Low Energy Availability with and without a High-Protein Diet Suppresses Bone Formation and Increases Bone Resorption in Men: A Randomized Controlled Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Preliminary Testing
2.4. Diet Preparation
2.5. Supplementation
2.6. Daily Exercise Prescription
2.7. Measurements and Assessments
2.8. Statistical Analyses
3. Results
3.1. Participant Characteristics and Compliance
3.2. Body Weight and Composition
3.3. Leptin, IGF-1 and IGFR
3.4. Markers of Bone Turnover
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Areta, J.L.; Taylor, H.L.; Koehler, K. Low energy availability: History, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur. J. Appl. Physiol. 2020, 1–21. [Google Scholar] [CrossRef]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Ihle, R.; Loucks, A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Miner. Res. 2004, 19, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Williams, N.I. Beyond hypoestrogenism in amenorrheic athletes: Energy deficiency as a contributing factor for bone loss. Curr. Sports Med. Rep. 2005, 4, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Kiechl, S.; Bonora, E.; Redlich, K.; Woloszczuk, W.; Oberhollenzer, F.; Jocher, J.; Dorizzi, R.; Muggeo, M.; Smolen, J.; et al. Serum leptin level and the risk of nontraumatic fracture. Am. J. Med. 2004, 117, 952–956. [Google Scholar] [CrossRef]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Donnell, S.M.; Corish, C.A. Low Energy Availability in Athletes 2020: An Updated Narrative Review of Prevalence, Risk, Within-Day Energy Balance, Knowledge, and Impact on Sports Performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simas, V.; Hing, W.; Pope, R.; Climstein, M. Effects of water-based exercise on bone health of middle-aged and older adults: A systematic review and meta-analysis. Open Access J. Sports Med. 2017, 8, 