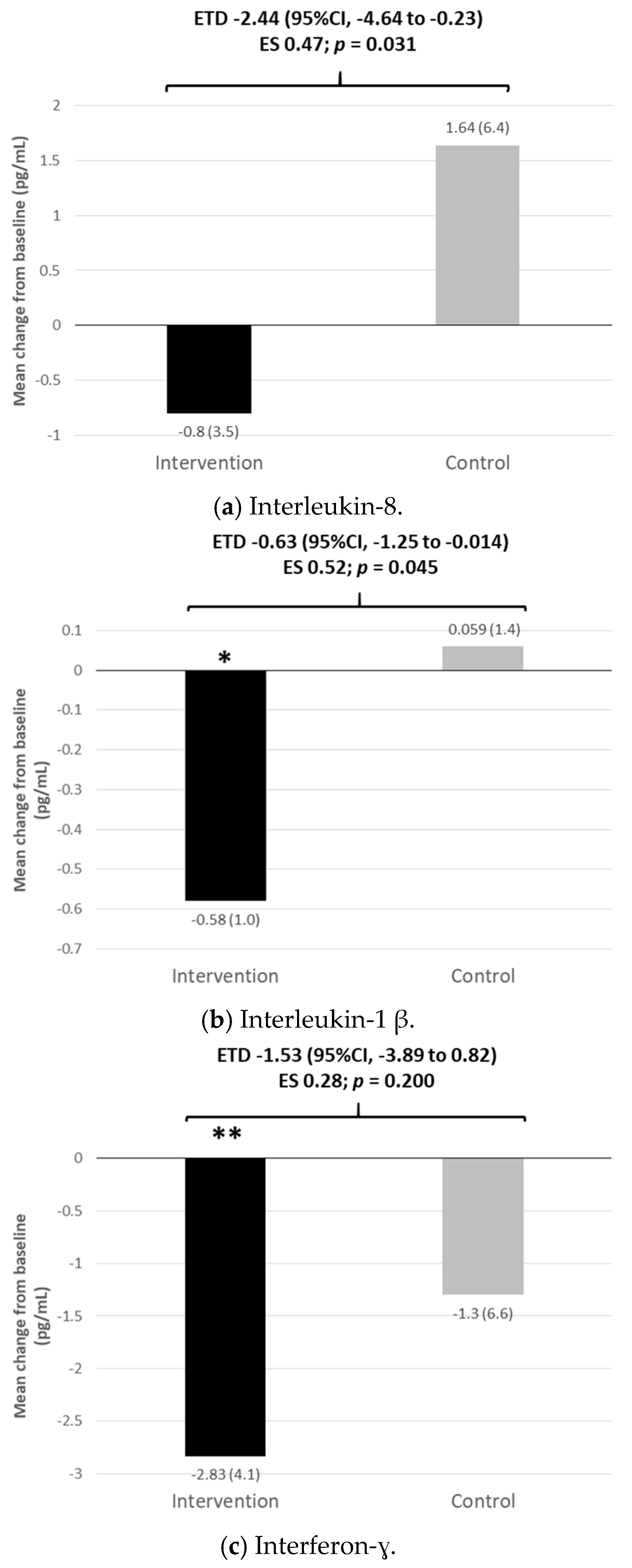
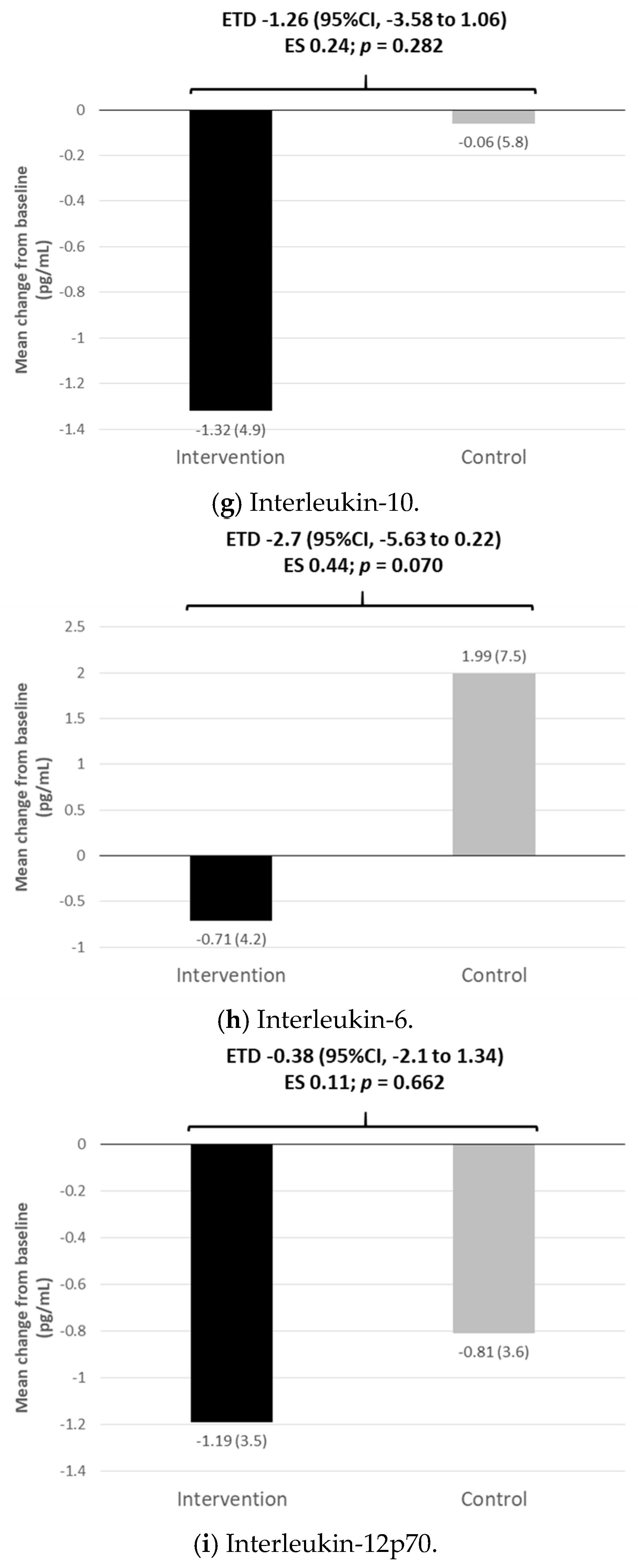
3.4. Inflammatory and Oxidative Stress Markers and Vascular Endothelial Growth Factor
3.5. Tolerability and Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of age-related macular degeneration in Europe: The past and the future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Lane, J.; Rohan, E.M.F.; Sabeti, F.; Essex, R.W.; Maddess, T.; Dawel, A.; Robbins, R.A.; Barnes, N.; He, X.; McKone, E. Impacts of impaired face perception on social interactions and quality of life in age-related macular degeneration: A qualitative study and new community resources. PLoS ONE 2018, 13, e0209218. [Google Scholar] [CrossRef] [Green Version]
- Elshout, M.; Webers, C.A.; van der Reis, M.I.; de Jong-Hesse, Y.; Schouten, J.S. Tracing the natural course of visual acuity and quality of life in neovascular age-related macular degeneration: A systematic review and quality of life study. BMC Ophthalmol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatziralli, I.; Mitropoulos, P.; Parikakis, E.; Niakas, D.; Labiris, G. Risk factors for poor quality of life among patients with age-related macular degeneration. Semin. Ophthalmol. 2017, 32, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2019, 3, CD005139. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Mones, J.; Richard, G.; Bandello, F.; et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- García-Layana, A.; García-Arumí, J.; Figueroa, M.S.; Arias Barquet, L.; Ruíz-Moreno, J.M.; Monclús-Arbona, L.; The Spanish AMD Multicenter Group. Management of wet age-related macular degeneration in Spain: Challenges for treat and extend implementation in routine clinical practice. J. Ophthalmol. 2019, 2019, 9821509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorusupudi, A.; Nelson, K.; Bernstein, P.S. The age-related eye disease 2 study: Micronutrients in the treatment of macular degeneration. Adv. Nutr. 2017, 8, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group; Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015, 133, 1415. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2014, 56, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eynard, A.R.; Repossi, G. Role of ω3 polyunsaturated fatty acids in diabetic retinopathy: A morphological and metabolically cross talk among blood retina barriers damage, autoimmunity and chronic inflammation. Lipids Health Dis. 2019, 18, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merle, B.M.; Benlian, P.; Puche, N.; Bassols, A.; Delcourt, C.; Souied, E.H.; Nutritional , A.M.D. Treatment Study Group. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2014, 55, 2010–2019. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Lin, Y.; Terry, R.; Nelson, K.; Bernstein, P.S. Role of long-chain and very-long-chain polyunsaturated fatty acids in macular degenerations and dystrophies. Clin. Lipidol. 2011, 6, 593–613. [Google Scholar] [CrossRef] [Green Version]
