The Ability of Exercise to Mitigate Caloric Restriction-Induced Bone Loss in Older Adults: A Structured Review of RCTs and Narrative Review of Exercise-Induced Changes in Bone Biomarkers
Abstract
1. Introduction
2. Structured Literature Review of Published RCTs
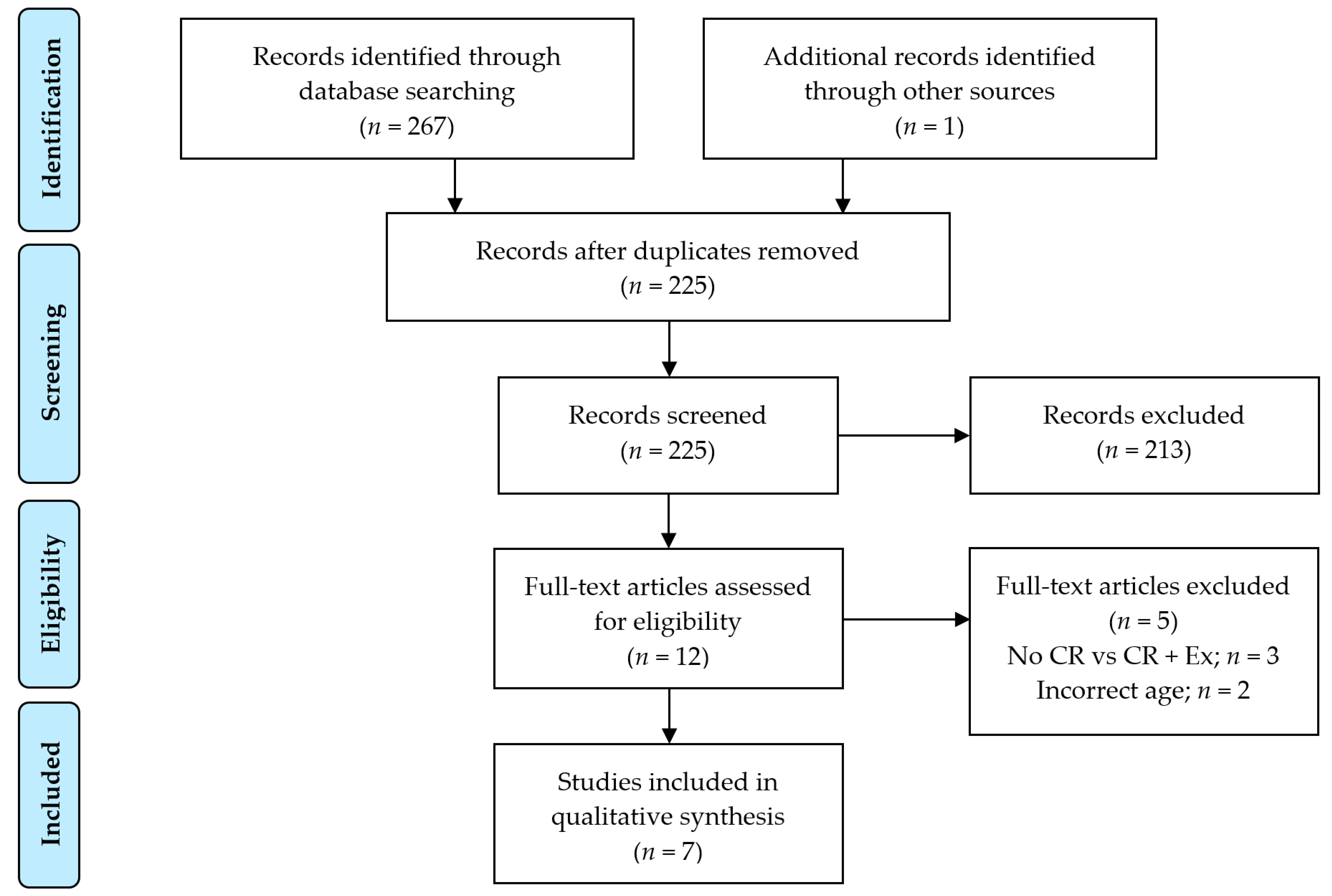
2.1. Search Strategy, Eligibility and Study Selection Criteria, and Data Extraction Process
2.2. Descriptive Characteristics of Included RCTs
2.3. Short-Term RCT Results
2.4. Long-Term RCT Results
2.5. Summary
3. The Effect of Exercise on Markers of Bone Resorption and Formation
3.1. Acute Exercise Effects on Markers of Bone Resorption and Bone Formation
3.2. Chronic Exercise Training Effects on Markers of Bone Resorption and Bone Formation
3.3. Summary
4. Conclusions and Future Research Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| aBMD | Areal bone mineral density |
| AT | Aerobic training |
| BMI | Body mass index |
| CR | Caloric restriction |
| CTX | C-telopeptide of type I collagen |
| DXA | Dual-energy x-ray absorptiometry |
| EX | Exercise |
| GRECC | Geriatric Research, Education, and Clinical Center |
| P1NP | Procollagen of type 1 n-terminal propeptide |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| PTH | Parathyroid hormone |
| QCT | Quantitative computed tomography |
| RCT | Randomized controlled trial |
| RT | Resistance training |
| WL | Weight loss |
Appendix A. Search Strategy Used to Identify RCTs Included in the Structured Component of the Review
| 1 | exercise’/exp |
| 2 | physical activity’/exp |
| 3 | (exercise:ti,ab,kw OR ‘physical activity, capacity’:ti,ab,kw) AND performance:ti,ab,kw |
| 4 | #1 OR #2 OR #3 |
| 5 | body weight loss’/exp |
| 6 | weight loss’:ti,ab,kw OR ‘weight losing’:ti,ab,kw OR ‘weight reduc*’:ti,ab,kw OR ‘weight decreas*’:ti,ab,kw |
| 7 | #5 OR #6 |
| 8 | bone’/exp |
| 9 | bone density’/exp |
| 10 | bone*:ti,ab,kw OR ‘bone densit*’:ti,ab,kw OR ‘bone mineral’:ti,ab,kw OR ‘bone loss’:ti,ab,kw OR ‘bone mass’:ti,ab,kw OR bmc:ti,ab,kw OR bmd:ti,ab,kw |
| 11 | #8 OR #9 OR #10 |
| 12 | #4 AND #7 AND #11 |
| 13 | #12 AND (‘randomized controlled trial’/de) AND ([aged]/lim OR [middle aged]/lim OR [very elderly]/lim) |
| 14 | (mouse:ti,ab,kw OR rat:ti,ab,kw OR leporidae:ti,ab,kw OR equidae:ti,ab,kw) |
| 15 | #13 NOT #14 |
| 1 | TI (exercis* or physical activit* or exert* or physical fitness) OR AB (exercis* or physical activit* or exert* or physical fitness) OR KW (exercis* or physical activit* or exert* or physical fitness) |
| 2 | TI (weight loss or losing weight or weight reduc* or weight decreas* or lose weight or weight management) OR AB (weight loss or losing weight or weight reduc* or weight decreas* or lose weight or weight management) OR KW (weight loss or losing weight or weight reduc* or weight decreas* or lose weight or weight management) |
| 3 | TI (bone* or bone density or bone mineral density or bone mineral content OR bone loss OR bone mass OR bone strength OR BMC OR BMD) OR AB (bone* or bone density or bone mineral density or bone mineral content OR bone loss OR bone mass OR bone strength OR BMC OR BMD) OR KW (bone* or bone density or bone mineral density or bone mineral content or bone loss or bone mass or bone strength OR BMC OR BMD) |
| 4 | TI (“randomi* controlled trial” OR “single blind*” OR “double blind*”) OR AB (“randomi* controlled trial” OR “single blind*” OR “double blind*”) OR KW (“randomi* controlled trial” OR “single blind*” OR “double blind*”) |
| 5 | #1 AND #2 AND #3 AND #4 |
References
- Anderson, L.A.; Goodman, R.A.; Holtzman, D.; Posner, S.F.; Northridge, M.E. Aging in the United States: Opportunities and challenges for public health. Am. J. Public Health 2012, 102, 393–395. [Google Scholar] [CrossRef]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Sambrook, P.N.; Eisman, J.A. Bone loss, physical activity, and weight change in elderly women: The Dubbo Osteoporosis Epidemiology Study. J. Bone Miner Res. 1998, 13, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.F.; Sigurdsson, S.; Karlsdottir, G.; Oskarsdottir, D.; Sigmarsdottir, A.; Chengshi, J.; Kornak, J.; Harris, T.B.; Sigurdsson, G.; Jonsson, B.Y.; et al. Age-related loss of proximal femoral strength in elderly men and women: The Age Gene/Environment Susceptibility Study—Reykjavik. Bone 2012, 50, 743–748. [Google Scholar] [CrossRef]
