Therapeutic Implications of Diet in Inflammatory Bowel Disease and Related Immune-Mediated Inflammatory Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Results
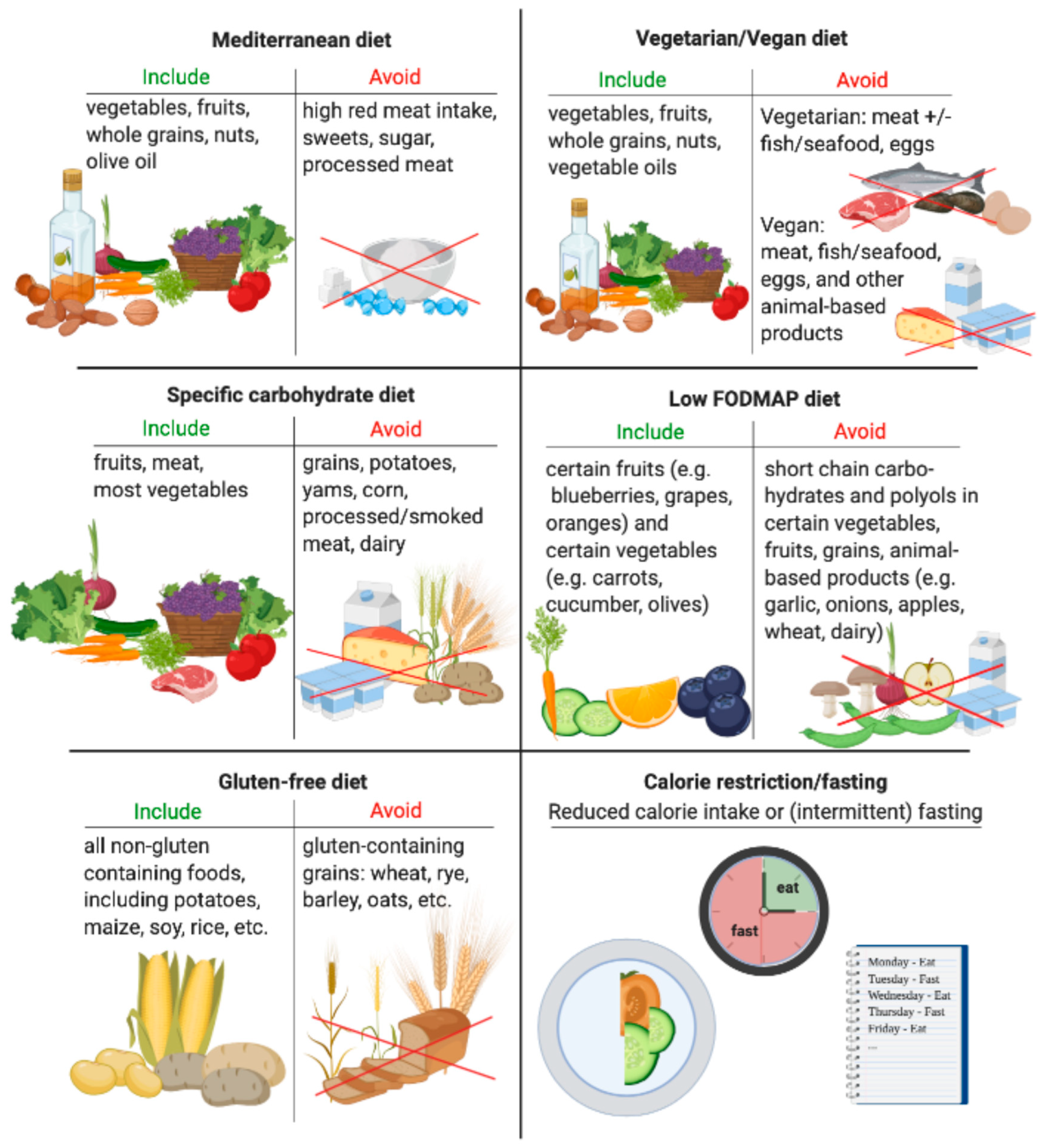
3.1. Mediterranean Diet
3.1.1. IBD
3.1.2. RA and Psoriasis
3.2. Vegetarian and Vegan Diet
3.2.1. RA and Psoriasis
3.2.2. IBD
3.3. Gluten-Free Diet
3.3.1. IBD
3.3.2. RA and Psoriasis
3.4. Calorie Restriction/Fasting
3.4.1. IBD
3.4.2. RA and Psoriasis
3.5. Specific Carbohydrate Diet
IBD
| Study | IBD Type | Design | N | Results |
|---|---|---|---|---|
| Mediterranean Diet (MD) | ||||
| Papada [30] | CD | Observational | 86 | Higher adherence with 6-month MD was associated with higher remission rates (p = 0.005). |
| Lo [31] | CD/UC | Prospective cohort study | 828 | Higher adherence with MD was associated with decreased mortality following IBD diagnosis (HR 0.69; 95% CI 0.49–0.98). |
| Marlow [32] | CD | Uncontrolled study | 8 | 6-week MD showed trend for normalization of microbiota, no effect on CRP (decrease less than 1 mg/L, p = 0.39). |
| Khalili [33] | CD/UC | Prospective cohort study | 83,147 | Higher adherence with MD was associated with a lower risk of developing CD (p = 0.03), but not UC (p = 0.61). |
| Albenberg [34] | CD | Prospective, controlled cohort study | 214 | Lower red and processed meat consumption were associated with lower relapse rates (42% vs. 62%) but no difference in time to relapse. |
| Vegetarian/Vegan Diet | ||||
| Chiba [45] | CD | Prospective controlled study | 22 | Lower relapse rate in patients on semi-vegetarian diet (1/16, 6%) vs. omnivorous diet (4/6, 67%) (p = 0.0003). |
| Jowett [44] | UC | Prospective cohort study | 191 | Higher consumption of meats (OR 3.2; 95% CI 1.3–7.8), particularly red and processed meat (OR 5.19; 95% CI 2.1–12.9), protein (OR 3.00; 95% CI 1.25–7.19), and alcohol (OR 2.71; 95% CI 1.1–6.67) increased the likelihood of relapse. |
| Amarapurkar [7] | CD/UC | Prospective case-control study | 1054 | Vegetarian diet was a protective factor for UC (OR 0.29; 95% CI 0.27–0.39) and a risk factor for CD (OR 1.179; 95% CI 0.88–1.57). |
| Gluten-Free Diet (GFD) | ||||
| Herfarth [46] | CD/UC | Cross-sectional questionnaire study | 1647 | 66% of participants report an improvement in clinical symptoms when on GFD, although the prevalence of celiac disease was only 0.6%. |
| Schreiner [47] | CD/UC | Prospective cohort study | 1254 | GFD was not associated with IBD activity, hospitalization, or surgery rates. |
| Calorie Restriction/Fasting | ||||