- García-Layana, A.; Guirao Navarro, M. The European perspective of the nutritional supplements in AMD prevention. In Proceedings of the 12th ISOPT Clinical the International Symposium on Ocula Pharmacology and Therapeutics, Berlin, Germany, 9–12 July 2015; pp. 7–11. [Google Scholar]
- Liu, Z.; Sun, L.; Zhu, L.; Jia, X.; Li, X.; Jia, H.; Wang, Y.; Weber, P.; Long, J.; Liu, J. Hydroxytyrosol protects retinal pigment epithelial cells from acrolein-induced oxidative stress and mitochondrial dysfunction. J. Neurochem. 2007, 103, 2690–2700. [Google Scholar] [CrossRef] [Green Version]
- Nagineni, C.N.; Raju, R.; Nagineni, K.K.; Kommineni, V.K.; Cherukuri, A.; Kutty, R.K.; Hooks, J.J.; Detrick, B. Resveratrol suppresses expression of VEGF by human retinal pigment epithelial cells: Potential nutraceutical for age-related macular degeneration. Aging Dis. 2014, 5, 88–100. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Cuadrado-Vives, C. Markers of lutein and zeaxanthin status in two age groups of men and women: Dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr. J. 2014, 13, 52. [Google Scholar] [CrossRef] [Green Version]
- Olmedilla-Alonso, B.; Estévez-Santiago, R.; Silván, J.M.; Sánchez-Prieto, M.; de Pascual-Teresa, S. Effect of Long-Term Xanthophyll and Anthocyanin Supplementation on Lutein and Zeaxanthin Serum Concentrations and Macular Pigment Optical Density in Postmenopausal Women. Nutrients 2018, 10, 959. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, M.; Recalde, S.; Garcia-Garcia, L.; Bezunartea, J.; Miskey, C.; Johnen, S.; Diarra, S.; Sebe, A.; Rodriguez-Madoz, J.R.; Pouillot, S.; et al. Preclinical evaluation of a cell-based gene therapy using the sleeping beauty transposon system in choroidal neovascularization. Mol. Ther. Methods Clin. Dev. 2019, 15, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Bondia-Pons, I.; Castellote, A.I.; López-Sabater, M.C. Comparison of conventional and fast gas chromatography in human plasma fatty acid determination. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 809, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kazis, L.E.; Anderson, J.J.; Meenan, R.F. Effect sizes for interpreting changes in health status. Med. Care 1989, 27, S178–S189. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; Sangiovanni, J.P.; Davis, M.D.; Ferris, F.L. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study. JAMA Ophthalmol. 