Abstract
The role of docosahexaenoic acid (DHA) and arachidonic acid (AA) in neurogenesis and brain development throughout the life cycle is fundamental. DHA and AA are long-chain polyunsaturated fatty acids (LCPUFA) vital for many human physiological processes, such as signaling pathways, gene expression, structure and function of membranes, among others. DHA and AA are deposited into the lipids of cell membranes that form the gray matter representing approximately 25% of the total content of brain fatty acids. Both fatty acids have effects on neuronal growth and differentiation through the modulation of the physical properties of neuronal membranes, signal transduction associated with G proteins, and gene expression. DHA and AA have a relevant role in neuroprotection against neurodegenerative pathologies such as Alzheimer’s disease and Parkinson’s disease, which are associated with characteristic pathological expressions as mitochondrial dysfunction, neuroinflammation, and oxidative stress. The present review analyzes the neuroprotective role of DHA and AA in the extreme stages of life, emphasizing the importance of these LCPUFA during the first year of life and in the developing/prevention of neurodegenerative diseases associated with aging.
1. Introduction
Over many years there has been growing interest about n-3 and n-6 long-chain polyunsaturated fatty acids (n-3 and n-6 LCPUFA), mainly related to their role in neural development and the prevention of neurodegenerative diseases. Lipids correspond to 60% of the dry weight of the mammalian brain, mostly in the form of phospholipids [1,2]. N-3 and n-6 LCPUFA are required for proper brain growth and development, specifically docosahexaenoic acid (C22:6n-3, DHA) and arachidonic acid (C20:4n-6, AA) [3]. DHA and AA represent approximately 25% of the total content of brain fatty acids [2,4] and are constituents of the lipids of cell membranes that form the gray matter [1,5]. DHA represent up to 90% of the total n-3 LCPUFA in the brain [6]. This LCPUFA can be obtained from its dietary precursor alpha-linolenic acid (C18:3n-3, ALA), which, after a complex process of enzymatic desaturation and elongation, is converted to DHA, or can be obtained from preformed DHA of marine dietary sources [7]. DHA is mainly found in the phospholipids of synaptic terminal membranes, and the vast majority of DHA is incorporated into the structure of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine [7,8,9]. The structural properties of DHA, such as its chain length and high unsaturation degree, give flexibility and fluidity to the neuronal plasma membrane, thus facilitating the signal transduction process into these cells [10,11]. These structural properties have a pivotal role in neuronal growth, migration, synaptogenesis, and synaptic plasticity [11,12,13]. In advanced stages of life, a decrease in plasma DHA levels is positively correlated with normal brain aging in healthy elderly individuals and also in patients diagnosed with neurogenerative diseases [14,15,16] such as Alzheimer’s disease (AD) [17,18]. It has also been observed that populations having a higher average dietary intake of DHA show a lower risk of developing cognitive impairment or AD [19,20]. Furthermore, a potential neuroprotective role of DHA in Parkinson’s disease (PD) through the increase in dopaminergic neurotransmission and the prevention of neuronal death is also actually recognized [21,22,23]. In this regard, the higher intake of n-3 LCPUFA (mostly DHA) trough supplementation may be effective to improve the nutritional status of the fatty acid and may play a role in maintaining brain health and in the prevention of brain aging symptoms [6,24,25].
AA is one of the most abundant n-6 LCPUFA in the brain and has a critical role in brain growth, being essential during the first months of life [26]. Ideally, during brain development, AA should be provided preformed in the diet because the low activity of the enzymes (Δ-5 and Δ-6 desaturases) involved in its formation from its dietary precursor linoleic acid (C18:2n-6, LA) results in a low conversion of LA to AA [27,28]. This fatty acid participates in many signaling pathways involved in cell division [29]. It is also a direct precursor of adrenic acid (C22:4n-6, AdA), a fatty acid necessary for neuronal development and enrichment of myelinic lipids [30,31]. A potential protective role of AA in neuronal aging through its participation in preserving the fluidity of the hippocampal cell membranes has been described [32,33,34,35]. However, opposite to this effect, a controversial role has been associated with AA in neuroinflammation and the genesis of cellular damage due to the pro-inflammatory action of some of AA metabolic derivatives [36,37,38]. According with this background, the present review analyzes the neurological role of DHA and AA in the extreme stages of life, emphasizing the importance of these LCPUFA during the first year of life and in the developing brain and in a context of age-related neurodegenerative disease prevention. Also, we discuss the eventual neuroprotective role of n-3 and n-6 LCPUFA in AD and PD.
2. DHA and AA: Biosynthesis, Metabolism, and Dietary Sources
ALA and LA are considered essential fatty acids because humans cannot synthesize them, so they must be supplied by the diet [39,40]. ALA is metabolized to eicosapentaenoic acid (C20:5 n-3, EPA) and subsequently to DHA, while LA is the precursor of AA [40]. ALA and LA are competitors in their respective metabolic pathways because both fatty acids are substrates for the same desaturase and elongase enzymes. In the synthesis of n-3 and n-6 LCPUFA in addition to the substrates ALA and LA, the participation of a complex enzymatic process which allows the desaturation and elongation of the 18 carbon atoms precursors (ALA and LA) is necessary [41]. This process occurs in microsomes and involves Δ-6 fatty acid desaturase 2 (FADS2), Δ-5 fatty acid desaturase 1 (FADS1), elongase 2 (ELOVL2) and elongase 5 (ELOVL5) enzymes [41]. The latter enzyme having a higher affinity for n-3 PUFA than for n-6 PUFA, favoring the transformation of ALA into DHA [42]. This less affinity of LA than ALA to desaturase and elongase enzymes has contributed to the recommendation of a 5:1 molar ratio for the dietary intake of n-6:n-3 PUFA, because when the dietary contribution of both fatty acids is similar, the formation of DHA is privileged compared to AA [43,44]. An interesting aspect about LCPUFA synthesis is the existence of polymorphisms in desaturase enzymes, which can influence both the ability to the synthesis of DHA or AA and the blood levels of these fatty acids [45]. For example, rs3834458 single nucleotide polymorphism in FADS2 may result in lower Δ-6 desaturase activity leading to higher ALA and lower DHA blood concentrations [46]. The presence of these polymorphisms can also influence lower levels of LCPUFA in breast milk, especially DHA, which could have impact on brain development [47,48,49]. The existence of polymorphisms has been associated with increased risk of developing insulin resistance, type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD), among other pathologies [50,51].
ALA is found in higher amounts in vegetable oils such as chia and flaxseed oil and in lower amounts in canola and soybean oils [52,53] The principal dietary sources of preformed DHA are fatty fish or blue fish, such as salmon, tuna, anchovy, sardine, and horse mackerel (Figure 1) [54,55,56]. The main dietary sources of LA are sunflower, soybean, and corn oils [52]. In contrast, the main dietary sources of AA are foods of animal origin, such as as beef, pork, lamb, chicken, turkey, and eggs [44]. Dietary sources of n-6 and n-3 polyunsaturated fatty acids influence brain development.
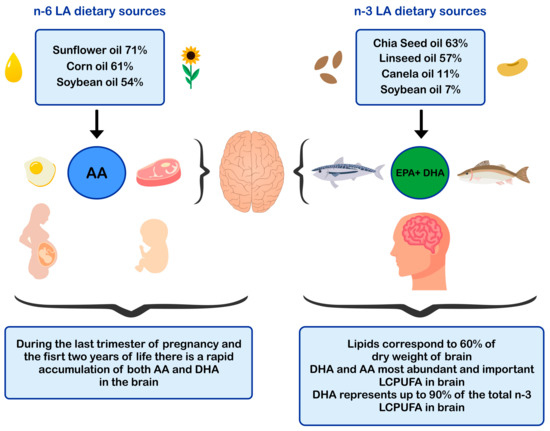
Figure 1.
Dietary sources of n-6 and n-3 polyunsaturated fatty acids and impact in brain development. AA: arachidonic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; LCPUFA: long-chain polyunsaturated fatty acid; LA: linoleic acid.
3. DHA and AA during Pregnancy and Breast Milk Period
During pregnancy and the first years of life, human beings have specific nutritional needs, which must be met to ensure proper development and growth [57]. Evolutionary changes about the biological mechanisms involved in transferring DHA and AA from mother to fetus during pregnancy [58,59] and lactation are well documented in the literature [60,61]. The chemical characteristics of human milk composition and its balance of nutrients and energy supply make it the optimal food source for infants [57]. In this regard, during the lactation stage the breast milk the lipid content increases from colostrum milk (2.36 ± 1.17 g/dL) to mature milk (3.39 ± 1.24 g/dL) [62], colostrum contains more LA than mature transitional milk, while colostrum contains less ALA than mature transitional milk [62]. Also, as lactation progresses, the proportions of DHA and AA decrease [62].
Human milk composition is the standard model to define adequate intakes of nutrients during the early stages of development in infants aged 0–2 years [63]. An example of these requirements occurs with AA, which is not considered an essential fatty acid for a healthy adult because habitual diet provides LA in amounts greater than 2.5% of the total caloric value per day requirement [44]. However, for infants aged 0-6 months, AA must be supplied in the diet within a range of 0.2% to 0.3% of the total caloric value per day requirement, establishing the concentration of preformed AA present in maternal milk as an adequate intake criterion which has been reported since the 1970s [44,64]. Both DHA and AA are always present in human milk, although in a variable quantity that is mainly determined by the mother’s diet [65,66] and the period of lactation [67]. Brenna et al., 2007 [66] carried out a systematic meta-analysis of the fatty acid composition of human breast milk obtained from 106 studies carried out in 33 countries, including a total of 2474 women, reporting a mean concentration of 0.47% for AA and 0.32% for DHA. Furthermore, the same group reported less variability in AA levels in breast milk compared to DHA levels with a coefficient of variation of 29% and 69%, respectively [55], indicating a greater variation in DHA synthesis than AA synthesis. This result may be due, in part, to the fact that approximately 90% of AA present in maternal milk fat is not derived from the diet, but from maternal body reserves [68]. However, DHA content is directly related to the mother’s dietary intake of ALA and/or DHA and, therefore, to the higher or lower access to foods that are a source of n-3 PUFA or n-3 LCPUFA [66]. Another study carried out by Fu et al., 2016 [69] provided information about 78 studies from 40 countries, and from 4163 samples of breast milk, reporting mean AA and DHA levels of 0.55% and 0.37%, respectively. Stability of AA levels in human milk takes an important biological significance when preformed AA is provided to infants who cannot obtain the fatty acid by de novo synthesis, or because its synthesis from the LA precursor is insufficient due to the low activity of desaturase enzymes [27,28,70]. Dietary DHA and AA, or tissue reserves of these LCPUFA must be transported to the placenta or to the breast incorporated into phospholipids and lysophospholipids [71]. Transport problems, not directly related to dietary deficiencies, may reduce the metabolic availability of LCPUFA, thus affecting normal brain development [71,72]. It has been estimated that during the first six months of the infant’s life, the mother supplies through breastfeeding around 3.9 kg of total lipids which is equivalent to 35,000 kcal [67]. This quantity is based on an average intake of infant lipids of 21.42 g/day, which provides 190 mg/day of AA and 130 mg/day of DHA [63,70]. These estimations were made using an average consumption of 850 mL/day of breast milk [73].