39–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, A.R.; Hackney, A.C.; Smith-Ryan, A.; Kucera, K.; Registar-Mihalik, J.; Ondrak, K. Prevalence of Low Energy Availability in Competitively Trained Male Endurance Athletes. Medicina 2019, 55, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viner, R.T.; Harris, M.; Berning, J.R.; Meyer, N.L. Energy Availability and Dietary Patterns of Adult Male and Female Competitive Cyclists with Lower Than Expected Bone Mineral Density. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 594–602. [Google Scholar] [CrossRef]
- Hector, A.J.; Phillips, S.M. Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Sutter, T.; Toumi, H.; Valery, A.; El Hage, R.; Pinti, A.; Lespessailles, E. Relationships between muscle mass, strength and regional bone mineral density in young men. PLoS ONE 2019, 14, e0213681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, A.A.; Houston, D.K.; Shapses, S.A.; Lyles, M.F.; Henderson, R.M.; Beavers, D.P.; Baker, A.C.; Beavers, K.M. Effect of a hypocaloric, nutritionally complete, higher-protein meal plan on bone density and quality in older adults with obesity: A randomized trial. Am. J. Clin. Nutr. 2019, 109, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzoli, R.; Bianchi, M.L.; Garabédian, M.; McKay, H.A.; Moreno, L.A. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 2010, 46, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Mantzoros, C. Bone metabolism in anorexia nervosa and hypothalamic amenorrhea. Metabolism 2018, 80, 91–104. [Google Scholar] [CrossRef]
- Smith, W.J.; Underwood, L.E.; Clemmons, D.R. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J. Clin. Endocrinol. Metab. 1995, 80, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Zanker, C.L.; Swaine, I.L. Responses of bone turnover markers to repeated endurance running in humans under conditions of energy balance or energy restriction. Eur. J. Appl. Physiol. 