Efficacy of Docosahexaenoic Acid for the Prevention of Necrotizing Enterocolitis in Preterm Infants: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
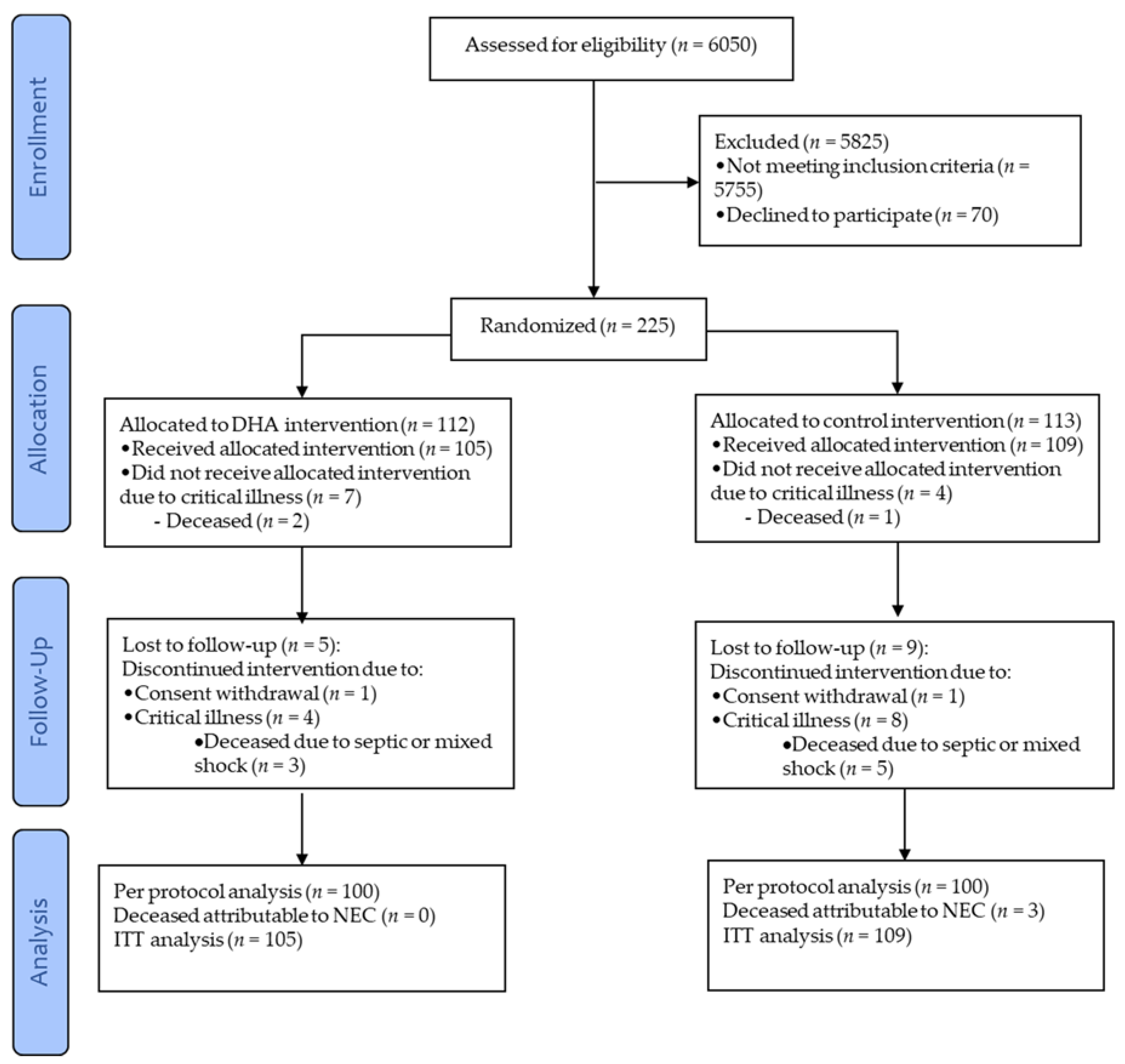
2.1. Study Design and Participants
2.2. Randomization and Intervention
2.3. Main Outcome Measure: Confirmed Necrotizing Enterocolitis
2.4. Adverse Events
2.5. Clinical Course and Management