| Tavakkoli [52] | CD/UC | Prospective cohort study | 60 | Ramadan fasting significantly improved symptoms (CAI reduction of 1.1) in UC (p = 0.005) but not in CD. |
| Specific Carbohydrate Diet (SCD) | ||||
| Cohen [60] | CD | Prospective, uncontrolled study | 10 | Significant improvement in disease activity in pediatric CD (PCDAI reduction of 13.3, p = 0.011). |
| Obih [61] | CD/UC | Retrospective chart review | 26 | Significant improvement in disease activity (PCDAI reduction 11.4 at 6 months, p = 0.03), in CRP (−0.9 mg/dL, p = 0.03) and calprotectin (−181 mcg/g, p = 0.03) in pediatric CD compared to control. No significant improvements in pediatric UC. |
| Kakodkar [62] | CD/UC | Case series | 50 | Patients in disease remission report the SCD to be effective in controlling acute flare symptoms (mean = 91.3%, range = 30% to 100%) and at maintaining remission (mean = 92.1%, range = 53% to 100%). |
| Suskind [63] | CD/UC | Survey study | 417 | 42% of patients report achieving remission at 6 and 12 months while on the diet. 47% of patients report improvement in abnormal lab values. |
| Low FODMAP Diet (LFD) | ||||
| Gearry [64] | CD/UC | Retrospective study | 72 | Improved symptoms after 3 months of LFD. |
| Prince [65] | CD/UC | Prospective study | 88 | Significant improvement in functional-like gastrointestinal symptoms compared to baseline (78% vs. 16% at baseline reporting satisfactory relief, p < 0.001). |
| Pedersen [66] | CD/UC | Controlled open-label study | 89 | LFD improved IBS symptoms (55 points lower IBS-SSS, p = 0.02) and health-related quality of life (SIBDQ 10 points higher, p < 0.01) compared to normal diet in IBD in remission. |
| Cox [67] | CD/UC | Single-blind study | 52 | LFD improved gut symptoms compared to control (52% reporting adequate relief on LFD vs. 16% on control, p = 0.007). |
| Cox [68] | CD/UC | Double-blinded, controlled, re-challenge study | 32 | Fructose challenge brought less relief of functional-like gastrointestinal symptoms compared with glucose (62.1% reported relief in the fructan group vs. 89.7 in glucose, p = 0.033). |
3.6. Low FODMAP
IBD
3.7. Other Diets Studied in IBD (e.g., Paleo, Atkins, etc.)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shivashankar, R.; Tremaine, W.J.; Harmsen, W.S.; Loftus, E.V. Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota from 1970 through 2010. Clin. Gastroenterol. Hepatol. 2017, 15, 857–863. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef] [PubMed]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A Prospective Study of Long-term Intake of Dietary Fiber and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; De Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2013, 63, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Nimptsch, K.; Wu, K.; Chan, A.T. High School Diet and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 2311–2319. [Google Scholar]