Traditionally the impact of LCPUFA intake during pregnancy and their effect in breast milk LCPUFA levels has been evaluated in normal-weight pregnant woman. However, the increase in obesity during pregnancy and breastfeeding as well as gestational diabetes, among other pathologies observed during the last decade, could alter the transport of LCPUFA to the placenta leading to a decrease in LCPUFA (mainly DHA) availability [72,74,75]. Currently, the potential impact in brain development of the transport of LCPUFA to the fetus or to the infant in maternal obesity and/or gestational diabetes, is a main study objective [71,75], findings that justify LCPUFA intake recommendations during pregnancy and lactation [71,75,76].
4. DHA and AA in Brain Development
The mammalian brain is a heterogeneous structure in which the maturation of the different brain regions varies over time [77]. The development of the human brain begins with the formation of the primitive neural tube during the first four weeks of gestation. The proliferation and cellular differentiation of neurons and glial cells (mainly astrocytes and oligodendrocytes) occurs between 5 and 18 weeks [77,78], a process in which are formed up to 200,000 neurons per minute [79,80]. Parallel to the multiplication of neurons, an active neuronal migration process occurs where glial cells act as auxiliary structures that guide neurons during their migration, facilitating neuronal mobility from the ventricular (central) germinal zone to the peripheral areas of the developing brain (neocortex) [81]. This is a transcendental event since migration defines the place where the neurons will be finally positioned in the different brain segments, thus determining the synaptic connection patterns that will be established and their future functionality [82,83]. Myelination begins during the third trimester of gestation, an active process that may extend until the third decade of life [2,77]. Also, during the last trimester of gestation, a significant increase in the number of synapses occurs. Neuronal cells become arborized and stratified in a laminar pattern that favors the connection, and up to 200 billion synapses can be formed [84]. This process is followed by programmed apoptosis; about 50% of neurons undergo programmed cell death, allowing the surviving neurons differentiation and specialization [82]. An explanation of this apoptotic phenomenon has been proposed; it would be a corrective mechanism to avoid erratic neuronal migration and/or due to the lack of trophic stimuli for some neurons that survive [11].
In humans, functional specialization of neurons occurs early from gestation and may even extend into early adulthood [83,85]. To quantify the stages of growth of the human brain, Dobbing and Sands (1973) analyzed 139 whole human brains of ages between 10 weeks of gestation until 7 years of life, and 9 adult brains [86]. The authors reported that the most significant proliferation of neuronal cells occurs during the period between 10 to 18 weeks of gestation, a period where may even reach the levels of neuronal proliferation that will be acquired in adulthood. Also, during the last trimester of pregnancy and the first two years of life, a rapid accumulation of both DHA and AA occurs in the developing brain [86]. In line with those results, Remko S.Kuipers et al. (2012) [87], determined that western infants accumulate fatty acids in the brain in the order AA> DHA > LA during all the stages of pregnancy, reaching the highest fatty acid accretion rate during the last five weeks of gestation [87]. During the second year of life, brain growth and neuronal structural reorganization continue rapidly [88]. The gray matter of the brain, which accumulates the greatest amount of DHA, increases in volume up to 5 years old [89]. It is known that 40–45% of the total lipids of the adult brain correspond to LCPUFA, with DHA and AA representing the vast majority (DHA 35–40% and AA 40–50%) (Figure 1) [11].
A study carried out by Lepping et al. (2019) [90] evaluated if supplementing with n-3 and n-6 LCPUFA during the first year of life could influence neurodevelopment. Newborns between 1 and 9 days old and up to 12 months old were randomly assigned to one of four terminal infant formula based on cow’s milk that had the same amounts of nutrients and ingredients except for LCPUFA and supplemented as follows: (i) control formula without DHA or AA; (ii) formula with 0.32% of fatty acids as DHA (17 mg/100 kcal); (iii) formula with 0.64% of fatty acids as DHA (34 mg/100 kcal); and (iv) formula with 0.96% of fatty acids as DHA (51 mg/100 kcal) [90]. All formulas containing DHA also provided 0.64% of fatty acids as AA (34 mg/100 kcal). The sources of DHA and AA were unicellular algae oils (Crypthecodinium cohnii) and fungi (Mortierella alpina), respectively [90]. The content of the other main fatty acids, including LA (16.9–17.5% of total fatty acids) and ALA (1.61–1.68% of total fatty acids), was similar for the four formulas; this study showed that the LCPUFA supplementation during infancy has lasting effects on brain structure, function, and neurochemical concentrations in regions associated with attention (parietal) and inhibition anterior cingulate cortex (ACC) [90]. Birch et al. (2010) [91] studied newborns that were randomly assigned to consume a formula that did not contain LCPUFA (control) or a formula where AA was 0.64% of total fatty acids and with variable amounts of DHA (0.32%, 0.64%, or 0.96% of total acids fatty) from birth until 12 months old [91]. At the age of 9 years, it was observed through functional magnetic resonance spectroscopy that there was greater brain activation in the ACC and parietal regions in children supplemented with n-3 LCPUFA [91]. The analysis showed that children belonging to the 0.64% DHA group exhibited greater connectivity between the prefrontal and parietal regions than all other groups [91]. Furthermore, a voxel-based analysis revealed that groups receiving 0.32% and 0.64% DHA had higher white matter volume in the ACC and parietal regions than controls and the 0.96% DHA group [90]. Thus, n-3 LCPUFA supplementation during childhood has long-lasting effects on brain structure, function, and neurochemical concentrations in regions associated with attention such as parietal) and ACC [90]. DHA/AA balance is an important variable to consider in the contribution of LCPUFA to cognitive and behavioral development in childhood. However, it has been described that blood DHA levels generally increase when increasing DHA supplementation, although these levels tend to stabilize when supplementation exceeds 0.64% [90]. This can contribute to explain why the infants fed the 0.96% DHA supplementation did not have more brain connectivity than the ones in the 0.64% DHA group [92]. AA levels showed a strong inverted U function in response to increased DHA supplementation when neural development was evaluated, i.e., at the highest DHA containing formula (0.96%) (DHA:AA ratio 1.5:1.0); it was observed that AA decreased in the blood, as compared to the two low and intermediate formulas (0.32% and 0.64% DHA) [92]. Today is a standard procedure the fortification of infant formulas with DHA and EPA, which are added as triglycerides, phospholipids, or fatty acid concentrates [76].
5. DHA and AA in Neuronal Development and Function
DHA and AA can modulate neuronal function by influencing: (i) the physical properties of neuronal membranes by modulating ion channels and vesicular transport for endo/exocytosis of membrane-bound proteins [11,93]; (ii) signal transduction, by modulating G protein-mediated second messenger systems; and (iii) gene expression, through direct binding to transcription factors [94] or through the regulation of signaling cascades by eicosanoids derived from AA and DHA-derived docosanoids. In this sense, DHA and AA are crucial for the metabolism, growth, and differentiation of neurons [11,95] (Figure 2).
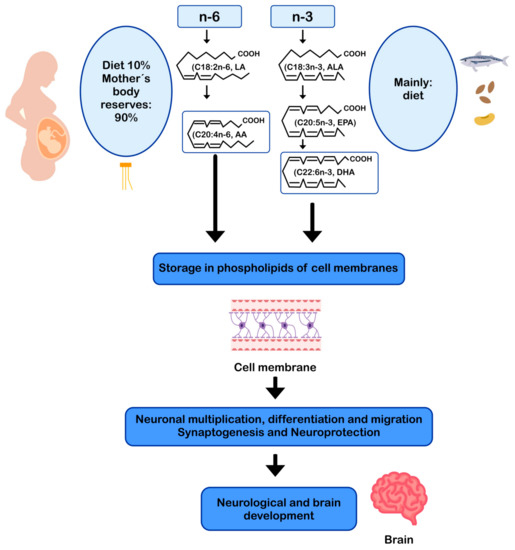
Figure 2.
AA and DHA in neuronal development and function. AA: arachidonic acid; ALA: alpha-linolenic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; LA: linoleic acid.
5.1. DHA in Neuronal Metabolism
DHA represents more than 90% of the n-3 LCPUFA in the brain [31], mainly as part of the membrane phospholipids in the brain gray matter [6,96], constituting 35% of the total fatty acids in the synaptic membranes [97]. DHA is primarily esterified to phosphatidylethanolamine, phosphatidylserine, and to a lesser extent phosphatidylcholine in neuronal membranes. The structural properties of DHA, such as the length of its carbon chain and its six double bonds, give flexibility and fluidity to the neuronal plasma membrane, facilitating signal transduction into the cell [10,11]. The fluidity of the neuronal membrane facilitates the lateral movement of receptors, G proteins, ion channels [98], enzymes [99], and neuroreceptors, increasing the efficiency in signal transduction [100,101,102]. In the brain, DHA is involved in neuronal growth, neuronal migration, synaptogenesis, synaptic plasticity, and gene expression [11,12,13,103]. Neurons cannot form DHA from its precursor ALA, but the glial cells, (especially astrocytes) can desaturate and elongate dietary ALA to convert it into DHA, which is subsequently transferred to most neurons [104,105]. DHA can also modulate the expression of genes related to neuronal energy generation involved in the function of the respiratory chain and ATP synthesis (adenosine triphosphate synthase) [103,104,105]. That function is relevant because approximately 50% of mitochondrial ATP is consumed by the Na+/K+ ATPase pump to maintain cell homeostasis and ionic gradients, a fundamental requirement for neuronal electrical excitability [103,104,105].