2000, 83, 434–440. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Elliott-Sale, K.J.; Parsons, A.; Tang, J.C.Y.; Greeves, J.P.; Fraser, W.D.; Sale, C. Effects of reduced energy availability on bone metabolism in women and men. Bone 2017, 105, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, N.R.; Di Marco, N.M.; Langley, S.; American Dietetics Association; Dietitians of Canada; American College of Sports Medicine. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, K.; Hoerner, N.R.; Gibbs, J.C.; Zinner, C.; Braun, H.; De Souza, M.J.; Schaenzer, W. Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. J. Sports Sci. 2016, 34, 1921–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, G.B. Body fat content influences the body composition response to nutrition and exercise. Ann. N. Y. Acad. Sci. 2000, 904, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S.; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.J.; Martin, B.R.; Boushey, C.J. Development and evaluation of a brief calcium assessment tool for adolescents. J. Am. Diet. Assoc. 2010, 110, 111–115. [Google Scholar] [CrossRef]
- Naspi, A.; Panasiti, V.; Abbate, F.; Roberti, V.; Devirgiliis, V.; Curzio, M.; Borghi, M.; Lozupone, F.; Carotti, S.; Morini, S.; et al. Insulin-like-growth-factor-binding-protein-3 (IGFBP-3) contrasts melanoma progression in vitro and in vivo. PLoS ONE 2014, 9, e98641. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of Leptin on the Skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef] [PubMed]
- Philippou, A.; Barton, E.R. Optimizing IGF-I for skeletal muscle therapeutics. Growth Horm. IGF Res. 2014, 24, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Veldhuis, J.D.; Frystyk, J.; Iranmanesh, A.; Orskov, H. Testosterone and estradiol regulate free insulin-like growth factor I (IGF-I), IGF binding protein 1 (IGFBP-1), and dimeric IGF-I/IGFBP-1 concentrations. J. Clin. Endocrinol. Metab. 