- Zhu, K.; Hunter, M.; James, A.; Lim, E.M.; Walsh, J.P. Associations between body mass index, lean and fat body mass and bone mineral density in middle-aged Australians: The Busselton Healthy Ageing Study. Bone 2015, 74, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; Watts, N.B.; Chapurlat, R.; Cooper, C.; Boonen, S.; Greenspan, S.; Pfeilschifter, J.; Silverman, S.; Diez-Perez, A.; Lindsay, R.; et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Obesity and Bone. Curr. Osteoporos. Rep. 2013, 11, 30–35. [Google Scholar] [CrossRef]
- Compston, J. Obesity and fractures in postmenopausal women. Curr. Opin. Rheumatol. 2015, 27, 414–419. [Google Scholar] [CrossRef]
- Nielson, C.M.; Srikanth, P.; Orwoll, E.S. Obesity and fracture in men and women: An epidemiologic perspective. J. Bone Miner Res. 2012, 27, 1–10. [Google Scholar] [CrossRef]
- Sukumar, D.; Schlussel, Y.; Riedt, C.S.; Gordon, C.; Stahl, T.; Shapses, S.A. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporos. Int. 2011, 22, 635–645. [Google Scholar] [CrossRef]
- Nielson, C.M.; Marshall, L.M.; Adams, A.L.; LeBlanc, E.S.; Cawthon, P.M.; Ensrud, K.; Stefanick, M.L.; Barrett-Connor, E.; Orwoll, E.S. Osteoporotic Fractures in Men Study Research, BMI and fracture risk in older men: The osteoporotic fractures in men study (MrOS). J. Bone Miner Res. 2011, 26, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Premaor, M.O.; Pilbrow, L.; Tonkin, C.; Parker, R.A.; Compston, J. Obesity and fractures in postmenopausal women. J. Bone Miner Res. 2010, 25, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, W.; Lunt, M.; Silman, A.J.; Cooper, C.; Lips, P.; Bhalla, A.K.; Cannata, J.B.; Eastell, R.; Felsenberg, D.; Gennari, C.; et al. Health-related quality of life and radiographic vertebral fracture. Osteoporos. Int. 2004, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Center, J.R.; Nguyen, T.V.; Schneider, D.; Sambrook, P.N.; Eisman, J.A. Mortality after all major types of osteoporotic fracture in men and women: An observational study. Lancet 1999, 353, 878–882. [Google Scholar] [CrossRef]
- Office of the Surgeon General (US). The Burden of Bone Disease. In Bone Health and Osteoporosis: A Report of the Surgeon General; Office of the Surgeon General (US): Rockville, MD, USA, 2004. [Google Scholar]
- Colon-Emeric, C.S.; Saag, K.G. Osteoporotic fractures in older adults. Best Pract. Res. Clin. Rheumatol. 2006, 20, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Ray, N.F.; Chan, J.K.; Thamer, M.; Melton, L.J., III. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: Report from the National Osteoporosis Foundation. J. Bone Miner Res. 1997, 12, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Fullman, R.L.; Barrett-Connor, E.; Cauley, J.A.; Stefanick, M.L.; Fink, H.A.; Lewis, C.E.; Orwoll, E. Voluntary weight reduction in older men increases hip bone loss: The osteoporotic fractures in men study. J. Clin. Endocrinol. Metab. 2005, 90, 1998–2004. [Google Scholar] [CrossRef]
- Dennison, E.; Eastell, R.; Fall, C.H.; Kellingray, S.; Wood, P.J.; Cooper, C. Determinants of bone loss in elderly men and women: A prospective population-based study. Osteoporos. Int. 1999, 10, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.T.; Felson, D.T.; Anderson, J.J. Bone mineral density in elderly men and women: Results from the Framingham osteoporosis study. J. Bone Miner Res. 1992, 7, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Knoke, J.D.; Barrett-Connor, E. Weight loss: A determinant of hip bone loss in older men and women. The Rancho Bernardo Study. Am. J. Epidemiol. 2003, 158, 1132–1138. [Google Scholar] [CrossRef]
- Shapses, S.A.; Sukumar, D. Bone metabolism in obesity and weight loss. Annu. Rev. Nutr. 2012, 32, 287–309. [Google Scholar] [CrossRef]
- Zibellini, J.; Seimon, R.V.; Lee, C.M.; Gibson, A.A.; Hsu, M.S.; Shapses, S.A.; Nguyen, T.V.; Sainsbury, A. Does Diet-Induced Weight Loss Lead to Bone Loss in Overweight or Obese Adults? A Systematic Review and Meta-Analysis of Clinical Trials. J. Bone Miner Res. 2015, 30, 2168–2178. [Google Scholar] [CrossRef]