2014, 132, 272. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Meng, Y.F.; Xing, Q.; Tao, J.J.; Lu, J. Fish consumption and age-related macular degeneration incidence: A meta-analysis and systematic review of prospective cohort studies. Nutrients 2016, 8, 743. [Google Scholar] [CrossRef] [Green Version]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Food groups and risk of age-related macular degeneration: A systematic review with meta-analysis. Eur. J. Nutr. 2019, 58, 2123–2143. [Google Scholar] [CrossRef]
- Lawrenson, J.G.; Evans, J.R. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2015, 2015, CD010015. [Google Scholar] [CrossRef] [Green Version]
- Souied, E.H.; Aslam, T.; Garcia-Layana, A.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C. Omega-3 fatty acids and age-related macular degeneration. Ophthalmic Res. 2015, 55, 62–69. [Google Scholar] [CrossRef]
- Čolak, E.; Ignjatović, S.; Radosavljević, A.; Žorić, L. The association of enzymatic and non-enzymatic antioxidant defense parameters with inflammatory markers in patients with exudative form of age-related macular degeneration. J. Clin. Biochem. Nutr. 2017, 60, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Haas, P.; Kubista, K.E.; Krugluger, W.; Huber, J.; Binder, S. Impact of visceral fat and pro-inflammatory factors on the pathogenesis of age-related macular degeneration. Acta Ophthalmol. 2015, 93, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Myers, C.E.; Cruickshanks, K.J.; Gangnon, R.E.; Danforth, L.G.; Sivakumaran, T.A.; Iyengar, S.K.; Tsai, M.Y.; Klein, B.E.K. Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: The Beaver Dam Eye Study. JAMA Ophthalmol. 2014, 132, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. Cellular senescence: Putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011, 21, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Biarnés Costa, M.; Gerhardinger, C. IL-1β is upregulated in the diabetic retina and retinal vessels: Cell-specific effect of high glucose and IL-1β autostimulation. PLoS ONE 2012, 7, e36949. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Bai, Y.; Xie, W.; Shi, X.; Li, F.; Yang, F.; Sun, Y.; Huang, L.; Li, X. Interleukin-1β level is increased in vitreous of patients with neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV). PLoS ONE 2015, 10, e0125150. [Google Scholar] [CrossRef] [Green Version]