- Amarapurkar, A.D.; Amarapurkar, D.N.; Rathi, P.; Sawant, P.; Patel, N.; Kamani, P.; Rawal, K.; Baijal, R.; Sonawane, A.; Narawane, N.; et al. Risk factors for inflammatory bowel disease: A prospective multi-center study. Indian J. Gastroenterol. 2018, 37, 189–195. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Amamou, A.; Savoye, G.; Ghosh, S. Inflammatory Bowel Diseases and Food Additives: To Add Fuel on the Flames! Nutrients 2019, 11, 1111. [Google Scholar] [CrossRef]
- Jadhav, P.; Jiang, Y.; Jarr, K.; Layton, C.; Ashouri, J.F.; Sinha, S.R. Efficacy of Dietary Supplements in Inflammatory Bowel Disease and Related Autoimmune Diseases. Nutrients 2020, 12, 2156. [Google Scholar] [CrossRef] [PubMed]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; Macdonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef]
- Gu, P.; Feagins, L.A. Dining With Inflammatory Bowel Disease: A Review of the Literature on Diet in the Pathogenesis and Management of IBD. Inflamm. Bowel Dis. 2019, 26, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet Alone Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Grover, Z.; Muir, R.; Lewindon, P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J. Gastroenterol. 2014, 49, 638–645. [Google Scholar] [CrossRef]
- Tsertsvadze, A.; Gurung, T.; Court, R.; Clarke, A.; Sutcliffe, P. Clinical effectiveness and cost-effectiveness of elemental nutrition for the maintenance of remission in Crohn’s disease: A systematic review and meta-analysis. Health Technol. Assess. 2015, 19, 1–138. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S. Effects of an Intermittent Reduced Calorie Diet on Crohn’s Disease. ID NCT04147585. Available online: https://clinicaltrials.gov/ct2/show/NCT04147585 (accessed on 30 November 2020).
- Sinha, S. The Influence of a Fasting Mimicking Diet on Ulcerative Colitis. ID NCT03615690. Available online: https://clinicaltrials.gov/ct2/show/NCT03615690 (accessed on 30 November 2020).
- Lewis, J.D. Trial of Specific Carbohydrate and Mediterranean Diets to Induce Remission of Crohn’s Disease (DINE-CD). ID NCT03058679. Available online: https://clinicaltrials.gov/ct2/show/NCT03058679 (accessed on 30 November 2020).
- Abreu, M. Diet Intervention for Crohn’s Disease Patient. ID NCT04213729. Available online: https://clinicaltrials.gov/ct2/show/NCT04213729 (accessed on 30 November 2020).