- Villareal, D.T.; Fontana, L.; Das, S.K.; Redman, L.; Smith, S.R.; Saltzman, E.; Bales, C.; Rochon, J.; Pieper, C.; Huang, M.; et al. Effect of Two-Year Caloric Restriction on Bone Metabolism and Bone Mineral Density in Non-Obese Younger Adults: A Randomized Clinical Trial. J. Bone Miner Res. 2016, 31, 40–51. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016, 230, R115–R130. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Johnson, K.C.; Kahn, S.E.; Shepherd, J.A.; Nevitt, M.C.; Peters, A.L.; Walkup, M.P.; Hodges, A.; Williams, C.C.; Bray, G.A.; et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: Results from the Look AHEAD randomized trial. J. Bone Miner Res. 2012, 27, 619–627. [Google Scholar] [CrossRef]
- Lipkin, E.W.; Schwartz, A.V.; Anderson, A.M.; Davis, C.; Johnson, K.C.; Gregg, E.W.; Bray, G.A.; Berkowitz, R.; Peters, A.L.; Hodges, A.; et al. The Look AHEAD Trial: Bone loss at 4-year follow-up in type 2 diabetes. Diabetes Care 2014, 37, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Ewing, S.K.; Stone, K.L.; Cauley, J.A.; Bowman, P.J.; Cummings, S.R. Study of Osteoporotic Fractures Research, G. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J. Am. Geriatr. Soc. 2003, 51, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.J.; Yildiz, V.O.; Wactawski-Wende, J.; Johnson, K.C.; Chen, Z.; Going, S.B.; Wright, N.C.; Cauley, J.A. Postmenopausal weight change and incidence of fracture: Post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. BMJ 2015, 350, h25. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.C.; Bray, G.A.; Cheskin, L.J.; Clark, J.M.; Egan, C.M.; Foreyt, J.P.; Garcia, K.R.; Glasser, S.; Greenway, F.L.; Gregg, E.W.; et al. The Effect of Intentional Weight Loss on Fracture Risk in Persons With Diabetes: Results From the Look AHEAD Randomized Clinical Trial. J. Bone Miner Res. 2017, 32, 2278–2287. [Google Scholar] [CrossRef]
- Lv, Q.-B.; Fu, X.; Jin, H.-M.; Xu, H.-C.; Huang, Z.-Y.; Xu, H.-Z.; Chi, Y.-L.; Wu, A.-M. The relationship between weight change and risk of hip fracture: Meta-analysis of prospective studies. Sci. Rep. 2015, 5, 16030. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Visser, M.; Davidovic, L.S.; Maggi, S.; Li, G.; Harris, T.B. Hip fracture risk in older white men is associated with change in body weight from age 50 years to old age. Arch. Intern. Med. 1998, 158, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Cauley, J.; Lipschutz, R.; Cummings, S.R. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch. Intern. Med. 1997, 157, 857–863. [Google Scholar] [CrossRef]
- Soltani, S.; Hunter, G.R.; Kazemi, A.; Shab-Bidar, S. The effects of weight loss approaches on bone mineral density in adults: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2016, 27, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Villalon, K.L.; Gozansky, W.S.; Van Pelt, R.E.; Wolfe, P.; Jankowski, C.M.; Schwartz, R.S.; Kohrt, W.M. A losing battle: Weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity 2011, 19, 2345–2350. [Google Scholar] [CrossRef]
- Avenell, A.; Richmond, P.R.; Lean, M.E.; Reid, D.M. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur. J. Clin. Nutr. 1994, 48, 561–566. [Google Scholar]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef]
- Waters, D.L.; Vawter, R.; Qualls, C.; Chode, S.; Armamento-Villareal, R.; Villareal, D.T. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J. Nutr. Health Aging 2013, 17, 3–7. [Google Scholar] [CrossRef]
- Kammire, D.E.; Walkup, M.P.; Ambrosius, W.T.; Lenchik, L.; Shapses, S.A.; Nicklas, B.J.; Houston, D.K.; Marsh, A.P.; Rejeski, W.J.; Beavers, K.M. Effect of Weight Change Following Intentional Weight Loss on Bone Health in Older Adults with Obesity. Obesity 2019, 27, 1839–1845. [Google Scholar] [CrossRef]
- Von Thun, N.L.; Sukumar, D.; Heymsfield, S.B.; Shapses, S.A. Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause 2014, 21, 501–508. [Google Scholar] [CrossRef]
- Hinton, P.S.; Rector, R.S.; Linden, M.A.; Warner, S.O.; Dellsperger, K.C.; Chockalingam, A.; Whaley-Connell, A.T.; Liu, Y.; Thomas, T.R. Weight-loss-associated changes in bone mineral density and bone turnover after partial weight regain with or without aerobic exercise in obese women. Eur. J. Clin. Nutr. 2012, 66, 606–612. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S.; American Society for Nutrition and NAASO. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes. Res. 2005, 13, 1849–1863. [Google Scholar] [CrossRef]
- Shapses, S.A.; Sukumar, D.; Schneider, S.H.; Schlussel, Y.; Sherrell, R.M.; Field, M.P.; Ambia-Sobhan, H. Vitamin D supplementation and calcium absorption during caloric restriction: A randomized double-blind trial. Am. J. Clin. Nutr. 2013, 97, 637–645. [Google Scholar] [CrossRef]