- Subhi, Y.; Krogh Nielsen, M.; Molbech, C.R.; Oishi, A.; Singh, A.; Nissen, M.H.; Sørensen, T.L. Plasma markers of chronic low-grade inflammation in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Acta Ophthalmol. 2019, 97, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Lavalette, S.; Raoul, W.; Houssier, M.; Camelo, S.; Levy, O.; Calippe, B.; Jonet, L.; Behar-Cohen, F.; Chemtob, S.; Guillonneau, X.; et al. Interleukin-1β inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am. J. Pathol. 2011, 178, 2416–2423. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Xu, X.; Chiou, G.C. Effect of interleukin-1 blockers, CK112, and CK116 on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro. J. Ocul. Pharmacol. Ther. 2006, 22, 19–25. [Google Scholar] [CrossRef]
- Barnes, T.C.; Anderson, M.E.; Moots, R.J. The many faces of interleukin-6: The role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011, 2011, 721608. [Google Scholar] [CrossRef] [Green Version]
- Roh, M.I.; Kim, H.S.; Song, J.H.; Lim, J.B.; Koh, H.J.; Kwon, O.W. Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with amd after bevacizumab treatment. Retina 2009, 29, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Staurenghi, G.; Lepre, T.; Missiroli, F.; Zampatti, S.; Cascella, R.; Borgiani, P.; Marsella, L.T.; Eandi, C.M.; Cusumano, A.; et al. Haplotypes in IL-8 gene are associated to age-related macular degeneration: A case-control study. PLoS ONE 2013, 8, e66978. [Google Scholar] [CrossRef]
- Mimura, T.; Funatsu, H.; Noma, H.; Shimura, M.; Kamei, Y.; Yoshida, M.; Kondo, A.; Watanabe, E.; Mizota, A. Aqueous humor levels of cytokines in patients with age-related macular degeneration. Ophthalmologica 2019, 241, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, Z.; Li, J.; Zhao, G. Associations of IL-8 gene polymorphisms and IL-8 levels with predisposition to age-related macular degeneration: A meta-analysis. Aging Clin. Exp. Res. 2020, 32, 2703. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Tao, Y.; Neumaier, M.; Findeisen, P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch. Ophthalmol. 2010, 128, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Tsujikawa, M.; Itabe, H.; Du, Z.J.; Xie, P.; Matsumura, N.; Fu, X.; Zhang, R.; Sonoda, K.-h.; Egashira, K.; et al. Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J. Cell Sci. 2012, 125, 2407–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touhami, S.; Beguier, F.; Augustin, S.; Charles-Messance, H.; Vignaud, L.; Nandrot, E.F.; Reichman, S.; Forster, V.; Mathis, T.; Sahel, J.-A.; et al. Chronic exposure to tumor necrosis factor alpha induces retinal pigment epithelium cell dedifferentiation. J. Neuroinflammation 2018, 15, 85. [Google Scholar] [CrossRef]
- Lichtlen, P.; Lam, T.T.; Nork, T.M.; Streit, T.; Urech, D.M. Relative contribution of VEGF and TNF-alpha in the cynomolgus laser-induced CNV model: Comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4738–4745. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Wittchen, E.S.; Hartnett, M.E. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol. Vis. 2016, 22, 116–128. [Google Scholar]