5.2. AA in Neuronal Metabolism
As already mentioned, AA is one of the most abundant fatty acids in the brain [26]. This n-6 LCPUFA is indispensable for brain growth and modulation of cell division and signaling [29]. During brain development, the concentration of AA increases rapidly [106]. It has been described in animal models that approximately 70–80% of AA concentration that is reached in adulthood is the result of its cerebral accumulation in the early postnatal period [2]. AA is an immediate precursor of adrenic acid (C22:4n-6, AdA), fatty acid found in large amounts in myelinic lipids, especially in phosphatidylethanolamine and phosphatidylcholine [106,107], suggesting the fundamental role of AA as a precursor of AdA in the development of neural tissue [106]. Conversion of AA to AdA may represent an important mechanism for supplying the high demand for AdA at the brain level, which is essential for neuronal myelinic lipid enrichment [2,106]. Wijendran et al. (2002) [108] investigated the metabolism of preformed AA in newborn baboons by the administration of a single oral dose of C13-labeled AA, reporting that 79–93% of AA consumed accumulates in brain membrane lipids and approximately 5% to 16% of AA is transformed into AdA [108]. Brain accumulation of AA and AdA occurs during the first month of life and represent 17% and 8% of the total n-6 LCPUFA, respectively [108].
Another brain function of AA is directly related to its participation in phosphatidylcholine (PC) structure [107]. Some intracellular phospholipid bilayers include AA-containing PC (AA-PC), a structure that plays a role as second messenger participating in the long-term enhancement of synapses in the CA1 region of the hippocampus [109]. Using image mass spectrometry, Yang et al. (2012) [110] characterized the distribution of AA-PC within neurons in cultured upper cervical nodes, finding an increasing gradient of AA-PC along the proximal to distal axonal axis suggesting that this structure is an important source of free AA [110]. Furthermore, it has been described that free AA can activate protein kinases and ion channels and inhibit neurotransmitter recycling [29], thus contributing to better control of synaptic transmission [70]. In addition to the role of AA in modulating neuronal excitability, AA is also essential in neuronal development in part because it is directly responsible for the activation of syntaxin-3, a protein of the neuronal membrane involved in the growth and neurite repair, an essential process in neurogenesis and subsequently in synaptic transmission [111].
5.3. DHA and AA as Precursors of Endocannabinoids
Remarkably, LCPUFA levels in the brain are highly correlated with dietary intake of PUFA and LCPUFA [112]. DHA and AA are precursors of many bioactive lipid mediators identified as docosanoids and eicosanoids, respectively, which are actively involved in regulatory responses in inflammation (eicosanoids such as prostaglandins, prostacyclins, thromboxanes, leucotrienes) and in resolution of inflammation (docosanoids such as resolvins and maresins) [113]. Both LCPUFA are also involved in the formation of endocannabinoids (eCB) with regulatory effects at the central nervous system (CNS) [114]. The endocannabinoid system consists of eCB, eCB receptors CB1 and CB2, and associated anabolic and catabolic enzymes [115]. Their functions are to maintain body energy homeostasis through the nutrient availability detection and the modulation of orexinergic inputs in selective regions of the CNS [116]. PUFA derived eicosanoids and eCB have been identified as independent ligands of CB1 and CB2 receptors [115]. Hammels et al. (2019) [115] reported that CB1 ligands synthesis in CNS depends on dietary intake of AA and DHA in a model of fatty acid desaturase 2-deficient mouse [115]. Moreover, DHA and AA have been recognized as ligands of the nuclear RxR receptor in the brain, being the PUFA ratio in the western diet, a critical nutritional parameter for numerous neurodegenerative diseases [115], which could increase neuroinflammation and over-stimulation of the endocannabinoid system [117]. AA bound to phospholipids determines the formation of eCB, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), molecules involved in the regulation of neuroinflammatory responses by microglia and astrocytes [114]. On the contrary, long-term supplementation with DHA and EPA reduces AEA and 2-AG synthesis [118]. N-3 and n-6 PUFA derived eCB are synthesized by lipoxygenases (LOX), cyclooxygenase 2 (COX-2), and cytochrome P450 epoxygenases (CYP450). Nevertheless, only LOX and CYP450 metabolites have been reported for a DHA eCB derivative; n-docosahexaenoylethanolamide (DHEA), and only CYP450 metabolites for an EPA eCB derivative; eicosapentaenoyl ethanolamide (EPEA) [116]. The physiological role of the metabolites generated by LOX and CYP450 remains to be elucidated to understand how these derivatives modulate cell signaling in health and disease [116]. A better understanding of the relationship between DHA, AA, and the endocannabinoid system is expected to lead to advances in the development of their therapeutic potential and the development of more specific treatment options for the prevention and treatment of neurodegenerative diseases [114].
6. DHA and AA in Neuroprotection
The most frequent neurodegenerative age-related diseases are Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) [119]. Although the etiopathogenesis and clinical characteristics of AD and PD are different, these diseases share common mechanisms of damage, such as mitochondrial dysfunction [120], neuroinflammation [121], and oxidative stress [122]. AD is characterized by dementia, memory loss, and cognitive decline, disorders that are worsened with aging [123]. In 2019, the World Alzheimer Report indicated that more than 50 million people live with dementia worldwide, a number that will increase up to 152 million by 2050 [124]. In this regard, every three seconds, a person develops dementia, and the current annual cost of dementia is estimated at US $ 1 trillion, which is estimated to double by 2030 [124].
DHA via enzyme 15-lipoxygenase (15-LOX) can be converted in oxylipins, including resolvins and neuroprotectins, which are potent lipid mediators [125,126]. Furthermore, DHA may undergo lipid peroxidation producing oxylipin metabolites, such as 4-hytoxyhexenal (4-HHE) [126,127]. These metabolites may have a role in modulating oxidative cell homeostasis by regulating the activity of the transcription factors nuclear factor kappa-B (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2), thus participating in the inflammatory and antioxidant response, and neuroprotection [126]. Osterman et al. (2019) [128] assigned healthy adults with low fish consumption (n = 121) to receive capsules with different doses of n-3 LCPUFA reflecting three patterns of fatty fish consumption: 1, 2, or 4 servings/week with 3.27 g of EPA + DHA (1:1.2) per serving or placebo. The authors reported that plasma oxylipins after 3 and 12 months increased linearly with the highest intake of EPA and DHA [128].
Aging is a normal process of the life cycle, and its progression is usually accompanied by the decrease of a wide range of body functions, including cognitive function, marked by decreased synaptic density, decreased neuronal survival, and loss of volume of the gray and white matter [105,129,130]. Alteration of lipid metabolism also occurs [131] and is associated with the dysfunction of fluidity and activity of brain cell membranes microdomains (or rafts) [132]. Exacerbation of alteration of lipid metabolism generates abnormal brain activity that can potentially lead to the development of neurodegenerative diseases [132,133]. Factors contributing to the early cognitive decline include diseases related to unhealthy lifestyles and metabolic syndrome [104]. Among them, atherosclerosis and hypertension are relevant because an altered blood flow generates hypoperfusion and vascular dysfunction, causing an impairment of the blood-brain barrier and triggering neurodegenerative processes that lead to cognitive deterioration and, ultimately, to the development of AD [131,132].
The pathogenesis of AD has not been fully resolved. However, it is known that the mechanisms that trigger the pathology are related to: (i) low levels of acetylcholine [134]; (ii) aggregation of insoluble β-amyloid (Aβ) peptides, a product of abnormal processing of the amyloidal precursor protein, in neuritic plaques, leading to Aβ accumulation in the CNS [135,136,137]; (iii) hyperphosphorylation of the tau protein associated with microtubules, causing intraneuronal accumulation of neurofibrillary tangles of tau protein and disruption of neuronal microtubules [138,139]; and (iv) oxidative stress, leading to inflammation, synaptic toxicity and accumulation of intraneuronal inclusions [136,140] (Figure 3). Regarding its clinical characteristics, AD is a slowly progressive disease and three stages can be recognized in its evolution: the first is characterized by memory failures; in the second, language disorders, apraxias, and Gerstmann syndrome (agraphia and agnosia) are frequently added; and in the third stage, the patient is physically disabled and prostrated [141,142].
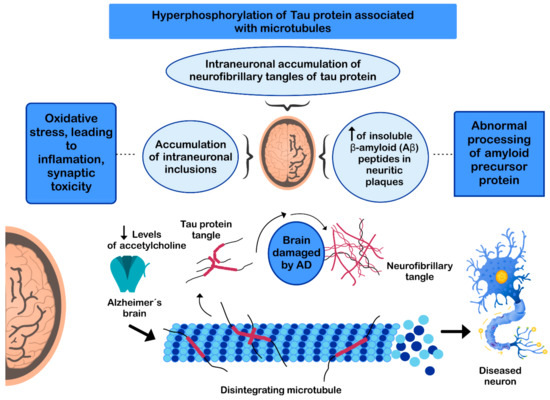
Figure 3.
Mechanisms involved in the pathogenesis of Alzheimer’s disease (AD).
PD is the second most common neurodegenerative disease after AD [143], affecting 2%-3% of the population ≥65 years old [144]. The etiopathogenesis of the disease includes: (i) intracellular aggregation of α-synuclein causing the formation of Lewy bodies [145,146]; (ii) neuronal loss of the substantia nigra pars compacta (SNPc) leading to a marked striatal dopamine deficiency [147,148]; (iii) mitochondrial dysfunction, due to defects in the activity and incorrect assembly of complex I of the mitochondrial electron transport chain [149,150] (inhibition of mitochondrial complex I induces neuronal cell death in the SNPc leading to dopaminergic decrease) [147,149]; (iv) oxidative stress [147], the nigral dopaminergic neurons are particularly vulnerable to metabolic diseases and oxidative stress [151,152,153,154]; and (v) neuroinflammation [147,155,156] (Figure 4). The clinical evolution of PD is characterized by motor disturbances due to progressive nigrostriatal dopaminergic neurodegeneration that occurs in parallel with decreased levels of striatal dopamine, dopaminergic synapses, and the density of dendritic spines in mid-striated spinal neurons [157]. Other non-motor symptoms of PD include cognitive impairment [158] and gastrointestinal dysfunctions [159,160].