- Ricci, T.A.; Chowdhury, H.A.; Heymsfield, S.B.; Stahl, T.; Pierson, R.N., Jr.; Shapses, S.A. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J. Bone Miner Res. 1998, 13, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, D.; Ambia-Sobhan, H.; Zurfluh, R.; Schlussel, Y.; Stahl, T.J.; Gordon, C.L.; Shapses, S.A. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: A randomized, controlled trial. J. Bone Miner Res. 2011, 26, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Bolam, K.A.; Skinner, T.L.; Jenkins, D.G.; Galvao, D.A.; Taaffe, D.R. The Osteogenic Effect of Impact-Loading and Resistance Exercise on Bone Mineral Density in Middle-Aged and Older Men: A Pilot Study. Gerontology 2015, 62, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Martyn-St James, M.; Carroll, S. Effects of different impact exercise modalities on bone mineral density in premenopausal women: A meta-analysis. J. Bone Miner. Metab. 2010, 28, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, M.; Xu, Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: A meta-analysis. Osteoporos. Int. 2015, 26, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, L.M.; Papaioannou, A.; Macintyre, N.J.; Ashe, M.C.; Heinonen, A.; Shipp, K.; Wark, J.; McGill, S.; Keller, H.; Jain, R.; et al. Too Fit To Fracture: Exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos. Int. 2014, 25, 821–835. [Google Scholar] [CrossRef]
- Beck, B.R.; Daly, R.M.; Singh, M.A.; Taaffe, D.R. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J. Sci. Med. Sport 2017, 20, 438–445. [Google Scholar] [CrossRef]
- Daly, R.M.; Dalla Via, J.; Duckham, R.L.; Fraser, S.F.; Helge, E.W. Exercise for the prevention of osteoporosis in postmenopausal women: An evidence-based guide to the optimal prescription. Braz. J. Phys. Ther. 2019, 23, 170–180. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Hosny, I.A.; Elghawabi, H.S.; Younan, W.B.; Sabbour, A.A.; Gobrial, M.A. Beneficial impact of aerobic exercises on bone mineral density in obese premenopausal women under caloric restriction. Skeletal Radiol. 2012, 41, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Nakata, Y.; Ohkawara, K.; Lee, D.J.; Okura, T.; Tanaka, K. Effects of additional resistance training during diet-induced weight loss on bone mineral density in overweight premenopausal women. J. Bone Miner. Metab. 2008, 26, 172–177. [Google Scholar] [CrossRef]
- Ryan, A.S.; Nicklas, B.J.; Dennis, K.E. Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J. Appl. Physiol. 1998, 84, 1305–1310. [Google Scholar] [CrossRef][Green Version]
- Villareal, D.T.; Fontana, L.; Weiss, E.P.; Racette, S.B.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Holloszy, J.O. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: A randomized controlled trial. Arch. Intern. Med. 2006, 166, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Shah, K.; Banks, M.R.; Sinacore, D.R.; Klein, S. Effect of Weight Loss and Exercise Therapy on Bone Metabolism and Mass in Obese Older Adults: A One-Year Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2008, 93, 2181–2187. [Google Scholar] [CrossRef]
- Anderson, R.E.; Wadden, T.A.; Herzog, R.J. Changes in bone mineral content in obese dieting women. Metabolism 1997, 46, 857–861. [Google Scholar] [CrossRef]
- Svendsen, O.L.; Hassager, C.; Christiansen, C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am. J. Med. 1993, 95, 131–140. [Google Scholar] [CrossRef]
- Daly, R.M.; Dunstan, D.W.; Owen, N.; Jolley, D.; Shaw, J.E.; Zimmet, P.Z. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos. Int. 2005, 16, 1703–1712. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Armamento-Villareal, R.; Parimi, N.; Chode, S.; Sinacore, D.R.; Hilton, T.N.; Napoli, N.; Qualls, C.; Villareal, D.T. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J. Bone Miner Res. 2011, 26, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Beavers, D.P.; Beavers, K.M.; Loeser, R.F.; Walton, N.R.; Lyles, M.F.; Nicklas, B.J.; Shapses, S.A.; Newman, J.J.; Messier, S.P. The independent and combined effects of intensive weight loss and exercise training on bone mineral density in overweight and obese older adults with osteoarthritis. Osteoarthr. Res. Soc. 2014, 22, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Jordan, R.C.; Frese, E.M.; Albert, S.G.; Villareal, D.T. Effects of Weight Loss on Lean Mass, Strength, Bone, and Aerobic Capacity. Med. Sci. Sports Exerc. 2017, 49, 206–217. [Google Scholar] [CrossRef]