- Mirshahi, A.; Hoehn, R.; Lorenz, K.; Kramann, C.; Baatz, H. Anti-tumor necrosis factor alpha for retinal diseases: Current knowledge and future concepts. J. Ophthalmic. Vis. Res. 2012, 7, 39–44. [Google Scholar]
- Fernández-Vega, B.; Fernández-Vega, Á.; Rangel, C.M.; Nicieza, J.; Villota-Deleu, E.; Vega, J.A.; Sanchez-Avila, R.M. Blockade of tumor necrosis factor-alpha: A role for adalimumab in neovascular age-related macular degeneration refractory to anti-angiogenesis therapy? Case Rep. Ophthalmol. 2016, 7, 154–162. [Google Scholar] [CrossRef]
- Wu, L.; Arevalo, J.F.; Hernandez-Bogantes, E.; Regatieri, C.V.; Roca, J.A.; Farah, M.E. Intravitreal tumor necrosis factor-alpha inhibitors for neovascular age-related macular degeneration suboptimally responsive to antivascular endothelial growth factor agents: A pilot study from the pan american collaborative retina study group. J. Ocul. Pharmacol. Ther. 2013, 29, 366–371. [Google Scholar] [CrossRef]
- Latruffe, N.; Lançon, A.; Frazzi, R.; Aires, V.; Delmas, D.; Michaille, J.J.; Djouadi, F.; Bastin, J.; Cherkaoui-Malki, M. Exploring new ways of regulation by resveratrol involving miRNAs, with emphasis on inflammation. Ann. N. Y. Acad. Sci. 2015, 1348, 97–106. [Google Scholar] [CrossRef]
- Hakobyan, S.; Harris, C.L.; Tortajada, A.; Goicochea de Jorge, E.; García-Layana, A.; Fernández-Robredo, P.; Rodríguez de Córdoba, S.; Morgan, B.P. Measurement of factor H variants in plasma using variant-specific monoclonal antibodies: Application to assessing risk of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1983–1990. [Google Scholar] [CrossRef] [Green Version]
- Aslam, T.; Delcourt, C.; Holz, F.; García-Layana, A.; Leys, A.; Silva, R.M.; Souied, E. European survey on the opinion and use of micronutrition in age-related macular degeneration: 10 years on from the Age-Related Eye Disease Study. Clin. Ophthalmol. 2014, 8, 2045–2053. [Google Scholar] [CrossRef] [Green Version]


| Characteristic | Intervention N = 50 | Control N = 59 |
|---|---|---|
| Age (years), mean (SD) | 78.4 (7.0) | 76.0 (8.0) |
| Sex (women), n (%) | 22 (44.0) | 31 (52.5) |
| BCVA, mean (SD) | 75.6 (11.1) | 76.2 (11.7) |
| Corneal alterations (yes), n (%) | 2 (4.0) | 2 (3.4) |
| AMD status | ||
| Presence of drusen, n (%) | 41 (82.0) | 42 (71.2) |
| Degree of drusen, n (%) | ||
| 1 | 8 (16.0) | 14 (23.7) |
| 2 | 17 (34.0) | 15 (25.4) |
| 3 | 10 (20.0) | 6 (10.2) |
| 4 | 6 (12.0) | 7 (11.9) |
| Missing | 8 (18.0) | 17 (28.8) |
| Pigmentary alterations, n (%) | ||