2005, 90, 2941–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.; Koehler, K. Caloric restriction induces anabolic resistance to resistance exercise. Eur. J. Appl. Physiol. 2020, 120, 1155–1164. [Google Scholar] [CrossRef]
- Wang, S.; Mu, R.; Zhang, X.; Yun, K.; Shang, H.; Zhao, M. Biological variation in serum bone turnover markers. Ann. Clin. Biochem. 2020, 57, 144–150. [Google Scholar] [CrossRef]
- Sukumar, D.; Ambia-Sobhan, H.; Zurfluh, R.; Schlussel, Y.; Stahl, T.J.; Gordon, C.L.; Shapses, S.A. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: A randomized, controlled trial. J. Bone Miner. Res. 2011, 26, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesudason, D.; Nordin, B.C.; Keogh, J.; Clifton, P. Comparison of 2 weight-loss diets of different protein content on bone health: A randomized trial. Am. J. Clin. Nutr. 2013, 98, 1343–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouvelioti, R.; Kurgan, N.; Falk, B.; Ward, W.E.; Josse, A.R.; Klentrou, P. Response of Sclerostin and Bone Turnover Markers to High Intensity Interval Exercise in Young Women: Does Impact Matter? Biomed Res. Int. 2018, 2018, 4864952. [Google Scholar] [CrossRef] [PubMed]
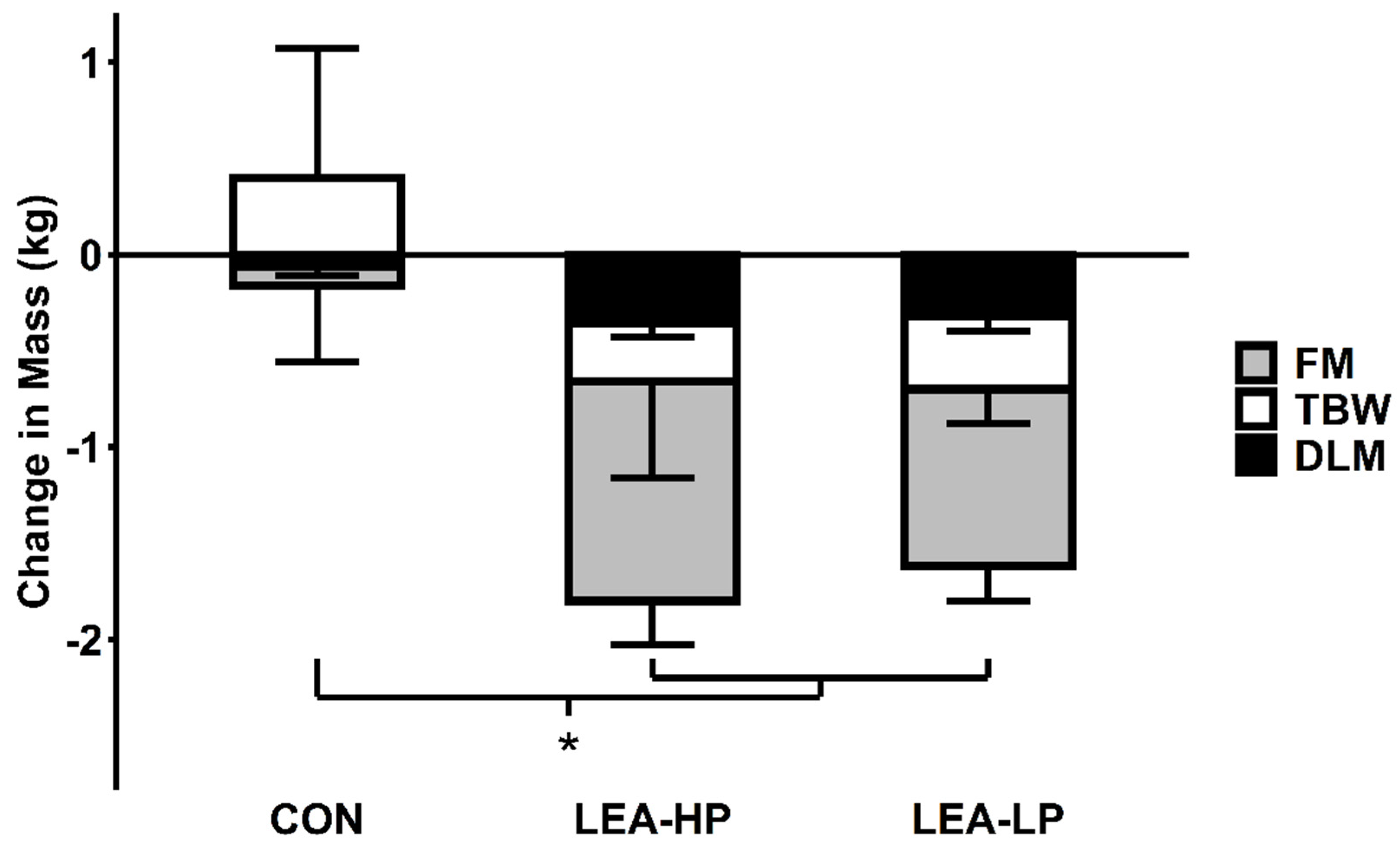
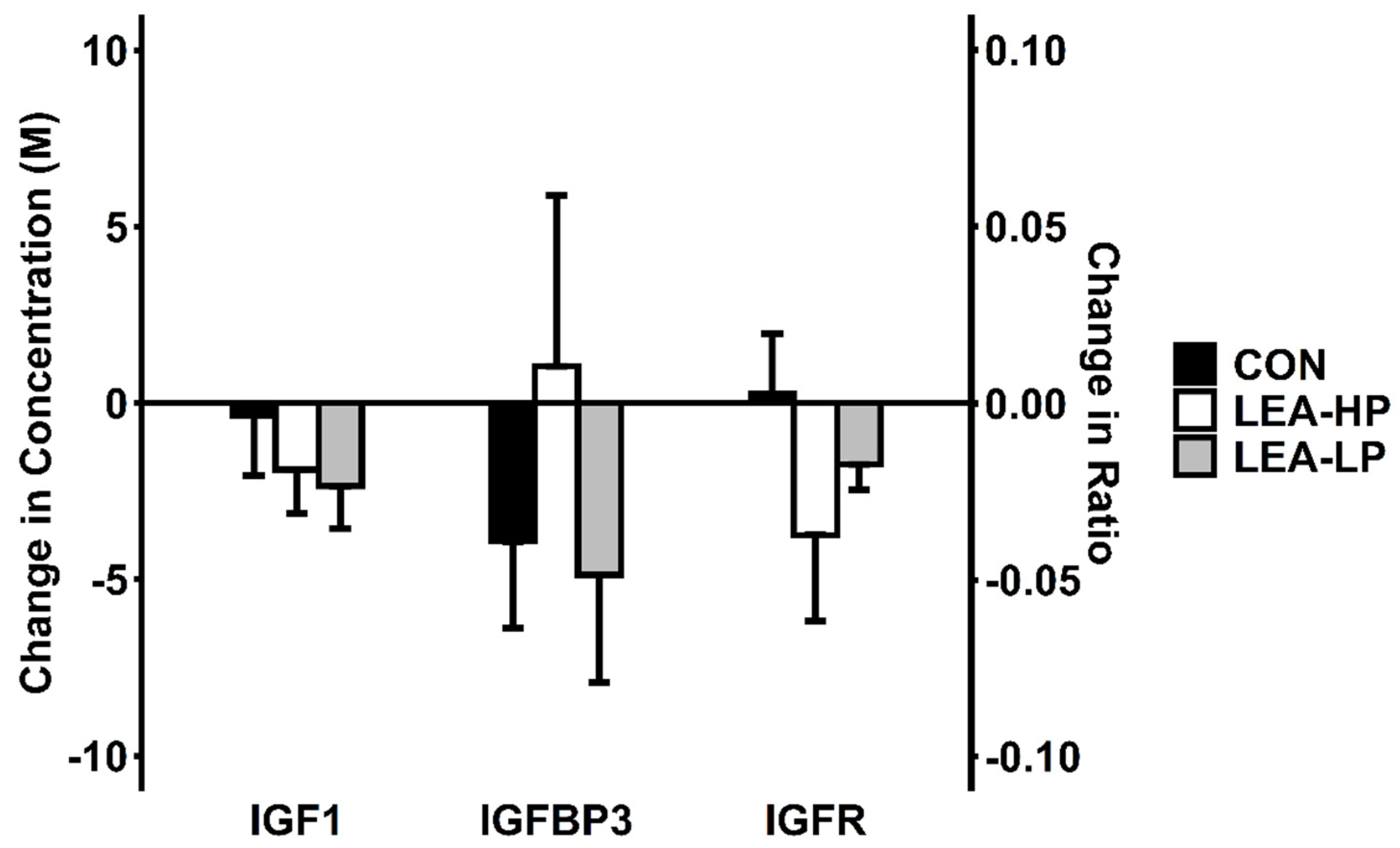
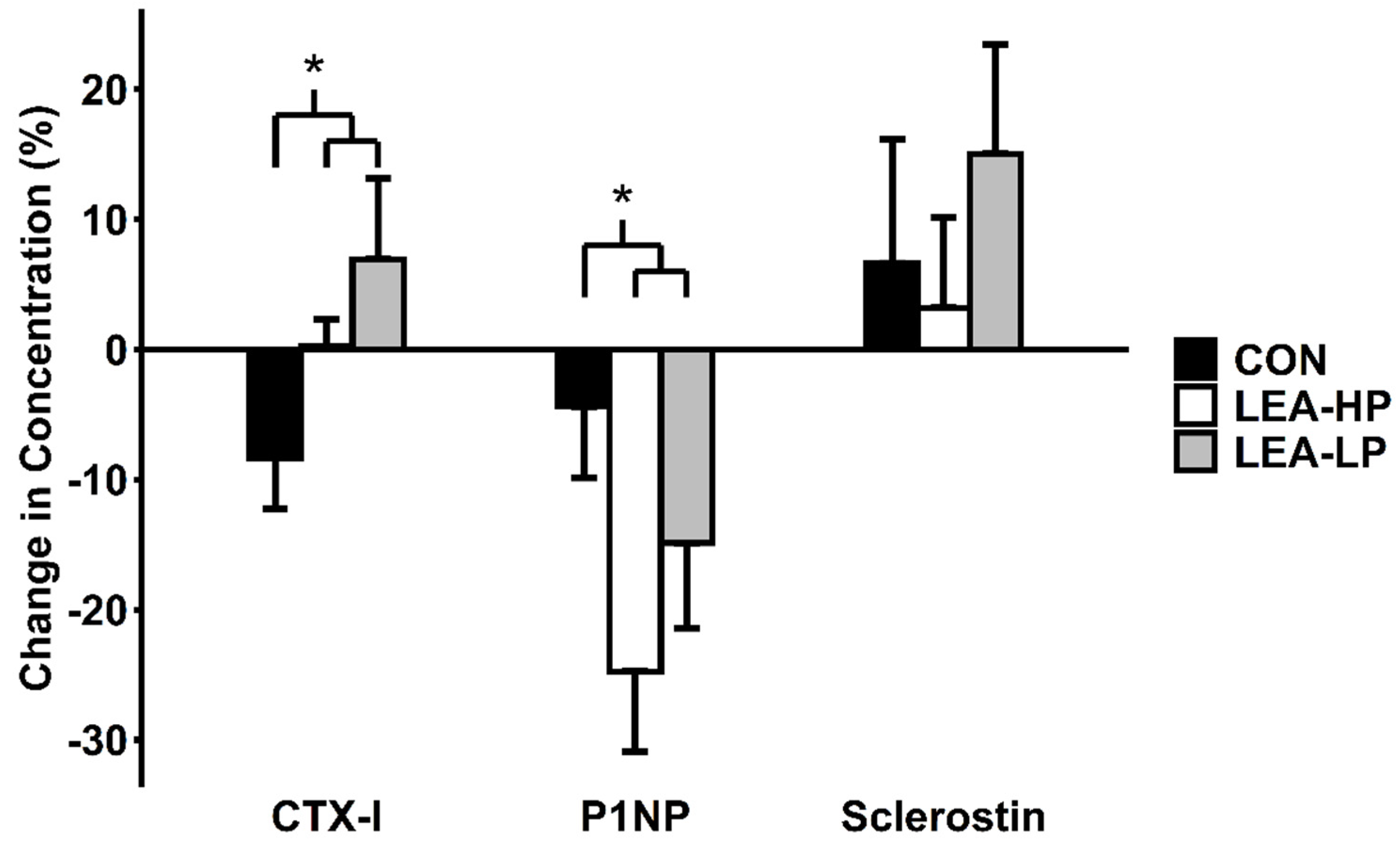
| Condition | LEA-LP | LEA-HP | CON |
|---|---|---|---|
| Energy Intake 1 | 30 | 30 | 55 |
| Exercise Energy Expenditure 1 | 15 | 15 | 15 |
| Energy Availability 1 | 15 | 15 | 40 |
| Protein Intake 2 | 0.8 | 1.7 | 1.7 |
| Hormone | Condition | Pre | Post | % Change | LEA vs. CON p Value |
|---|---|---|---|---|---|
| P1NP (μg/L) | LEA-LP | 90.9 ± 11.7 | 77.9 ± 12.2 | −14.9 ± 6.5% | 0.04 |
| LEA-HP | 85.4 ± 11.8 | 61.7 ± 7.6 | −24.8 ± 6.2% | ||
| CON | 85.0 ± 8.2 | 80.8 ± 8.7 | −4.4 ± 5.5% | ||