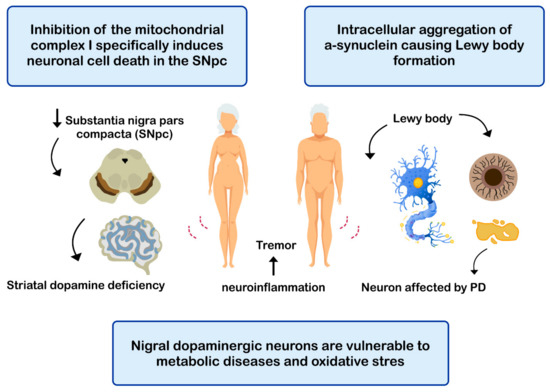
Figure 4.
Mechanisms related with the pathogenesis of Parkinson’s disease (PD).
7. Dietary DHA in Alzheimer and Parkinson Diseases
Various mechanisms have been described by which DHA would exert a neuroprotective effect on AD. It has been described that populations having a higher intake of DHA show a lower risk of developing cognitive impairment or AD [20,21]. DHA can activate synapsin-1, a protein that promotes synapsis in excitatory glutamatergic neurons in the hippocampus, thus contributing to synaptic plasticity and improving cognitive function [161,162]. Furthermore, synapsin-1 is involved in the release of neurotransmitters, axonal elongation, and maintenance of synaptic contacts [163,164], whose synthesis and further phosphorylation are determined by the brain neurotrophic factor [158,160], which is increased by DHA, thus facilitating synaptic transmission [161]. On the other hand, DHA inhibits tau protein phosphorylation, preventing the intraneuronal microtubule disassembling and the neurofibrillary tangles accumulation [136]. DHA can also reduce neuronal Aβ load by preventing its formation [163,164,165] and accumulation [163,166], thus reducing the toxicity produced by Aβ [165] and neuronal apoptosis [167]. A recent study suggests that DHA, but not AA, can suppress the production of oligomeric Aβ (oAβ) and the oAβ-induced increase of phosphorylated cytosolic phospholipase A2, synthesis of inducible nitric oxide synthase, production of reactive oxygen species, and activation of tumor necrosis factor α in microglia cells [168]. DHA can modulate AA metabolism in oAβ-stimulated microglia by suppressing oxidative and inflammatory pathways and upregulating the antioxidative pathway involving Nrf2/heme oxygenase-1 [168]. Moreover, DHA can prevent Aβ-induced necroptosis of THP-1 cells via the receptor interacting protein kinase-1 and -3 (RIPK1/RIPK3) signaling pathway. DHA treatment (0.5 μM, 1 μM) restored migration of THP-1 monocytes which was impaired by Aβ25-35 [169]. Also, the anti-inflammatory effect of DHA derived docosanoids, i.e., resolvins, prevent brain damage adjacent to the inflammatory state [170,171,172]. The OmegAD study (2015) evaluated the effect of the supplementation with EPA (0.6 g/day) and DHA (1.7 g/day) on the production of resolvins for six months in subjects with AD [168]. Analysis of mononuclear cells from peripherical blood of patients showed that supplementation with EPA and DHA maintained the production of resolvins, preventing deleterious changes in cognitive function [170]. Understanding the mechanisms underlying the effects of DHA on microglia should contribute to developing a nutraceutical therapy with DHA for the prevention and/or treatment of AD [168].
Clinical trials have shown that DHA supplementation allows to preserve cognitive abilities in healthy individuals (learning, memory, and verbal fluency), while in AD results are still not so clear. It must be considered that (i) the age of the subjects, (ii) the state of evolution of the disease, (iii) the daily amount of DHA ingested as a supplement, and (iv) the time of intervention, are relevant to clearly identify the benefits of DHA supplementation during aging and AD [173,174].
DHA has also been linked to the maintenance of synaptic homeostasis and neuronal activity by modulating the expression of nuclear α-synuclein proteins in PD [11,123]. DHA produces an increase in the expression of brain trophic factors [161], including glial cell-derived neurotrophic factor (GDNF) [162,175], contributing to the prevention of glial cell dysfunction, which is related to the early appearance of neurodegenerative pathologies [176]. GDNF has a protective effect against dopaminergic injury in substantia nigra by promoting anti-inflammatory pathways linked to the inhibition of the expression of transcription factor Nuclear Factor-κB (NF-κB) [177]. On the other hand, the administration of DHA to humans and mice may increase (i) dopaminergic neurotransmission, (ii) synaptic membrane formation, and (iii) dendritic spine density, thus preventing neuronal death and motor symptoms, such as the disturbance and impairment of the motor and non-motor coordination which are characteristic in PD [21,22,175,178,179,180,181,182,183,184]. The beneficial effects of DHA interventions have been demonstrated even when the fatty acid is administered after the almost full development of PD symptoms in humans [172]. This DHA effect is an antagonist to the action of l-3,4-dihydroxyphenylalanine (l-DOPA), the drug most used in the treatment of PD, which compensates for the loss of dopaminergic cells by improving the synthesis of dopamine at the synaptic terminals [185] but does not prevent the degeneration of dopaminergic neurons and has no effect on non-motor symptoms, which are determinative in the quality of life of PD patients [186].
8. Dietary AA in Alzheimer and Parkinson Diseases
The role of AA in neurodegenerative pathologies, such as AD and PD, has not been fully elucidated. Some studies carried out on the effect of AA-enriched diets on AD mouse models observed that these diets could prevent cognitive dysfunction caused by abnormal processing of the amyloid precursor protein (APP), contributing to reducing the formation of insoluble Aβ in neuritic plaques [184,187]. In contrast, Amtul et al. (2012) reported an increase in Aβ production and deposition in mice exposed to an AA-enriched diet [188]. The contrast in the results may be due to the different AA doses used, since dietary AA content was ten times higher in the study conducted by Amtul et al., 2012 [184,187,188]. On the other hand, regarding the neuroinflammation of AD, it has been widely documented in the literature that AD patients overexpress the enzyme cyclooxygenase 2 (COX-2) in the cortex and the hippocampus [36,37,38,189], thus increasing the formation of pro-inflammatory prostaglandins PGD2 and PGE2 [190]. However, although AA is a substrate for COX-2 [178], currently, there is insufficient and nonconclusive evidence linking dietary AA with the appearance of neuroinflammation [191]. Regarding synaptic function, studies in mice indicate that dietary AA has positive effects on cognition and synaptic plasticity in aging animals [106,192], which may establish a positive relationship between abilities of spatial memory and AA content in the hippocampus [179,193,194]. On the other hand, Kotania S. et al. (2006) reported that the supplementation of 240 mg/day of AA and DHA produced a significant improvement in immediate memory and attention score in a group of patients with mild cognitive dysfunction and organic brain lesions but with no diagnosis of AD (no-AD) [195]. However, no significant differences were observed when compared to the AD group. A possible mechanism that would explain this improvement in cognitive function observed in control and AD patients proposes that AA would act at the post-synaptic level through the long-term enhancement of the synaptic transmission and the expansion of this at the pre-synaptic level by exerting a direct modulation of voltage-dependent potassium channels, thus stimulating exocytosis of neurotransmitters [35,196,197,198,199].
AA role in PD is controversial. Lakkapa et al. (2016) proposed that epoxyeicosatrienoic acids (EETs), which are AA metabolites, could constitute a therapeutic target for PD since they are widely distributed in the brain and have shown anti-inflammatory and antioxidant effects [200]. Concordantly, the same authors reported that EETs increased the expression of antioxidant enzymes and attenuated oxidative stress and inflammation in a Drosophila model of PD [201]. On the other hand, it is known that protein α-synuclein self-assembles into toxic β-sheet aggregates in PD, while it assembles into α-helical oligomers in healthy neurons [202]. Iljina et al. (2016) studied α-synuclein multimers conformation in the presence of AA in vitro. The authors reported that AA induces the α-helical assembling of α-synuclein, leading to lower neuronal damage than the β-sheet multimers formed without AA treatment [202].
On the contrary, AA is a ligand of the fatty acid-binding protein 3 (FABP3) [203], which has been described as an early biomarker of dementia and PD and highly expressed in dopaminergic neurons [203]. In the presence of AA, the fatty acid binds to AA inducing α-synuclein oligomerization and aggregation in Neuro-2A cells [203]. In line with that result, Julien et al. (2006) described that patients with PD had higher AA brain levels evaluated postmortem [204]. Finally, a meta-analysis was conducted in 2019 to assess the influences of dietary fat in PD and concluded that cholesterol and AA intake increase PD risk [205].
9. Conclusions
In recent years, substantial progress has been made in understanding the cellular and molecular mechanisms underlying the neuroprotective effect of DHA and AA in the extreme stages of life. The relevance of DHA and AA during the first months of life is widely accepted because these LCPUFA play a fundamental role in neural development. However, although DHA in advanced stages of life is associated with neuroprotective effects, both in healthy humans and in patients with AD or PD, the role of AA in these pathologies is still controversial. Analysis of currently available information suggests directing future research towards understanding the role of AA in aging and neurodegenerative pathologies. Another pending research challenge will be to establish the dose-response relationship for DHA in future treatments of patients with AD or PD since, according to these results, the use of DHA could be an alternative or a complement to the pharmacological treatment of these pathologies in the early stages, as well as a selective and promising agonist in the prevention of premature brain aging and its associated pathologies. The role of personalized medicine, in the near future, together with a better knowledge of bioavailability, pharmacodynamics, pharmacokinetic and metabolic effects of DHA and AA challenges toward the development of more effective forms to supply these LCPUFA in benefit of neuroprotection throughout the life cycle.