- Levine, A.; Rhodes, J.M.; Lindsay, J.O.; Abreu, M.T.; Kamm, M.A.; Gibson, P.R.; Gasche, C.; Silverberg, M.S.; Mahadevan, U.; Boneh, R.S.; et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1381–1392. [Google Scholar] [CrossRef]
- Damas, O.M.; Garces, L.; Abreu, M.T. Diet as Adjunctive Treatment for Inflammatory Bowel Disease: Review and Update of the Latest Literature. Curr. Treat. Options Gastroenterol. 2019, 17, 313–325. [Google Scholar] [CrossRef]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Casteele, N.V. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; McInnes, I.B.; Siebert, S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology 2019, 58, i43–i54. [Google Scholar] [CrossRef]
- Danese, S.; Semeraro, S.; Papa, A.; Roberto, I.; Scaldaferri, F.; Fedeli, G.; Gasbarrini, G.; Gasbarrini, A. Extraintestinal manifestations in inflammatory bowel disease. World J. Gastroenterol. 2005, 11, 7227–7236. [Google Scholar] [CrossRef] [PubMed]
- Cottone, M.; Sapienza, C.; Macaluso, F.S.; Cannizzaro, M. Psoriasis and Inflammatory Bowel Disease. Dig. Dis. 2019, 37, 451–457. [Google Scholar] [CrossRef]
- Bassukas, I.D.; Gaitanis, G.; Katsanos, K.H.; Christodoulou, D.K.; Tsianos, E.; Vlachos, C. Psoriasis and inflammatory bowel disease: Links and risks. Psoriasis Targets Ther. 2016, 6, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Sánchez-Villegas, A. The emerging role of Mediterranean diets in cardiovascular epidemiology: Monounsaturated fats, olive oil, red wine or the whole pattern? Eur. J. Epidemiol. 2003, 19, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Kuchkuntla, A.R.; Limketkai, B.; Nanda, S.; Hurt, R.T.; Mundi, M.S. Fad Diets: Hype or Hope? Curr. Nutr. Rep. 2018, 7, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Papada, E.; Amerikanou, C.; Forbes, A.; Kaliora, A.C. Adherence to Mediterranean diet in Crohn’s disease. Eur. J. Nutr. 2020, 59, 1115–1121. [Google Scholar] [CrossRef]
- Lo, C.-H.; Khalili, H.; Song, M.; Lochhead, P.; Burke, K.E.; Richter, J.M.; Giovannucci, E.L.; Chan, A.T.; Ananthakrishnan, A.N. Healthy Lifestyle Is Associated With Reduced Mortality in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 87–95.e4. [Google Scholar] [CrossRef]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A Diet Low in Red and Processed Meat Does Not Reduce Rate of Crohn’s Disease Flares. Gastroenterology 2019, 157, 128–136.e5. [Google Scholar] [CrossRef]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.A.; Capell, H.A. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef]
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D.; on behalf of the EIRA Study Group. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. Arthritis Res. Ther. 2018, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.; Touvier, M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Wolkenstein, P.; Chosidow, O.; Ezzedine, K.; Sbidian, E. Association Between Mediterranean Anti-inflammatory Dietary Profile and Severity of Psoriasis: Results From the NutriNet-Sante Cohort. JAMA Dermatol. 2018, 154, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; Di Somma, C.; Macchia, P.E.; Napolitano, M.; Savanelli, M.C.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Transl. Med. 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Borchgrevink, C.; Laerum, E.; Haugen, M.; Eek, M.; Førre, O.; Mowinkel, P.; Hovi, K. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Førre, Ø. Vegetarian diet for patients with rheumatoid arthritis—Status: Two years after introduction of the diet. Clin. Rheumatol. 1994, 13, 475–482. [Google Scholar] [CrossRef]