| CTX-I (ng/mL) | LEA-LP | 1.30 ± 0.16 | 1.39 ± 0.19 | 6.9 ± 6.2% | 0.04 |
| LEA-HP | 1.22 ± 0.10 | 1.23 ± 0.10 | 0.2 ± 2.1% | ||
| CON | 1.47 ± 0.19 | 1.32 ± 0.14 | −8.3 ± 3.9% | ||
| Sclerostin (pmol/L) | LEA-LP | 31.4 ± 2.9 | 36.4 ± 4.7 | 15.0 ± 8.4% | 0.81 |
| LEA-HP | 30.4 ± 3.6 | 30.8 ± 3.5 | 3.2 ± 6.9% | ||
| CON | 28.6 ± 5.4 | 30.3 ± 5.6 | 6.6 ± 9.5% | ||
| Leptin (μg/L) | LEA-LP | 3.28 ± 1.77 | 1.44 ± 0.89 | −65.5 ± 4.4% | 0.02 |
| LEA-HP | 2.50 ± 1.21 | 1.23 ± 0.75 | −54.3 ± 16.7% | ||
| CON | 3.03 ± 1.24 | 2.57 ± 1.51 | −25.4 ± 11.4% | ||
| IGF-1 (ng/mL) | LEA-LP | 228 ± 30 | 200 ± 27 | −11.8 ± 5.4% | 0.14 |
| LEA-HP | 202 ± 29 | 180 ± 21 | −8.1 ± 7.3% | ||
| CON | 225 ± 33 | 221 ± 20 | 2.9 ± 9.4% | ||
| IGFBP-3 (ng/mL) | LEA-LP | 2418 ± 131 | 2281 ± 111 | −5.2 ± 3.6% | 0.61 |
| LEA-HP | 2282 ± 186 | 2311 ± 80 | 4.1 ± 7.3% | ||
| CON | 2612 ± 132 | 2502 ± 117 | −3.9 ± 2.7% | ||
| IGFR (no units) | LEA-LP | 0.34 ± 0.05 | 0.32 ± 0.04 | - | 0.15 |
| LEA-HP | 0.34 ± 0.07 | 0.28 ± 0.04 | - | ||
| CON | 0.32 ± 0.06 | 0.32 ± 0.04 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, C.; Bilek, L.D.; Koehler, K. Low Energy Availability with and without a High-Protein Diet Suppresses Bone Formation and Increases Bone Resorption in Men: A Randomized Controlled Pilot Study. Nutrients 2021, 13, 802. https://doi.org/10.3390/nu13030802
Murphy C, Bilek LD, Koehler K. Low Energy Availability with and without a High-Protein Diet Suppresses Bone Formation and Increases Bone Resorption in Men: A Randomized Controlled Pilot Study. Nutrients. 2021; 13(3):802. https://doi.org/10.3390/nu13030802
Chicago/Turabian StyleMurphy, Chaise, Laura D. Bilek, and Karsten Koehler. 2021. "Low Energy Availability with and without a High-Protein Diet Suppresses Bone Formation and Increases Bone Resorption in Men: A Randomized Controlled Pilot Study" Nutrients 13, no. 3: 802. https://doi.org/10.3390/nu13030802