Author Contributions
Conceptualization, R.V. and A.V.; writing—original draft preparation, V.S., F.E., A.V., R.C.-W., and R.V.; writing—review and editing, A.V., R.C.-W., and R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (National Fund for Scientific and Technological Research), FONDECYT, University of Chile and University of Toronto.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Svennerholm, L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968, 9, 570–579. [Google Scholar] [CrossRef]
- Crawford, M.A.; Broadhurst, C.L. The role of docosahexaenoic and the marine food web as determinants of evolution and hominid brain development: The challenge for human sustainability. Nutr. Health 2012, 21, 17–39. [Google Scholar] [CrossRef]
- Carlson, S.E.; Colombo, J. Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Adv. Pediatrics 2016, 63, 453–471. [Google Scholar] [CrossRef]
- Belkind-Gerson, J.; Carreón-Rodríguez, A.; Contreras-Ochoa, C.O.; Estrada-Mondaca, S.; Parra-Cabrera, M.S. Fatty acids and neurodevelopment. J. Pediatr. Gastroenterol. Nutr. 2008, 47, S7–S9. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrinets 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Diau, G.Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 247–250. [Google Scholar] [CrossRef]
- Carrié, I.; Clément, M.; de Javel, D.; Francès, H.; Bourre, J.-M. Specific phospholipid fatty acid composition of brain regions in mice: Effects of n–3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472. [Google Scholar] [CrossRef]
- Garcia, M.C.; Ward, G.; Ma, Y.-C.; Salem, N.; Kim, H.-Y. Effect of Docosahexaenoic Acid on the Synthesis of Phosphatidylserine in Rat Brain Microsomes and C6 Glioma Cells. J. Neurochem. 2002, 70, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Gawrisch, K.; Eldho, N.V.; Holte, L.L. The structure of DHA in phospholipid membranes. Lipids 2003, 38, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Morales, J.; Sanhueza, J.; Valenzuela, A. Docosahexaenoic acid (DHA), an essential fatty acid at the brain. Rev. Chil. Nutr. 2013, 40, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Katakura, M.; Hashimoto, M.; Shahdat, H.; Gamoh, S.; Okui, T.; Matsuzaki, K.; Shido, O. Docosahexaenoic acid promotes neuronal differentiation by regulating basic helix–loop–helix transcription factors and cell cycle in neural stem cells. Neuroscience 2009, 160, 651–660. [Google Scholar] [CrossRef]
- Jumpsen, J.; Lien, E.L.; Goh, Y.K.; Clandinin, M.T. Small changes of dietary (n-6) and (n-3)/fatty acid content ration alter phosphatidylethanolamine and phosphatidylcholine fatty acid composition during development of neuronal and glial cells in rats. J. Nutr. 1997, 127, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Kyle, D.J.; Schaefer, E.; Patton, G.; Beiser, A. Low serum docosahexaenoic acid is a significant risk factor for alzheimer’s dementia. Lipids 1999, 34, S245. [Google Scholar] [CrossRef] [PubMed]
- Heude, B.; Ducimetière, P.; Berr, C. Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA Study. Am. J. Clin. Nutr. 2003, 77, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Kaufman, J.S.; A Satia, J.; Rosamond, W.; Folsom, A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: The Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2007, 85, 1103–1111. [Google Scholar] [CrossRef]
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 2000, 35, 1305–1312. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Alexander, D.D.; van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of Fish and n-3 Fatty Acids and Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef]
- Chang, Y.L.; Chen, S.J.; Kao, C.L.; Hung, S.C.; Ding, D.C.; Yu, C.C.; Chen, Y.J.; Ku, H.H.; Lin, C.P.; Lee, K.H.; et al. Docosahexaenoic acid promotes dopaminergic differentiation in induced pluripotent stem cells and inhibits teratoma formation in rats with Parkinson-like pathology. Cell Transplant. 2012, 21, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.; Parlak, H.; Tanriover, G.; Dilmac, S.; Ulker, S.N.; Birsen, L.; Agar, A. The protective mechanism of docosahexaenoic acid in mouse model of Parkinson: The role of heme oxygenase. Neurochem. Int. 2016, 101, 110–119. [Google Scholar] [CrossRef]
- Mori, M.A.; Delattre, A.M.; Carabelli, B.; Pudell, C.; Bortolanza, M.; Staziaki, P.V.; Visentainer, J.V.; Montanher, P.F.; del Bel, E.A.; Ferraz, A.C. Neuroprotective effect of omega-3 polyunsaturated fatty acids in the 6-OHDA model of Parkinson’s disease is mediated by a reduction of inducible nitric oxide synthase. Nutr. Neurosci. 2018, 21, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients 2018, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Kiesewetter, D.; Chang, L.; Rapoport, S.I.; Igarashi, M. Quantifying conversion of linoleic to arachidonic and other n-6 polyunsaturated fatty acids in unanesthetized rats. J. Lipid Res. 2010, 51, 2940–2946. [Google Scholar] [CrossRef]
- Brenna, J.T. Arachidonic acid needed in infant formula when docosahexaenoic acid is present. Nutr. Rev. 2016, 74, 329–336. [Google Scholar] [CrossRef]
- Harauma, A.; Hatanaka, E.; Yasuda, H.; Nakamura, M.T.; Salem, N.; Moriguchi, T. Effects of arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid on brain development using artificial rearing of delta-6-desaturase knockout mice. Prostaglandins Leukot. Essent. Fat. Acids 2017, 127, 32–39. [Google Scholar] [CrossRef]
- Wilson, R.; Sargent, J.R.X. Lipid and fatty acid composition of brain tissue from adrenoleukodystrophy patients. J. Neurochem. 1993, 61, 290–297. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 545–551. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef]
- Rickman, C.; Davletov, B. Arachidonic Acid Allows SNARE Complex Formation in the Presence of Munc18. Chem. Biol. 2005, 12, 545–553. [Google Scholar] [CrossRef]
- Connell, E.; Darios, F.; Broersen, K.; Gatsby, N.; Peak-Chew, S.; Rickman, C.; Davletov, B. Mechanism of arachidonic acid action on syntaxin–Munc18. EMBO Rep. 2007, 8, 414–419. [Google Scholar] [CrossRef]
- Latham, C.F.; Osborne, S.L.; Cryle, M.J.; Meunier, F.A. Arachidonic acid potentiates exocytosis and allows neuronal SNARE complex to interact with Munc18a. J. Neurochem. 2006, 100, 1543–1554. [Google Scholar] [CrossRef]
- Tokuda, H.; Kontani, M.; Kawashima, H.; Kiso, Y.; Shibata, H.; Osumi, N. Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neurosci. Res. 2014, 88, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Purohit, D.; Haroutunian, V.; Luterman, J.D.; Willis, F.; Naslund, J.; Buxbaum, J.D.; Mohs, R.C.; Aisen, P.S.; Pasinetti, G.M. Neuronal Cyclooxygenase 2 Expression in the Hippocampal Formation as a Function of the Clinical Progression of Alzheimer Disease. Arch. Neurol. 2001, 58, 487–492. [Google Scholar] [CrossRef]
- Fujimi, K.; Noda, K.; Sasaki, K.; Wakisaka, Y.; Tanizaki, Y.; Iida, M.; Kiyohara, Y.; Kanba, S.; Iwaki, T. Altered Expression of COX-2 in Subdivisions of the Hippocampus during Aging and in Alzheimer’s Disease: The Hisayama Study. Dement. Geriatr. Cogn. Disord. 2007, 23, 423–431. [Google Scholar] [CrossRef]
- Ching, Y.K.; Chin, Y.S.; Appukutty, M.; Ramanchadran, V.; Yu, C.Y.; Ang, G.Y.; Gan, W.Y.; Chan, Y.M.; Teh, L.K.; Salleh, M.Z. Interaction of Dietary Linoleic Acid and α-Linolenic Acids with rs174547 in FADS1 Gene on Metabolic Syndrome Components among Vegetarians. Nutrinets 2019, 11, 1686. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Soto, M.; Mutch, D.M. Diet Regulation of Long-Chain PUFA Synthesis: Role of Macronutrients, Micronutrients, and Polyphenols on Δ-5/Δ-6 Desaturases and Elongases 2/5. Adv. Nutr. 2020, 142. [Google Scholar] [CrossRef]
- Kang, J.X. The Importance of Omega-6/Omega-3 Fatty Acid Ratio in Cell Function. World Rev. Nutr. Diet. 2003, 92, 23–36. [Google Scholar] [CrossRef]
- Valenzuela, R.; Videla, L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Fatty Acids in Human Nutrition. Report of an Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; p. 91. [Google Scholar]
- Brayner, B.; Kaur, G.; Keske, M.A.; Livingstone, K.M. FADS Polymorphism, Omega-3 Fatty Acids and Diabetes Risk: A Sys-tematic Review. Nutrients 2018, 13, 10758. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Zhang, Z.; Zheng, X.; Wang, Y.; Yu, M.; Liu, G. Effects of the rs3834458 Single Nucleotide Polymorphism in FADS2 on Levels of n-3 Long-chain Polyunsaturated Fatty Acids: A Meta-analysis. Prostaglandins Leukot. Essent. Fat. Acids 2019, 150, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Innis, S.M. Genetic Variants of the FADS1 FADS2 Gene Cluster Are Associated with Altered (n-6) and (n-3) Essential Fatty Acids in Plasma and Erythrocyte Phospholipids in Women during Pregnancy and in Breast Milk during Lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.D.; Smith, G.D.; Emmett, P.M.; Hibbeln, J.R.; Golding, J. FADS2 Polymorphisms Modify the Effect of Breastfeeding on Child IQ. PLoS ONE 2010, 5, e11570. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Duan, Q.L.; Moraes, T.J.; Becker, A.B.; Mandhane, P.J.; E Turvey, S.; Lefebvre, D.L.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD Cohort Study. Am. J. Clin. Nutr. 2019, 110, 1370–1383. [Google Scholar] [CrossRef]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Z.; Liu, S.; Xiao, Y.; Miao, M.; Dong, Q.; Xin, Y. Association of Nonalcoholic Fatty Liver Disease and Coronary Artery Disease with FADS2 rs3834458 Gene Polymorphism in the Chinese Han Population. Gastroenterol. Res. Pr. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Morales, G.; González, M.; Morales, J.; Sanhueza, J.; Valenzuela, A. N-3 long chain polyunsaturated fatty acids and cardiovascular disease. Rev. Chil. Nutr. 2014, 41, 319–327. [Google Scholar]