- Beavers, K.M.; Walkup, M.P.; Weaver, A.A.; Lenchik, L.; Kritchevsky, S.B.; Nicklas, B.J.; Ambrosius, W.T.; Stitzel, J.D.; Register, T.C.; Shapses, S.A.; et al. Effect of Exercise Modality during Weight Loss on Bone Health in Older Adults With Obesity and Cardiovascular Disease or Metabolic Syndrome: A Randomized Controlled Trial. J. Bone Miner Res. 2018, 33, 2140–2149. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Eastell, R.; Lui, L.Y.; Wu, L.A.; de Papp, A.E.; Grauer, A.; Marin, F.; Cauley, J.A.; Bauer, D.C.; Black, D.M.; et al. Change in Bone Density and Reduction in Fracture Risk: A Meta-Regression of Published Trials. J. Bone Miner Res. 2019, 34, 632–642. [Google Scholar] [CrossRef]
- Black, D.M.; Bauer, D.C.; Vittinghoff, E.; Lui, L.Y.; Grauer, A.; Marin, F.; Khosla, S.; de Papp, A.; Mitlak, B.; Cauley, J.A.; et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: Meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020, 8, 672–682. [Google Scholar] [CrossRef]
- Heaney, R.P. The bone-remodeling transient: Implications for the interpretation of clinical studies of bone mass change. J. Bone Miner Res. 2009, 9, 1515–1523. [Google Scholar] [CrossRef]
- Messier, S.P.; Legault, C.; Mihalko, S.; Miller, G.D.; Loeser, R.F.; DeVita, P.; Lyles, M.; Eckstein, F.; Hunter, D.J.; Williamson, J.D.; et al. The intensive diet and exercise for arthritis (IDEA) trial: Design and rationale. BMC Musculoskelet. Disord. 2009, 10, 93. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Armamento-Villareal, R.; Aguirre, L.; Waters, D.L.; Napoli, N.; Qualls, C.; Villareal, D.T. Effect of aerobic or resistance exercise, or both, on bone mineral density and bone metabolism in obese older adults while dieting: A randomized controlled trial. J. Bone Miner Res. 2019, 35, 430–439. [Google Scholar] [CrossRef]
- Burton, E.; Hill, A.M.; Pettigrew, S.; Lewin, G.; Bainbridge, L.; Farrier, K.; Airey, P.; Hill, K.D. Why do seniors leave resistance training programs? Clin. Interv. Aging 2017, 12, 585–592. [Google Scholar] [CrossRef]
- Endo, Y.; Nourmahnad, A.; Sinha, I. Optimizing Skeletal Muscle Anabolic Response to Resistance Training in Aging. Front Physiol. 2020, 11, 874. [Google Scholar] [CrossRef]
- Turner, C.H.; Robling, A.G. Mechanisms by which exercise improves bone strength. J. Bone Miner Metab. 2005, 23, 16–22. [Google Scholar] [CrossRef]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; van Rietbergen, B. Bone remodelling in humans is load-driven but not lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef] [PubMed]
- Bass, S.L.; Eser, P.; Daly, R. The effect of exercise and nutrition on the mechanostat. J. Musculoskelet. Neuronal Interact. 2005, 5, 239–254. [Google Scholar] [PubMed]
- Frost, H.M. Bone “mass″ and the “mechanostat″: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Stein, E.M.; Bessler, M.; Della Badia, M.; Restuccia, N.; Olivero-Rivera, L.; McMahon, D.J.; Silverberg, S.J. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J. Clin. Endocrinol. Metab. 2008, 93, 3735–3740. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Robling, A.G. Designing exercise regimens to increase bone strength. Exerc. Sport Sci. Rev. 2002, 31, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, A.L.; Rockwell, D.; Kulund, D.N.; Harrison, R.B. Bone mass in lifetime tennis athletes. JAMA 1980, 244, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Krahl, H.; Michaelis, U.; Pieper, H.G.; Quack, G.; Montag, M. Stimulation of bone growth through sports. A radiologic investigation of the upper extremities in professional tennis players. Am. J. Sports Med. 1994, 22, 751–757. [Google Scholar] [CrossRef]
- Robling, A.G.; Burr, D.B.; Turner, C.H. Recovery periods restore mechanosensitivity to dynamically loaded bone. J. Exp. Biol. 2001, 204, 3389–3399. [Google Scholar]
- Robling, A.G.; Hinant, F.M.; Burr, D.B.; Turner, C.H. Shorter, more frequent mechanical loading sessions enhance bone mass. Med. Sci. Sports Exerc. 2002, 34, 196–202. [Google Scholar] [CrossRef]
- Robling, A.G.; Hinant, F.M.; Burr, D.B.; Turner, C.H. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J. Bone Miner Res. 2002, 17, 1545–1554. [Google Scholar] [CrossRef]