- McDougall, J.; Bruce, B.; Spiller, G.; Westerdahl, J.; McDougall, M. Effects of a Very Low-Fat, Vegan Diet in Subjects with Rheumatoid Arthritis. J. Altern. Complement. Med. 2002, 8, 71–75. [Google Scholar] [CrossRef]
- Hafstrom, I.; Ringertz, B.; Spångberg, A.; Von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef]
- Afifi, L.; Danesh, M.J.; Lee, K.M.; Beroukhim, K.; Farahnik, B.; Ahn, R.S.; Yan, D.; Singh, R.K.; Nakamura, M.; Koo, J.; et al. Dietary Behaviors in Psoriasis: Patient-Reported Outcomes from a U.S. National Survey. Dermatol. Ther. 2017, 7, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef]
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a Gluten-free Diet and Improvement of Clinical Symptoms in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef]
- Schreiner, P.; Yilmaz, B.; Rossel, J.-B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, G.; Gerdén, B.; Hagforsen, E.; Nilsson, B.; Pihl-Lundin, I.; Kraaz, W.; Hjelmquist, G.; Lööf, L. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br. J. Dermatol. 2000, 142, 44–51. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, R.; Gabrielli, M.; Lora, L.; Napoli, L.; Tosetti, C.; Pirrotta, E.; Ubaldi, E.; Bertolusso, L.; Zamparella, M.; De Polo, M.; et al. Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study. Dermatology 2015, 230, 156–160. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Haghdani, S.; Emami, M.H.; Adilipour, H.; Tavakkoli, M.; Tavakkoli, M. Ramadan fasting and inflammatory bowel disease. Indian J. Gastroenterol. 2009, 27, 239–241. [Google Scholar]
- Davidovici, B.B.; Sattar, N.; Jörg, P.C.; Puig, L.; Emery, P.; Barker, J.N.; Van De Kerkhof, P.; Ståhle, M.; Nestle, F.O.; Girolomoni, G.; et al. Psoriasis and Systemic Inflammatory Diseases: Potential Mechanistic Links between Skin Disease and Co-Morbid Conditions. J. Investig. Dermatol. 2010, 130, 1785–1796. [Google Scholar] [CrossRef]
- Jensen, P.; Zachariae, C.; Christensen, R.; Geiker, N.R.; Schaadt, B.K.; Stender, S.; Hansen, P.R.; Astrup, A.; Skov, L. Effect of weight loss on the severity of psoriasis: A randomized clinical study. JAMA Dermatol. 2013, 149, 795–801. [Google Scholar] [CrossRef]
- Jensen, P.; Christensen, R.; Zachariae, C.; Geiker, N.R.; Schaadt, B.K.; Stender, S.; Hansen, P.R.; Astrup, A.; Skov, L. Long-term effects of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: A prospective observational follow-up study. Am. J. Clin. Nutr. 2016, 104, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, E.; Bilberg, A.; Björkman, S.; Hedberg, M.; Jacobsson, L.; Forsblad-D’Elia, H.; Carlsten, H.; Eliasson, B.; Larsson, I. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: An interventional study. Arthritis Res. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Abendroth, A.; Michalsen, A.; Lüdtke, R.; Rüffer, A.; Musial, F.; Dobos, G.J.; Langhorst, J. Changes of Intestinal Microflora in Patients with Rheumatoid Arthritis during Fasting or a Mediterranean Diet. Forsch. Komplement. Res. Complement. Med. 2010, 17, 307–313. [Google Scholar] [CrossRef]
- Michalsen, A.; Riegert, M.; Lüdtke, R.; Bäcker, M.; Langhorst, J.; Schwickert, M.; Dobos, G.J. Mediterranean diet or extended fasting’s influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: An observational study. BMC Complement. Altern. Med. 2005, 5, 22. [Google Scholar] [CrossRef]
- Michalsen, A. Effectiveness of Therapeutic Fasting and Specific Diet in Patients with Rheumatoid Arthritis (NutriFast). ID NCT03856190. Available online: https://clinicaltrials.gov/ct2/show/NCT03856190 (accessed on 30 November 2020).