- Morales, J.; Valenzuela, R.; González, D.; González, M.; Tapia, G.; Sanhueza, J. New dietary sources of alpha-linolenic acid: A critical view. Rev. Chil. Nutr. 2012, 39, 79–87. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, pol-yunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Lefevre, M.; Toner, C.D.; Colombo, J.; Cunnane, S.C.; Holden, J.M.; Klurfeld, D.M.; Morris, M.C.; Whelan, J. Towards Establishing Dietary Reference Intakes for Eicosapentaenoic and Docosahexaenoic Acids. J. Nutr. 2009, 139, 804S–819S. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Lopez-Alarcon, M.G.; Garza, C. Nutrient Adequacy of Exclusive Breastfeeding for the Term Infant During the First Six Months of Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Lauritzen, L.; Carlson, S.E. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child. Nutr. 2011, 7, 41–58. [Google Scholar] [CrossRef]
- Koletzko, B.; Larqué, E.; Demmelmair, H. Placental transfer of long-chain polyunsaturated fatty acids (LC-PUFA). J. Peérinat. Med. 2007, 35, S5–S11. [Google Scholar] [CrossRef]
- Demmelmair, H.; Kuhn, A.; Dokoupil, K.; Hegele, V.; Sauerwald, T.; Koletzko, B. Human lactation: Oxidation and maternal transfer of dietary 13C-labelled α-linolenic acid into human milk. Isot. Environ. Health Stud. 2015, 52, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Cetin, I.; Brenna, J.T.; Perinatal Lipid Intake Working Group; Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology, Hepatology and Nutrition, Committee on Nutrition; International Federation of Placenta Associations; et al. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, L.; Bao, W.; Rong, S. Nutritional composition of breast milk in Chinese women: A systematic review. Asia Pac. J. Clin. Nutr. 2018, 27, 491–502. [Google Scholar] [CrossRef]
- Forsyth, S.; Gautier, S.; Salem, N. The importance of dietary DHA and ARA in early life: A public health perspective. In Proceedings of the Nutrition Society; Amsterdam University Press: Amsterdam, The Netherlands, 2017; Volume 76, pp. 568–573. [Google Scholar]
- Hall, B. Uniformity of human milk. Am. J. Clin. Nutr. 1979, 32, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.; Jung, B.-M.; Yi, H.; Jung, J.A.; Chang, N. Breast milk fatty acid composition and fatty acid intake of lactating mothers in South Korea. Br. J. Nutr. 2017, 117, 556–561. [Google Scholar] [CrossRef]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef]
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2016, 69, 28–40. [Google Scholar] [CrossRef]
- Del Prado, M.; Villalpando, S.; Elizondo, A.; Rodríguez, M.; Demmelmair, H.; Koletzko, B. Contribution of dietary and newly formed arachidonic acid to human milk lipids in women eating a low-fat diet. Am. J. Clin. Nutr. 2001, 74, 242–247. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, X.; Zhou, B.; Jiang, A.C.; Chai, L. An updated review of worldwide levels of docosahexaenoic and arachidonic acid in human breast milk by region. Public Health Nutr. 2016, 19, 2675–2687. [Google Scholar] [CrossRef]
- Lauritzen, L.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid. Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Elshani, B.; Kotori, V.; Daci, A. Role of omega-3 polyunsaturated fatty acids in gestational diabetes, maternal and fetal insights: Current use and future directions. J. Matern. Neonatal. Med. 2021, 34, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Muñoz, Y.; Ortiz, M.; Maliqueo, M.; Chouinard-Watkins, R.; Valenzuela, R. Impact of Maternal Obesity on the Metabolism and Bioavailability of Polyunsaturated Fatty Acids during Pregnancy and Breastfeeding. Nutrients 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Project, F.T.E.C.O.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef]
- Barrera, C.; Valenzuela, R.; Chamorro, R.; Bascuñán, K.; Sandoval, J.; Sabag, N.; Valenzuela, F.; Valencia, M.P.; Puigrredon, C.; Valenzuela, A. The Impact of Maternal Diet during Pregnancy and Lactation on the Fatty Acid Composition of Erythro-cytes and Breast Milk of Chilean Women. Nutrients 2018, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Devarshi, P.P.; Grant, R.W.; Ikonte, C.J.; Hazels-Mitmesser, S. Maternal Omega-3 Nutrition, Placental Transfer and Fetal Brain De-velopment in Gestational Diabetes and Preeclampsia. Nutrients 2019, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.G.; Legette, L.L.; Ikonte, C.J.; Mitmesser, S.H. Choline and DHA in Maternal and Infant Nutrition: Synergistic Implications in Brain and Eye Health. Nutrients 2019, 11, 1125. [Google Scholar] [CrossRef]
- Rodier, P.M. Chronology of Neuron Development: Animal Studies and their Clinical Implications. Dev. Med. Child. Neurol. 2008, 22, 525–545. [Google Scholar] [CrossRef]
- Vasung, L.; Turk, E.A.; Ferradal, S.L.; Sutin, J.; Stout, J.N.; Ahtam, B.; Lin, P.-Y.; Grant, P.E. Exploring early human brain development with structural and physiological neuroimaging. NeuroImage 2019, 187, 226–254. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Miura, M. How to form and close the brain: Insight into the mechanism of cranial neural tube closure in mammals. Cell. Mol. Life Sci. 2012, 70, 3171–3186. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Lim, K.O. Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci. Biobehav. Rev. 2006, 30, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Kelava, I.; Lancaster, M.A. Stem Cell Models of Human Brain Development. Cell Stem Cell 2016, 18, 736–748. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, M.; Cai, H.; Hualin, C.; Jiang, P.; Dang, R.; Liu, Y.; He, X.; Xue, Y.; Cao, L.; et al. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis. 2016, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.; Obeid, R. Choline, Neurological Development and Brain Function: A Systematic Review Focusing on the First 1000 Days. Nutrients 2020, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Owji, S.; Shoja, M.M. The History of Discovery of Adult Neurogenesis. Clin. Anat. 2020, 33, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Dobbing, J.; Sands, J. Quantitative growth and development of human brain. Arch. Dis. Child. 1973, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cocas, L.A.; Fernandez, G.; Barch, M.; Doll, J.; Diaz, I.Z.; Pleasure, S.J. Cell Type-Specific Circuit Mapping Reveals the Presynaptic Connectivity of Developing Cortical Circuits. J. Neurosci. 2016, 36, 3378–3390. [Google Scholar] [CrossRef]
- Lien, V.W.; Clandinin, M.T. Dietary assessment of arachidonic acid and docosahexaenoic acid intake in 4–7 year-old children. J. Am. Coll. Nutr. 2009, 28, 7–15. [Google Scholar] [CrossRef]
- Lepping, R.J.; Honea, R.A.; Martin, L.E.; Liao, K.; Choi, I.-Y.; Lee, P.; Papa, V.B.; Brooks, W.M.; Shaddy, D.J.; Carlson, S.E.; et al. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev. Psychobiol. 2019, 61, 5–16. [Google Scholar] [CrossRef]
- Birch, E.E.; Carlson, S.E.; Hoffman, D.R.; Fitzgerald-Gustafson, K.M.; Fu, V.L.; Drover, J.R.; Castañeda, Y.S.; Minns, L.; Wheaton, D.K.; Mundy, D.; et al. The DIAMOND (DHA Intake and Measurement of Neural Development) Study: A double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am. J. Clin. Nutr. 2010, 91, 848–859. [Google Scholar] [CrossRef]
- Colombo, J.; Shaddy, D.J.; Kerling, E.H.; Gustafson, K.M.; Carlson, S.E. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot. Essent. Fat. Acids 2017, 121, 52–56. [Google Scholar] [CrossRef]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Peérinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef]
- Talamonti, E.; Sasso, V.; To, H.; Haslam, R.P.; Napier, J.A.; Ulfhake, B.; Pernold, K.; Asadi, A.; Hessa, T.; Jacobsson, A.; et al. Impairment of DHA synthesis alters the expression of neuronal plasticity markers and the brain inflammatory status in mice. FASEB J. 2019, 34, 2024–2040. [Google Scholar] [CrossRef]
- Wainwright, P.E.; Xing, H.-C.; Mutsaers, L.; McCutcheon, D.; Kyle, D. Arachidonic Acid Offsets the Effects on Mouse Brain and Behavior of a Diet with a Low (n-6):(n-3) Ratio and Very High Levels of Docosahexaenoic Acid. J. Nutr. 1997, 127, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Giusto, N.M.; Salvador, G.A.; Castagnet, P.I.; Pasquaré, S.J.; Ilincheta de Boschero, M.G. Age-associated changes in central nervous system glycerolipid composition and metabolism. Neurochem. Res. 2002, 27, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Biology of metabolism in growing animals. In Essential Fatty Acid Metabolism During Early Development; Burrin, D.G., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 235–274. [Google Scholar]
- Poling, J.S.; Karanian, J.W.; Salem, N.; Vicini, S. Time- and voltage-dependent block of delayed rectifier potassium channels by docosahexaenoic acid. Mol. Pharmacol. 1995, 47, 381–390. [Google Scholar] [PubMed]
- Strokin, M.; Chechneva, O.; Reymann, K.G.; Reiser, G. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose dep-rivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phos-pholipids by calcium independent phospholipase A2. Neuroscience 2006, 140, 547–553. [Google Scholar] [CrossRef] [PubMed]
- De Lion, S.; Chalon, S.; Guilloteau, D.; Besnard, J.-C.; Durand, G. α-Linolenic Acid Dietary Deficiency Alters Age-Related Changes of Dopaminergic and Serotoninergic Neurotransmission in the Rat Frontal Cortex. J. Neurochem. 2002, 66, 1582–1591. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, T.; Shi, H.; Chen, X.; Wang, H.; Zheng, K.; Huang, X.-F.; Yu, Y. DHA reduces hypothalamic inflammation and improves central leptin signaling in mice. Life Sci. 2020, 257, 118036. [Google Scholar] [CrossRef]
- Chalon, S.; Delion-Vancassel, S.; Belzung, C.; Guilloteau, D.; Leguisquet, A.M.; Besnard, J.C.; Durand, G. Dietary fish oil affects mon-oaminergic neurotransmission and behavior in rats. J. Nutr. 1998, 128, 2512–2519. [Google Scholar] [CrossRef]