- Scott, J.P.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running. J. Clin. Endocrinol. Metab. 2010, 95, 3918–3925. [Google Scholar] [CrossRef]
- Scott, J.P.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. Effect of fasting versus feeding on the bone metabolic response to running. Bone 2012, 51, 990–999. [Google Scholar] [CrossRef]
- Barry, D.W.; Hansen, K.C.; van Pelt, R.E.; Witten, M.; Wolfe, P.; Kohrt, W.M. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med. Sci. Sports Exerc. 2011, 43, 617–623. [Google Scholar] [CrossRef]
- Barry, D.W.; Kohrt, W.M. Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium. Calcif. Tissue Int. 2007, 80, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Wherry, S.J.; Wolfe, P.; Sherk, V.D.; Wellington, T.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Maintenance of Serum Ionized Calcium During Exercise Attenuates Parathyroid Hormone and Bone Resorption Responses. J. Bone Miner Res. 2018, 33, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Wolfe, P.; Sherk, V.D.; Wherry, S.J.; Wellington, T.; Melanson, E.L.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Dermal Calcium Loss Is Not the Primary Determinant of Parathyroid Hormone Secretion during Exercise. Med. Sci. Sports Exerc. 2019, 51, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Sherk, V.D.; Wherry, S.J.; Barry, D.W.; Shea, K.L.; Wolfe, P.; Kohrt, W.M. Calcium Supplementation Attenuates Disruptions in Calcium Homeostasis during Exercise. Med. Sci. Sports Exerc. 2017, 49, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Wherry, S.J.; Swanson, C.M.; Wolfe, P.; Wellington, T.; Boxer, R.S.; Schwartz, R.S.; Kohrt, W.M. Bone Biomarker Response to Walking under Different Thermal Conditions in Older Adults. Med. Sci. Sports Exerc. 2019, 51, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Haakonssen, E.C.; Ross, M.L.; Knight, E.J.; Cato, L.E.; Nana, A.; Wluka, A.E.; Cicuttini, F.M.; Wang, B.H.; Jenkins, D.G.; Burke, L.M. The effects of a calcium-rich pre-exercise meal on biomarkers of calcium homeostasis in competitive female cyclists: A randomised crossover trial. PLoS ONE 2015, 10, e0123302. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.L.; Barry, D.W.; Sherk, V.D.; Hansen, K.C.; Wolfe, P.; Kohrt, W.M. Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med. Sci. Sports Exerc. 2014, 46, 2007–2013. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Scott, J.P.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J. Appl. Physiol. 2011, 110, 423–432. [Google Scholar] [CrossRef]
- Townsend, R.; Elliott-Sale, K.J.; Pinto, A.J.; Thomas, C.; Scott, J.P.; Currell, K.; Fraser, W.D.; Sale, C. Parathyroid Hormone Secretion Is Controlled by Both Ionized Calcium and Phosphate During Exercise and Recovery in Men. J. Clin. Endocrinol. Metab. 2016, 101, 3231–3239. [Google Scholar] [CrossRef]
- Grabowski, P. Physiology of bone. Endocr. Dev. 2009, 16, 32–48. [Google Scholar] [CrossRef]
- Silva, B.C.; Costa, A.G.; Cusano, N.E.; Kousteni, S.; Bilezikian, J.P. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J. Endocrinol. Investig. 2011, 34, 801–810. [Google Scholar] [CrossRef]
- Szent-Gyorgyi, A.G. Calcium regulation of muscle contraction. Biophys. J. 1975, 15, 707–723. [Google Scholar] [CrossRef]
- Sherk, V.D.; Barry, D.W.; Villalon, K.L.; Hansen, K.C.; Wolfe, P.; Kohrt, W.M. Bone loss over 1 year of training and competition in female cyclists. Clin. J. Sport Med. 2014, 24, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.P.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. Treadmill running reduces parathyroid hormone concentrations during recovery compared with a nonexercising control group. J. Clin. Endocrinol. Metab. 2014, 99, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Rantalainen, T.; Heinonen, A.; Linnamo, V.; Komi, P.V.; Takala, T.E.; Kainulainen, H. Short-term bone biochemical response to a single bout of high-impact exercise. J. Sports Sci. Med. 2009, 8, 553–559. [Google Scholar] [PubMed]
- Prawiradilaga, R.S.; Madsen, A.O.; Jorgensen, N.R.; Helge, E.W. Acute response of biochemical bone turnover markers and the associated ground reaction forces to high-impact exercise in postmenopausal women. Biol. Sport 2020, 37, 41–48. [Google Scholar] [CrossRef]
- Barry, D.W.; Kohrt, W.M. Exercise and the preservation of bone health. J. Cardiopulm. Rehabil. Prev. 2008, 28, 153–162. [Google Scholar] [CrossRef]