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and Mucosal Improvement With Specific Carbohydrate Diet in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef]
- Kakodkar, S.; Farooqui, A.J.; Mikolaitis, S.L.; Mutlu, E.A. The Specific Carbohydrate Diet for Inflammatory Bowel Disease: A Case Series. J. Acad. Nutr. Diet. 2015, 115, 1226–1232. [Google Scholar] [CrossRef]
- Suskind, D.L.; Wahbeh, G.; Cohen, S.A.; Damman, C.J.; Klein, J.; Braly, K.; Shaffer, M.; Lee, D. Patients Perceive Clinical Benefit with the Specific Carbohydrate Diet for Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 3255–3260. [Google Scholar] [CrossRef]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—a pilot study. J. Crohns Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef]
- Prince, A.C.; Myers, C.E.; Joyce, T.; Irving, P.; Lomer, M.; Whelan, K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1129–1136. [Google Scholar] [CrossRef]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable Carbohydrates [FODMAPs] Exacerbate Functional Gastrointestinal Symptoms in Patients With Inflammatory Bowel Disease: A Randomised, Double-blind, Placebo-controlled, Cross-over, Re-challenge Trial. J. Crohns Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E. Study design considerations for irritable bowel syndrome clinical trials. Ann. Gastroenterol. 2014, 27, 338–345. [Google Scholar] [PubMed]
- Konijeti, G.G.; Kim, N.; Lewis, J.D.; Groven, S.; Chandrasekaran, A.; Grandhe, S.; Diamant, C.; Singh, E.; Oliveira, G.; Wang, X.; et al. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2054–2060. [Google Scholar] [CrossRef]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr. J. 2014, 13, 5. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nat. Cell Biol. 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Robinson, J.L.; Elias, J.E.; et al. Gut Microbiota-Targeted Diets Modulate Human Immune Status. bioRxiv 2020. [Google Scholar] [CrossRef]
- Madsen, K.L.; Malfair, D.; Gray, D.; Doyle, J.S.; Jewell, L.D.; Fedorak, R.N. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 1999, 5, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Taurog, J.D.; Richardson, J.A.; Croft, J.T.; Simmons, W.A.; Zhou, M.; Fernández-Sueiro, J.L.; Balish, E.; Hammer, R.E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 1994, 180, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi-Roodsaz, S.; Joosten, L.A.; Koenders, M.I.; Devesa, I.; Roelofs, M.F.; Radstake, T.R.; Heuvelmans-Jacobs, M.; Akira, S.; Nicklin, M.J.; Ribeiro-Dias, F.; et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Investig. 2008, 118, 205–216. [Google Scholar] [CrossRef]
- Wu, H.-J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-Residing Segmented Filamentous Bacteria Drive Autoimmune Arthritis via T Helper 17 Cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Sakaguchi, N.; Kobayashi, K.; Brown, G.D.; Tagami, T.; Sakihama, T.; Hirota, K.; Tanaka, S.; Nomura, T.; Miki, I.; et al. A role for fungal {beta}-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 2005, 201, 949–960. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Peeters, M.; Hiele, M.; Vantrappen, G.; Pennincx, F.; Aerts, R.; Kerremans, R.; Goboes, K. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 1991, 338, 771–774. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 1–25. [Google Scholar] [CrossRef]
- Sinha, S.R.; Nguyen, L.P.; Tropini, C.; Haileselassie, Y.; Becker, L.S.; Sim, D.; Bittinger, K.; Sonnenburg, J.L.; Habtezion, A. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. SSRN Electron. J. 2018, 27, 659–670.e5. [Google Scholar] [CrossRef]
- Laing, B.B.; Lim, A.G.; Ferguson, L.R. A Personalised Dietary Approach-A Way Forward to Manage Nutrient Deficiency, Effects of the Western Diet, and Food Intolerances in Inflammatory Bowel Disease. Nutrients 2019, 11, 1532. [Google Scholar] [CrossRef]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A Metabolism: An Update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef]
- Bailey, R.; Cooper, J.D.; Zeitels, L.; Smyth, D.J.; Yang, J.H.; Walker, N.M.; Hyppönen, E.; Dunger, D.B.; Ramos-Lopez, E.; Badenhoop, K.; et al. Association of the Vitamin D Metabolism Gene CYP27B1 With Type 1 Diabetes. Diabetes 2007, 56, 2616–2621. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef]
| Study | Disease | Design | N | Results |