- Feltham, B.A.; Louis, X.L.; Eskin, A.M.N.; Suh, M. Docosahexaenoic Acid: Outlining the Therapeutic Nutrient Potential to Combat the Prenatal Alcohol-Induced Insults on Brain Development. Adv. Nutr. 2020, 11, 724–735. [Google Scholar] [CrossRef]
- Singh, R.B.; Gupta, S.; Dherange, P.; de Meester, F.; Wilczynska, A.; Alam, S.E.; Pella, D.; Wilson, D.W. Metabolic syndrome: A brain disease. Can. J. Physiol. Pharmacol. 2012, 90, 1171–1183. [Google Scholar] [CrossRef]
- Masliah, E.; Crews, L.; Hansen, L. Synaptic remodeling during aging and in Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 91–99. [Google Scholar] [CrossRef]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Hsieh, A.T.; Anthony, J.C.; Diersen-Schade, D.A.; Rumsey, S.C.; Lawrence, P.; Li, C.; Nathanielsz, P.W.; Brenna, J.T. The Influence of Moderate and High Dietary Long Chain Polyunsaturated Fatty Acids (LCPUFA) on Baboon Neonate Tissue Fatty Acids. Pediatr. Res. 2007, 61, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Lawrence, P.; Diau, G.-Y.; Boehm, G.; Nathanielsz, P.; Brenna, J. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J. Lipid Res. 2002, 43, 762–767. [Google Scholar] [CrossRef]
- Alashmali, S.M.; Kitson, A.P.; Lin, L.; Lacombe, R.J.S.; Bazinet, R.P. Maternal dietary n-6 polyunsaturated fatty acid deprivation does not exacerbate post-weaning reductions in arachidonic acid and its mediators in the mouse hippocampus. Nutr. Neurosci. 2017, 22, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Sugiura, Y.; Ikegami, K.; Konishi, Y.; Setou, M. Axonal Gradient of Arachidonic Acid-containing Phosphatidylcholine and Its Dependence on Actin Dynamics. J. Biol. Chem. 2012, 287, 5290–5300. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Davletov, B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nat. Cell Biol. 2006, 440, 813–817. [Google Scholar] [CrossRef]
- Novak, E.M.; Dyer, R.A.; Innis, S.M. High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 2008, 1237, 136–145. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflam-mation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Dyall, S.C. Interplay between n-3 and n-6 Long-Chain Polyunsaturated Fatty Acids and the Endocannabinoid System in Brain Protection and Repair. Lipids 2017, 52, 885–900. [Google Scholar] [CrossRef]
- Hammels, I.; Binczek, E.; Schmidt-Soltau, I.; Jenke, B.; Thomas, A.; Vogel, M.; Thevis, M.; Filipova, D.; Papadopoulos, S.; Stoffel, W. Novel CB1-ligands maintain homeostasis of the endocannabinoid system in ω3- and ω6-long-chain-PUFA deficiency. J. Lipid Res. 2019, 60, 1396–1409. [Google Scholar] [CrossRef]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Wood, J.T.; Williams, J.S.; Pandarinathan, L.; Janero, D.R.; Lammi-Keefe, C.J.; Makriyannis, A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J. Lipid Res. 2010, 51, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 11, 889–909. [Google Scholar] [CrossRef]
- Camilleri, A.; Vassallo, N. The Centrality of Mitochondria in the Pathogenesis and Treatment of Parkinson’s Disease. CNS Neurosci. Ther. 2014, 20, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guajardo, V.; Tentillier, N.; Romero-Ramos, M. The relation between α-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience 2015, 302, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared Mechanisms of Neurodegeneration in Alzheimer’s Disease and Parkinson’s Disease. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Du, Q.Y.; Chen, L.D. Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as multi-targeted compounds against Alzheimer’s disease. Eur. J. Med. Chem. 2016, 116, 200–209. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019. [Google Scholar]
- Shalini, S.-M.; Ho, C.F.-Y.; Ng, Y.-K.; Tong, J.-X.; Ong, E.-S.; Herr, D.R.; Dawe, G.S.; Ong, W.-Y. Distribution of Alox15 in the Rat Brain and Its Role in Prefrontal Cortical Resolvin D1 Formation and Spatial Working Memory. Mol. Neurobiol. 2018, 55, 1537–1550. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahex-aenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent Fatty Acids. 2018, 136, 3–13. [Google Scholar] [CrossRef]
- Ishikado, A.; Morino, K.; Nishio, Y.; Nakagawa, F.; Mukose, A.; Sono, Y.; Yoshioka, N.; Kondo, K.; Sekine, O.; Yoshizaki, T.; et al. 4-Hydroxy Hexenal Derived from Docosahexaenoic Acid Protects Endothelial Cells via Nrf2 Activation. PLoS ONE 2013, 8, e69415. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, A.I.; West, A.L.; Schoenfeld, K.; Browning, L.M.; Walker, C.G.; A Jebb, S.; Calder, P.C.; Schebb, N.H. Plasma oxylipins respond in a linear dose-response manner with increased intake of EPA and DHA: Results from a randomized controlled trial in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Van Skike, C.E.; Lin, A.L.; Roberts-Burbank, R.; Halloran, J.J.; Hernandez, S.F.; Cuvillier, J.; Soto, V.Y.; Hussong, S.A.; Jahrling, J.B.; Javors, M.A.; et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell. 2020, 19, e13057. [Google Scholar] [CrossRef]
- Tisserand, D.J.; Pruessner, J.C.; Sanz-Arigita, E.J.; van Boxtel, M.P.; Evans, A.C.; Jolles, J.; Uylings, H.B. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage 2002, 17, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Fabelo, N.; Fernández-Echevarría, C.; Canerina-Amaro, A.; Rodríguez-Barreto, D.; Quinto-Alemany, D.; Mesa-Herrera, F.; Díaz, M. Lipid Raft Alterations in Aged-Associated Neuropathologies. Curr. Alzheimer Res. 2016, 13, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Aureli, M.; Grassi, S.; Mauri, L.; Prioni, S.; Prinetti, A. Lipid Rafts in Neurodegeneration and Neuroprotection. Mol. Neurobiol. 2014, 50, 130–148. [Google Scholar] [CrossRef]
- Santos, G.; Díaz, M.; Torres, N.V. Lipid Raft Size and Lipid Mobility in Non-raft Domains Increase during Aging and Are Exac-erbated in APP/PS1 Mice Model of Alzheimer’s Disease. Predictions from an Agent-Based Mathematical Model. Front Physiol. 2016, 7, 90. [Google Scholar] [CrossRef][Green Version]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing. Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef]
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid alterations in lipid rafts from Alzheimer’s disease human brain cortex. J. Alzheimers Dis. 2010, 19, 489–502. [Google Scholar] [CrossRef] [PubMed]
- García-Ayllón, M.-S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P-tau and β-amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Feldman, H.H.; Schneider, L.S.; Wilcock, G.K.; Frisoni, G.B.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Wischik, D.J.; et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 2016, 388, 2873–2884. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Wilson, C.S.; Lipton, R.B.; Arreto, C.-D. Evolution of the Research Literature and the Scientific Community of Alzheimer’s Disease from 1983–2017: A 35-Year Survey. J. Alzheimer’s Dis. 2020, 75, 1105–1134. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Logroscino, G.; Rizzo, G.; Copetti, M.; Arcuti, S.; Martino, D.; Fontana, A. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Author Response. Neurology 2016, 87, 237–238. [Google Scholar] [CrossRef]
- Muller, M.; Tang, M.X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement. Geriatr. Cogn. Disord. 2007, 24, 185–192. [Google Scholar] [CrossRef]
- Twelves, D.; Perkins, K.S.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Disord. 2003, 18, 19–31. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017, 23, 17013. [Google Scholar] [CrossRef]
- Kim, W.S.; Kågedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Warner, T.T.; Schapira, A.H. Genetic and Environmental Factors in the Cause of Parkinson’s Disease. Ann. Neurol. 2003, 53 (Suppl. 3), S16–S23; discussion S23–S25. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Keeney, P.M.; Xie, J.; Capaldi, R.A.; Bennett, J.P., Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006, 10, 5256–5264. [Google Scholar] [CrossRef]
- Bolam, J.P.; Pissadaki, E.K. Living on the edge with too many mouths to feed: Why dopamine neurons die. Mov. Disord. 2012, 27, 1478–1483. [Google Scholar] [CrossRef]
- Pissadaki, E.K.; Bolam, J.P. The energy cost of action potential propagation in dopamine neurons: Clues to susceptibility in Parkinson’s disease. Front Comput. Neurosci. 2013, 7, 13. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Guzman, J.N.; Sanchez-Padilla, J.; Schumacker, P.T. The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson’s disease. Neuroscience 2011, 198, 221–231. [Google Scholar] [CrossRef]
- Mosharov, E.V.; Larsen, K.E.; Kanter, E.; Phillips, K.A.; Wilson, K.; Schmitz, Y.; Krantz, D.E.; Kobayashi, K.; Edwards, R.H.; Sulzer, D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009, 62, 218–229. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Rodrigues, T.M.; Castro-Caldas, A.; Ferreira, J.J. Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: Systematic review and meta-analysis. Parkinsonism Relat Disord. 2016, 27, 25–34. [Google Scholar] [CrossRef]
- Wissel, B.D.; Dwivedi, A.K.; Merola, A.; Chin, D.; Jacob, C.; Duker, A.P.; Vaughan, J.E.; Lovera, L.; la Faver, K.; Levy, A.; et al. Functional neurological disorders in Parkinson disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, 566–571. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Curr. Treat. Options Neurol. 2018, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Cole, G.M.; Ma, Q.-L.; Frautschy, S.A. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 213–221. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.O.; Basun, H.; et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef]