- Kelley, G.A. Exercise and regional bone mineral density in postmenopausal women: A meta-analytic review of randomized trials. Am. J. Phys. Med. Rehabil. 1998, 77, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Kohrt, W.M. Exercise and bone mineral density in men: A meta-analysis of randomized controlled trials. Bone 2013, 53, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Nelson, M.E.; Yingling, V.R.; American College of Sports, M. American College of Sports Medicine Position Stand: Physical activity and bone health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef]
- Alam, I.; Warden, S.J.; Robling, A.G.; Turner, C.H. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J. Bone Miner Res. 2005, 20, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Gatti, D.; Viapiana, O.; Fiore, C.E.; Nuti, R.; Luisetto, G.; Ponte, M.; Rossini, M.; Group, B.S. Physical activity and bone turnover markers: A cross-sectional and a longitudinal study. Calcif. Tissue Int. 2008, 83, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S.; Rouzi, A.A.; Qari, M.H. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: A cross-sectional and a longitudinal study. J. Clin. Endocrinol. Metab. 2012, 97, 3691–3699. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.R.; Vukovich, M.D. Osteogenic index and changes in bone markers during a jump training program: A pilot study. Med. Sci. Sports Exerc. 2010, 42, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bemben, M.G.; Knehans, A.W.; Bemben, D.A. Effects of an 8-Month Ashtanga-Based Yoga Intervention on Bone Metabolism in Middle-Aged Premenopausal Women: A Randomized Controlled Study. J. Sports Sci. Med. 2015, 14, 756–768. [Google Scholar] [PubMed]
- Alghadir, A.H.; Aly, F.A.; Gabr, S.A. Effect of Moderate Aerobic Training on Bone Metabolism Indices among Adult Humans. Pak. J. Med. Sci. 2014, 30, 840–844. [Google Scholar] [CrossRef]
- Roghani, T.; Torkaman, G.; Movasseghe, S.; Hedayati, M.; Goosheh, B.; Bayat, N. Effects of short-term aerobic exercise with and without external loading on bone metabolism and balance in postmenopausal women with osteoporosis. Rheumatol. Int. 2013, 33, 291–298. [Google Scholar] [CrossRef]
- Vincent, K.R.; Braith, R.W. Resistance exercise and bone turnover in elderly men and women. Med. Sci. Sports Exerc. 2002, 34, 17–23. [Google Scholar] [CrossRef]
- Lester, M.E.; Urso, M.L.; Evans, R.K.; Pierce, J.R.; Spiering, B.A.; Maresh, C.M.; Hatfield, D.L.; Kraemer, W.J.; Nindl, B.C. Influence of exercise mode and osteogenic index on bone biomarker responses during short-term physical training. Bone 2009, 45, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Glover, S.J.; Eastell, R.; McCloskey, E.V.; Rogers, A.; Garnero, P.; Lowery, J.; Belleli, R.; Wright, T.M.; John, M.R. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone 2009, 45, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Almirol, E.A.; Chi, L.Y.; Khurana, B.; Hurwitz, S.; Bluman, E.M.; Chiodo, C.; Matzkin, E.; Baima, J.; LeBoff, M.S. Short-term effects of teriparatide versus placebo on bone biomarkers, structure, and fracture healing in women with lower-extremity stress fractures: A pilot study. J. Clin. Transl. Endocrinol. 2016, 5, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Sahni, S.; Xu, H.; McLean, R.R.; Broe, K.E.; Hannan, M.T.; Boyd, S.K.; Bouxsein, M.L.; Kiel, D.P.; Samelson, E.J. Long-Term and Recent Weight Change Are Associated With Reduced Peripheral Bone Density, Deficits in Bone Microarchitecture, and Decreased Bone Strength: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2018, 33, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Inman, C.L.; Warren, G.L.; Hogan, H.A.; Bloomfield, S.A. Mechanical loading attenuates bone loss due to immobilization and calcium deficiency. J. Appl. Physiol. 1999, 87, 189–195. [Google Scholar] [CrossRef] [PubMed]
| Modality | Frequency | Intensity | Example Exercises |
|---|---|---|---|
| Resistance Training * | ≥2 days/week | ≥2 set of 8–12 repetitions 1–3 min rest intervals 1 exercise per major muscle group * Can progress to high-velocity contractions with low to moderate intensity (30–70% maximum). | Compound exercises including (but not limited to): squats, lunges, hip ab/adduction, leg press, thoracic/lumbar extension, abdominal/postural exercises, or bent-over row. |