| Hyperpigmentation | 15 (30.0) | 13 (22.0) |
| Hypo-/hyperpigmentation | 15 (30.0) | 15 (25.4) |
| Hypopigmentation | 3 (6.0) | 5 (8.5) |
| No alterations | 17 (34.0) | 15 (42.4) |
| Geographic atrophy (yes), n (%) | 9 (18.0) | 4 (6.8) |
| LENS status | ||
| Phakic | 30 (60.0) | 37 (62.7) |
| Pseudophakic | 20 (40.0) | 22 (37.3) |
| Intervention | Control | Mean Difference (Intervention-Control) | p-Value | 95% CI | Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean Change | SD | N | Mean Change | SD | Student’s t-Test | Lower | Upper | Cohen’s d | |
| CAROTENOIDS (μg/dL) | |||||||||||
| Lutein | 42 | 24.41 ** | 27.93 | 43 | −1.57 | 6.58 | 26.0 | <0.001 | 17.08 | 34.91 | 1.29 |
| Zeaxanthin | 42 | 2.88 ** | 3.52 | 43 | −0.09 | 1.29 | 2.98 | <0.001 | 1.83 | 4.13 | 1.13 |
| POLYUNSATURATED FATTY ACIDS (as % of total fatty acids in plasma) | |||||||||||
| DHA | 34 | 0.74 | 0.59 | 35 | 0.04 | 0.66 | 0.701 | <0.001 | 0.4 | 1.004 | 1.12 |
| Σ n-3 PUFAs | 34 | 0.82 | 1.1 | 35 | 0.05 | 1.04 | 0.763 | 0.004 | 0.248 | 1.279 | 0.72 |
| Σ n-6 PUFAs | 34 | −0.97 | 4.4 | 35 | 2.47 | 7.28 | −3.444 | 0.021 | −6.347 | −0.541 | 0.57 |
| Σ n-3 LCPUFAs | 34 | 0.79 | 1.11 | 35 | 0.08 | 1 | 0.715 | 0.006 | 0.207 | 1.222 | 0.67 |
| Σ n-6 LCPUFAs | 34 | −0.35 | 1.15 | 35 | 0.3 | 2.19 | −0.654 | 0.126 | −1.5 | 0.188 | 0.37 |
| Ratio of n-6/n-3 PUFAs | 34 | −2.18 | 2.59 | 35 | 1.05 | 3.35 | −3.227 | <0.001 | −4.7 | −1.785 | 1.08 |
| Ratio of LCn-6:LCn-3 PUFAs | 34 | −0.6 | 0.64 | 35 | 0.18 | 0.89 | −0.783 | <0.001 | −1.155 | −0.412 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Layana, A.; Recalde, S.; Hernandez, M.; Abraldes, M.J.; Nascimento, J.; Hernández-Galilea, E.; Olmedilla-Alonso, B.; Escobar-Barranco, J.J.; Zapata, M.A.; Silva, R.; et al. A Randomized Study of Nutritional Supplementation in Patients with Unilateral Wet Age-Related Macular Degeneration. Nutrients 2021, 13, 1253. https://doi.org/10.3390/nu13041253
García-Layana A, Recalde S, Hernandez M, Abraldes MJ, Nascimento J, Hernández-Galilea E, Olmedilla-Alonso B, Escobar-Barranco JJ, Zapata MA, Silva R, et al. A Randomized Study of Nutritional Supplementation in Patients with Unilateral Wet Age-Related Macular Degeneration. Nutrients. 2021; 13(4):1253. https://doi.org/10.3390/nu13041253
Chicago/Turabian StyleGarcía-Layana, Alfredo, Sergio Recalde, Maria Hernandez, Maximino J. Abraldes, João Nascimento, Emiliano Hernández-Galilea, Begoña Olmedilla-Alonso, Jose Juan Escobar-Barranco, Miguel Angel Zapata, Rufino Silva, and et al. 2021. "A Randomized Study of Nutritional Supplementation in Patients with Unilateral Wet Age-Related Macular Degeneration" Nutrients 13, no. 4: 1253. https://doi.org/10.3390/nu13041253