- Thornton, E.; Vink, R.; Blumbergs, P.C.; Heuvel, C.V.D. Soluble amyloid precursor protein α reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res. 2006, 1094, 38–46. [Google Scholar] [CrossRef]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C.-M. Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 2011, 58, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Sugioka, K.; Yamasaki, H.; Fujii, Y.; Ishibashi, Y.; Oka, J.; Shido, O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002, 81, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Yang, B.; Li, R.; Teng, T.; Ladu, M.J.; Sun, G.Y.; Greenlief, C.M.; Lee, J.C. Effects of Docosahexaenoic Acid and Its Peroxidation Product on Amyloid-β Peptide-Stimulated Microglia. Mol. Neurobiol. 2020, 57, 1085–1098. [Google Scholar] [CrossRef]
- Yuan, S.; Li, H.; Yang, C.; Xie, W.; Wang, Y.; Zhang, J.; Cai, Z.; Mao, Z.; Xie, W.; Lü, T. DHA attenuates Aβ-induced necroptosis through the RIPK1/RIPK3 signaling pathway in THP-1 monocytes. Biomed. Pharmacother. 2020, 126, 110102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hjorth, E.; Vedin, I.; Eriksdotter, M.; Freund-Levi, Y.; Wahlund, L.-O.; Cederholm, T.; Palmblad, J.; Schultzberg, M. Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: The OmegAD study. J. Lipid Res. 2015, 56, 674–681. [Google Scholar] [CrossRef]
- Vedin, I.; Cederholm, T.; Freund-Levi, Y.; Basun, H.; Hjorth, E.; Irving, G.F.; Eriksdotter-Jönhagen, M.; Schultzberg, M.; Wahlund, L.-O.; Palmblad, J. Reduced prostaglandin F2α release from blood mononuclear leukocytes after oral supplementation of ω3 fatty acids: The OmegAD study. J. Lipid Res. 2010, 51, 1179–1185. [Google Scholar] [CrossRef]
- Vedin, I.; Cederholm, T.; Levi, Y.F.; Basun, H.; Garlind, A.; Irving, G.F.; Jönhagen, M.E.; Vessby, B.; Wahlund, L.-O.; Palmblad, J. Effects of docosahexaenoic acid–rich n−3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: The OmegAD study. Am. J. Clin. Nutr. 2008, 87, 1616–1622. [Google Scholar] [CrossRef]
- Yassine, H.N.; Braskie, M.N.; Mack, W.J.; Castor, K.J.; Fonteh, A.N.; Schneider, L.S.; Harrington, M.G.; Chui, H.C. Association of Do-cosahexaenoic Acid Supplementation with Alzheimer Disease Stage in Apolipoprotein E ε4 Carriers: A Review. JAMA Neurol. 2017, 74, 339–347. [Google Scholar] [CrossRef]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Tanriover, G.; Seval-Celik, Y.; Ozsoy, O.; Akkoyunlu, G.; Savcioglu, F.; Hacioglu, G.; Demir, N.; Agar, A. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia Histochem. Cytobiol. 2010, 48, 434–441. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J. Neuroinflammat. 2016, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Oguro, A.; Ishihara, Y.; Siswanto, F.M.; Yamazaki, T.; Ishida, A.; Imaishi, H.; Imaoka, S. Contribution of DHA diols (19,20-DHDP) produced by cytochrome P450s and soluble epoxide hydrolase to the beneficial effects of DHA supplementation in the brains of rotenone-induced rat models of Parkinson’s disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158858. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J.; Cansev, M.; Sakamoto, T.; Ulus, I.H. Nutritional modifiers of aging brain function: Use of uridine and other phosphatide precursors to increase formation of brain synapses. Nutr. Rev. 2010, 68, S88–S101. [Google Scholar] [CrossRef]
- Okaichi, Y.; Ishikura, Y.; Akimoto, K.; Kawashima, H.; Toyodaono, Y.; Kiso, Y.; Okaichi, H. Arachidonic acid improves aged rats’ spatial cognition. Physiol. Behav. 2005, 84, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pooler, A.M.; Albrecht, M.A.; Wurtman, R.J. Dietary uridine-5′-monophosphate supplementation increases potassi-um-evoked dopamine release and promotes neurite outgrowth in aged rats. J. Mol. Neurosci. 2005, 27, 137–145. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Dodiya, H.B.; Broersen, L.M.; Douna, H.; van Wijk, N.; Lopes da Silva, S.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. Gut-brain and brain-gut axis in Parkinson’s disease models: Effects of a uridine and fish oil diet. Nutr. Neurosci. 2018, 21, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, G.; Seval-Celik, Y.; Tanriover, G.; Ozsoy, O.; Saka-Topcuoglu, E.; Balkan, S.; Agar, A. Docosahexaenoic acid provides pro-tective mechanism in bilaterally MPTP-lesioned rat model of Parkinson’s disease. Folia Histochem. Cytobiol. 2012, 50, 228–238. [Google Scholar] [CrossRef]
- Ozsoy, O.; Seval-Celik, Y.; Hacioglu, G.; Yargicoglu, P.; Demir, R.; Agar, A.; Aslan, M. The influence and the mechanism of do-cosahexaenoic acid on a mouse model of Parkinson’s disease. Neurochem. Int. 2011, 59, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Hosono, T.; Mouri, A.; Nishitsuji, K.; Jung, C.-G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Shibata, H.; Suzuki, T.; Nabehsima, T.; et al. Arachidonic or Docosahexaenoic Acid Diet Prevents Memory Impairment in Tg2576 Mice. J. Alzheimer’s Dis. 2015, 48, 149–162. [Google Scholar] [CrossRef]
- Schrag, A.; Quinn, N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 2000, 123, 2297–2305. [Google Scholar] [CrossRef]
- Lee, H.M.; Koh, S.-B. Many Faces of Parkinson’s Disease: Non-Motor Symptoms of Parkinson’s Disease. J. Mov. Disord. 2015, 8, 92–97. [Google Scholar] [CrossRef]
- Hosono, T.; Nishitsuji, K.; Nakamura, T.; Jung, C.G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Kiso, Y.; Suzuki, T.; Michikawa, M. Arachidonic acid diet attenuates brain Aβ deposition in Tg2576 mice. Brain Res. 2015, 1613, 92–99. [Google Scholar] [CrossRef]
- Amtul, Z.; Uhrig, M.; Wang, L.; Rozmahel, R.F.; Beyreuther, K. Detrimental effects of arachidonic acid and its metabolites in cellular and mouse models of Alzheimer’s disease: Structural insight. Neurobiol. Aging. 2012, 33, 831.e21–831.e31. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Bazan, N.G. Cyclooxygenase 2 RNA message abundance, stability, and hypervariability in sporadic alzheimer neocortex. J. Neurosci. Res. 1997, 50, 937–945. [Google Scholar] [CrossRef]
- Mohri, I.; Kadoyama, K.; Kanekiyo, T.; Sato, Y.; Kagitani-Shimono, K.; Saito, Y.; Suzuki, K.; Kudo, T.; Takeda, M.; Urade, Y.; et al. Hematopoietic Prostaglandin D Synthase and DP1 Receptor Are Selectively Upregulated in Microglia and Astrocytes Within Senile Plaques From Human Patients and in a Mouse Model of Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2007, 66, 469–480. [Google Scholar] [CrossRef]
- Thomas, M.H.; Pelleieux, S.; Vitale, N.; Olivier, J.L. Dietary arachidonic acid as a risk factor for age-associated neurodegenerative diseases: Potential mechanisms. Biochimistry 2016, 130, 168–177. [Google Scholar] [CrossRef]
- Fukaya, T.; Gondaira, T.; Kashiyae, Y.; Kotani, S.; Ishikura, Y.; Fujikawa, S.; Kiso, Y.; Sakakibara, M. Arachidonic acid preserves hip-pocampal neuron membrane fluidity in senescent rats. Neurobiol. Aging. 2007, 28, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Kotani, S.; Nakazawa, H.; Tokimasa, T.; Akimoto, K.; Kawashima, H.; Toyoda-Ono, Y.; Kiso, Y.; Okaichi, H.; Sakakibara, M. Synaptic plasticity preserved with arachidonic acid diet in aged rats. Neurosci. Res. 2003, 46, 453–461. [Google Scholar] [CrossRef]
- Clements, M.; Bliss, T.; Lynch, M. Increase in arachidonic acid concentration in a postsynaptic membrane fraction following the induction of long-term potentiation in the dentate gyrus. Neuroscience 1991, 45, 379–389. [Google Scholar] [CrossRef]
- Kotani, S.; Sakaguchi, E.; Warashina, S.; Matsukawa, N.; Ishikura, Y.; Kiso, Y.; Sakakibara, M.; Yoshimoto, T.; Guo, J.; Yamashima, T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci. Res. 2006, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mejia, R.O.; Mucke, L. Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim. Biophys. Acta. 2010, 1801, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Roseth, S.; Fykse, E.M.; Fonnum, F. The effect of arachidonic acid and free fatty acids on vesicular uptake of glutamate and gamma-aminobutyric acid. Eur. J. Pharmacol. 1998, 341, 281–288. [Google Scholar] [CrossRef]
- Carta, M.; Lanore, F.; Rebola, N.; Szabo, Z.; da Silva, S.V.; Lourenço, J.; Verraes, A.; Nadler, A.; Schultz, C.; Blanchet, C.; et al. Membrane lipids tune synaptic transmission by direct modulation of presynaptic potassium channels. Neuron 2014, 81, 787–799. [Google Scholar] [CrossRef]
- Angelova, P.R.; Müller, W.S. Arachidonic acid potently inhibits both postsynaptic-type Kv4.2 and presynaptic-type Kv1.4 IApotassium channels. Eur. J. Neurosci. 2009, 29, 1943–1950. [Google Scholar] [CrossRef]
- Lakkappa, N.; Krishnamurthy, P.T.; Hammock, B.D.; Velmurugan, D.; Bharath, M.S. Possible role of Epoxyeicosatrienoic acid in prevention of oxidative stress mediated neuroinflammation in Parkinson disorders. Med. Hypotheses 2016, 93, 161–165. [Google Scholar] [CrossRef]
- Lakkappa, N.; Krishnamurthy, P.T.; Pandareesh, M.D.; Hammock, B.D.; Hwang, S.H.; Pandareesh, M. Soluble epoxide hydrolase inhibitor, APAU, protects dopaminergic neurons against rotenone induced neurotoxicity: Implications for Parkinson’s disease. NeuroToxicology 2019, 70, 135–145. [Google Scholar] [CrossRef]
- Iljina, M.; Tosatto, L.; Choi, M.L.; Sang, J.C.; Ye, Y.; Hughes, C.D. Arachidonic acid mediates the formation of abundant al-pha-helical multimers of alpha-synuclein. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Cheng, A.; Shinoda, Y.; Yamamoto, T.; Miyachi, H.; Fukunaga, K. Development of FABP3 ligands that inhibit arachidonic ac-id-induced α-synuclein oligomerization. Brain Res. 2019, 1707, 190–197. [Google Scholar] [CrossRef]
- Julien, C.; Berthiaume, L.; Hadj-Tahar, A.; Rajput, A.H.; Bédard, P.J.; di Paolo, T. Postmortem brain fatty acid profile of levo-dopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem. Int. 2006, 48, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Qu, Y.; Chen, X.; Xu, M.-M. Relationship between high dietary fat intake and Parkinson’s disease risk: A meta-analysis. Neural. Regen. Res. 2019, 14, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).