| Impact Training * | 4–7 days/week | 3–5 sets of 10–20 repetitions 2–4x body weight 1–2 min rest intervals 50–100 jumps per session. * Begin with RT and progress to impact training after 6–12 weeks. | Jump, hop, skipping, and stepping derivatives. Intensity can be increased via increased jump/step height or via external resistance (e.g., weighted vest) |
| Balance/Mobility Training * | ≥2–3 h/week | Start with static exercises and progress to dynamic exercises. 15–20 min/day | Static: One-legged stand, tandem stand, etc. Dynamic: walking on toes, backwards walking, etc. |
| Aerobic Training | 3–5 days/week | Moderate to vigorous 30–60 min/day | Walking, dancing, etc. |
| Author (Year) | N (% Female) | Age (Years) | BMI (kg/m2) | Duration (Months) | Intervention Characteristics | WL (%) | DXA aBMD Percent Change from Baseline | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | n | Brief Description of Intervention | TB | LS | TH | FN | ||||||
| Svendsen et al. (1993) [61] | 121 (100) | 54 | 29.7 | 3 | Control | 21 | Maintained normal lifestyle | 0.7β | −1.2 | −0.4 | -- | -- |
| CR | 51 | ~1000 kcal/day target | −12.2 | −1.9 | −1.6 | -- | -- | |||||
| CR + EX | 49 | ~1000 kcal/day target + RT and AT 3 d/week | −13.2 | −1.9 | −2.4↓ | -- | -- | |||||
| Daly et al. (2005) [62] | 29 (45) | 67 | 32 | 0 to 6 6 to 12 | CR | 13 | Mo. 0–6: 0.25 kg WL/wk Mo. 6–12: no dietary guidance | −3.3↓ 1.6↑ | −0.9↓β −0.6↓ | −0.6 −1.5 | -- -- | −1.1 −1.0 |
| CR + RT | 16 | Mo. 0–6: 0.25 kg WL/week + center-based RT 3 d/week Mo. 6–12: No dietary guidance + at-home RT 3 d/week | −2.7↓ 1.7↑ | −0.3 −0.4 | −0.1 −0.7 | -- -- | −0.5 −0.4 | |||||
| Villareal et al. (2011) [63] | 107 (63) | 70 | 37 | 12 | Control | 27 | Maintained normal lifestyle | <−1.0 β | 0.5 | 0.6 | −0.7 ↓D | 0.0 |
| Shah et al. (2011) [64] | CR | 26 | ~500–750 kcal/day deficit | −10.0↓ | −0.4 | 0.9 | −2.6↓ | −2.1↓C | ||||
| EX | 26 | Multimodal exercise (AT, RT, FT, BT) 3 d/wk | −1.0 β | 0.6 | 1.0 | 1.4 ↑β | 1.2D | |||||
| CR + EX | 28 | ~500–750 kcal/day deficit + multimodal exercise 3 d/wk | −9.0↓ | 0.8 | 0.7 | −1.1↓ | −0.9E | |||||
| Beavers et al. (2014) [65] | 284 (74) | 66 | 33 | 18 | EX | 95 | Multimodal exercise (AT, RT) 3 d/week. | −1.4↓ | -- | 0.7 | −0.2β | −0.1β |
| CR | 88 | ~800–1000 kcal/day deficit | −9.7↓ | -- | 0.5 | −2.4 | −1.7 | |||||
| CR + EX | 101 | ~800–1000 kcal/day deficit + Walk + RE + Walk 3 d/week | −10.4↓ | -- | 0.1 | −2.0 | −1.6 | |||||
| Weiss et al. (2017) [66] | 52 (75) | 57 | 27.7 | 3.5 | CR | 17 | 20% energy deficit for 12–14 wks | −6.9↓ | 0.1 | 0.3 | −0.3 | -- |
| EX | 16 | 60 min AT ~7 d/week | −7.2↓ | −0.1 | 1.1 | −0.7 | -- | |||||
| CR + EX | 19 | Half of CR and EX prescriptions | −7.4↓ | −0.4 | −0.9 | 0.0 | -- | |||||
| Beavers et al. (2018) [67] | 187 (70) | 67 | 34.5 | 18 | CR | 60 | ~330 kcal/day deficit | −5.9↓β | -- | 1.0 | −2.2 | −0.9 |
| CR + AT | 67 | ~330 kcal/day deficit + 45 min AT 4 d/week | −10.6↓ | -- | −0.5 | −2.7 | 0.4 | |||||
| CR + RT | 60 | ~330 kcal + RT 4 day/week | −10.4↓ | -- | 0.6 | −2.5 | 0.4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wherry, S.J.; Miller, R.M.; Jeong, S.H.; Beavers, K.M. The Ability of Exercise to Mitigate Caloric Restriction-Induced Bone Loss in Older Adults: A Structured Review of RCTs and Narrative Review of Exercise-Induced Changes in Bone Biomarkers. Nutrients 2021, 13, 1250. https://doi.org/10.3390/nu13041250
Wherry SJ, Miller RM, Jeong SH, Beavers KM. The Ability of Exercise to Mitigate Caloric Restriction-Induced Bone Loss in Older Adults: A Structured Review of RCTs and Narrative Review of Exercise-Induced Changes in Bone Biomarkers. Nutrients. 2021; 13(4):1250. https://doi.org/10.3390/nu13041250
Chicago/Turabian StyleWherry, Sarah J., Ryan M. Miller, Sarah H. Jeong, and Kristen M. Beavers. 2021. "The Ability of Exercise to Mitigate Caloric Restriction-Induced Bone Loss in Older Adults: A Structured Review of RCTs and Narrative Review of Exercise-Induced Changes in Bone Biomarkers" Nutrients 13, no. 4: 1250. https://doi.org/10.3390/nu13041250
APA StyleWherry, S. J., Miller, R. M., Jeong, S. H., & Beavers, K. M. (2021). The Ability of Exercise to Mitigate Caloric Restriction-Induced Bone Loss in Older Adults: A Structured Review of RCTs and Narrative Review of Exercise-Induced Changes in Bone Biomarkers. Nutrients, 13(4), 1250. https://doi.org/10.3390/nu13041250