|---|---|---|---|---|
| McKellar [35] | RA | Prospective | 130 | 6-week intervention to MD focused diet showed improvement in patient global assessment (p = 0.002), pain scores (p = 0.049) and morning stiffness (p = 0.041) at 6 months when compared to control. |
| Johansson [36] | RA | Population case control | 5388 | Higher adherence to MD was associated with decreased odds in developing seropositive RA (OR 0.79; 95% CI 0.65–0.96). |
| Phan [37] | Ps * | Population survey | 35,735 | Higher adherence to MD was associated with lower psoriasis disease activity (OR 0.71; 95% CI 0.55–0.92). |
| Barrea [38] | Ps | Case control | 124 | Psoriasis severity scores associated with adherence to MD, r = −0.6 (p < 0.001). |
| Vegetarian/Vegan: Positive Outcomes in RA Studies Suggest Possible Benefits in IBD, Where Data on This Diet Have Been Quite Limited. | ||||
| Study | Disease | Design | N | Results |
| Kjeldsen-Kragh [39,40] | RA | Randomized trial | 53 | Improvement in ESR (−4 mm/h)/CRP (−6 mg/L) (p < 0.002 and p < 0.005) seen in intervention group. However, significant dropout in study (~60% completed). |
| McDougall [41] | RA | Single-arm intervention | 24 | Improvement in RA pain scores (p < 0.004), swollen joints (p < 0.02) after switch to vegan low fat diet. |
| Hafstrom [42] | RA | Randomized trial | 66 | Higher prevalence of fulfilling ACR improvement criteria in those in the vegan diet free of gluten group (40% vs. 4%). 60% intervention group completed the 9-month follow-up. |
| Afifi [43] | Ps | Survey | 1206 | Self-reported improvement in skin symptoms in 70% of those on a vegan diet. |
| Gluten-free +: Benefit of this diet seen in only a subset of psoriasis patients with gliadin antibodies, which makes it difficult to extrapolate to patients with IBD. | ||||
| Study | Disease | Design | N | Results |
| Michaelsson [48] | Ps | Single-arm intervention | 39 | Gluten-free diet led to an improvement in PASI (5.5 before vs. 3.6 after) in those with gliadin antibodies (p = 0.001). |
| Study | Disease | Design | N | Results |
|---|---|---|---|---|
| Jensen [54] | Ps | Randomized trial | 60 | Caloric restriction group showed significant weight loss (−15.4 kg) (p < 0.001) and reduction in PASI (−2.0) (p = 0.06) compared to regular diet group. |
| Jensen [55] | Ps | Prospective observational | 38 | Long-term (>1 year) benefits in both weight loss and PASI for those who underwent a 16-week caloric reduction (PASI reduction mean −2.9; 95% CU −3.9, −1.9). |
| Klingberg [56] | PsA * | Single-arm intervention | 46 | Treatment with caloric restriction led to weight loss and significant improvement in multiple symptoms (e.g., VAS pain p = 0.004, swollen joints score p = 0.021), CRP (−2.0 mg/L) in those with psoriatic arthritis (p = 0.041). |
| Abendroth [57] | RA | Non-randomized Observational | 50 | Of the 22 who participated in fasting, there were decreased disease activity scores (−1.6) when compared to baseline before dietary intervention at day 13 (p < 0.001). |
| Michalsen [58] | RA | Non-randomized Observational | 51 | Of the nine patients who fasted, there was a significant improvement in disease activity at 2 weeks compared to baseline (p = 0.007). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Jarr, K.; Layton, C.; Gardner, C.D.; Ashouri, J.F.; Abreu, M.T.; Sinha, S.R. Therapeutic Implications of Diet in Inflammatory Bowel Disease and Related Immune-Mediated Inflammatory Diseases. Nutrients 2021, 13, 890. https://doi.org/10.3390/nu13030890
Jiang Y, Jarr K, Layton C, Gardner CD, Ashouri JF, Abreu MT, Sinha SR. Therapeutic Implications of Diet in Inflammatory Bowel Disease and Related Immune-Mediated Inflammatory Diseases. Nutrients. 2021; 13(3):890. https://doi.org/10.3390/nu13030890
Chicago/Turabian StyleJiang, Yan, Karolin Jarr, Cosima Layton, Christopher D. Gardner, Judith F. Ashouri, Maria T. Abreu, and Sidhartha R. Sinha. 2021. "Therapeutic Implications of Diet in Inflammatory Bowel Disease and Related Immune-Mediated Inflammatory Diseases" Nutrients 13, no. 3: 890. https://doi.org/10.3390/nu13030890
APA StyleJiang, Y., Jarr, K., Layton, C., Gardner, C. D., Ashouri, J. F., Abreu, M. T., & Sinha, S. R. (2021). Therapeutic Implications of Diet in Inflammatory Bowel Disease and Related Immune-Mediated Inflammatory Diseases. Nutrients, 13(3), 890. https://doi.org/10.3390/nu13030890