2.6. Fatty Acid Profile in Erythrocytes and Human Milk
2.7. Sample Size and Statistical Analysis
3. Results
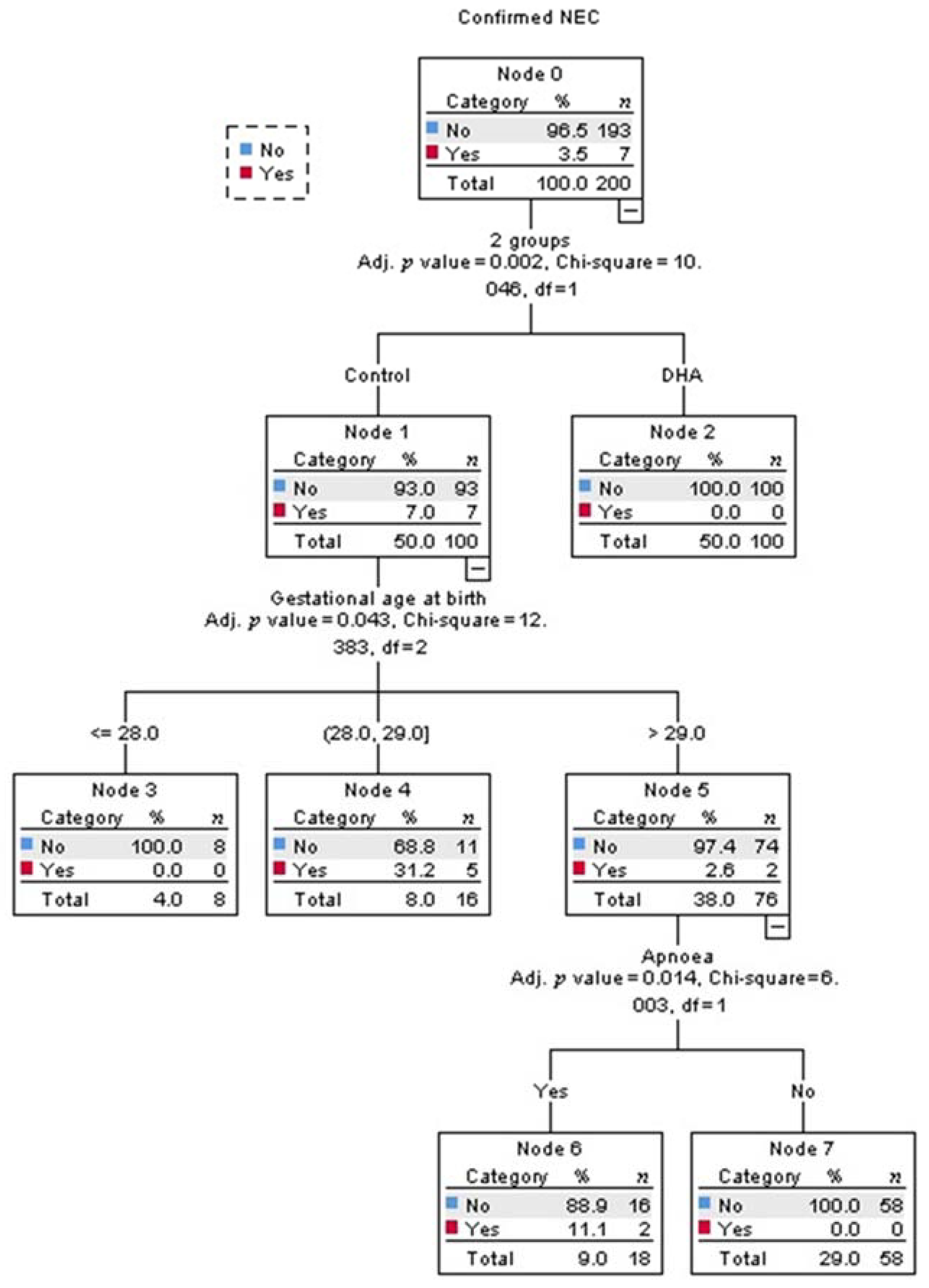
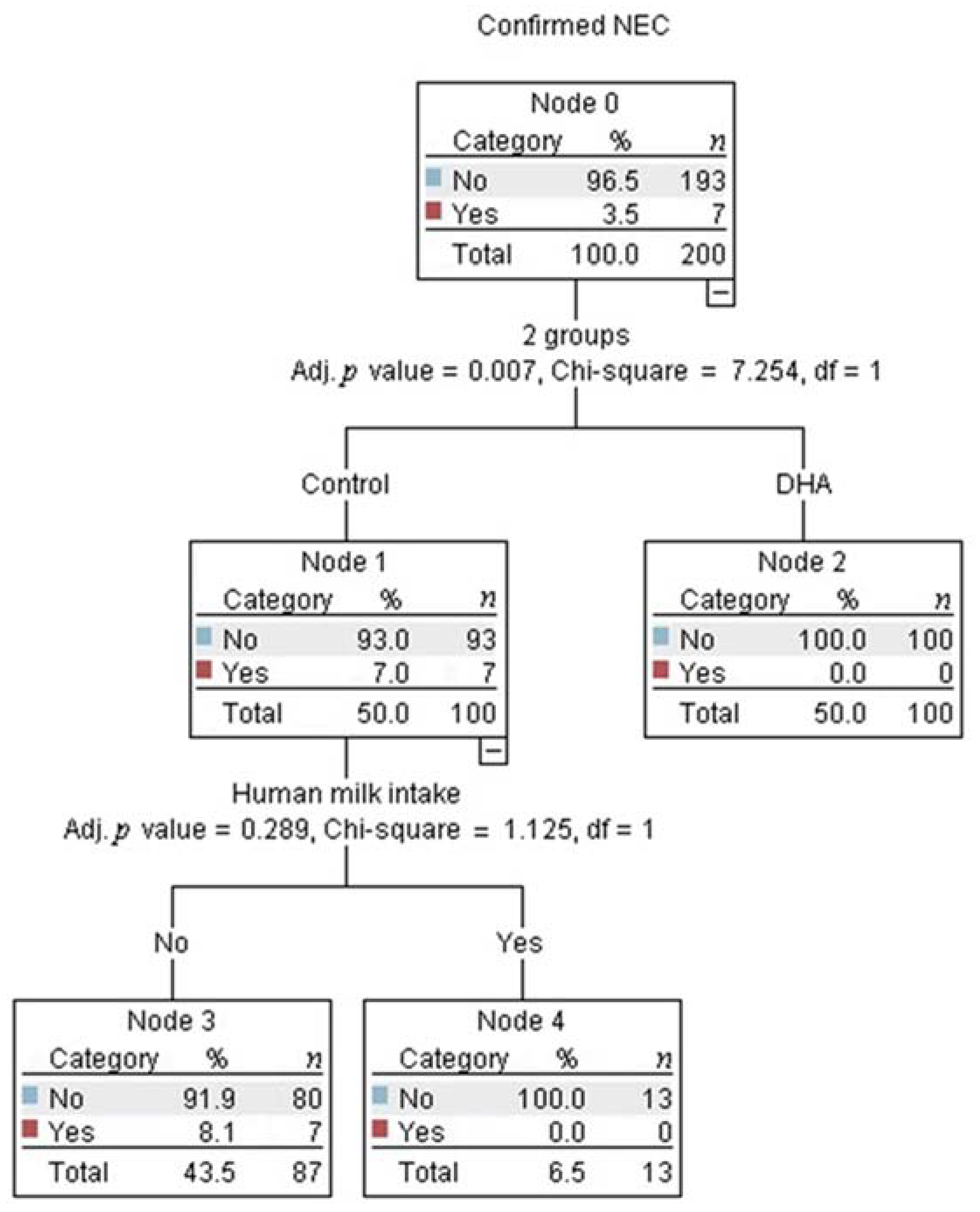
3.1. Confirmed Necrotizing Enterocolitis
3.2. Adverse Events
3.3. Clinical Course and Management
3.4. Fatty Acid Profile in Erythrocytes and Human Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Clinical Trial registration ID
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Paria, A. Etiology and medical management of NEC. Early Hum. Dev. 2016, 97, 17–23. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Jones, I.H.; Hall, N.J. Contemporary outcomes for infants with necrotizing enterocolitis-a systematic review. J. Pediatr. 2020, 220, 86–92.e83. [Google Scholar] [CrossRef]
- Bazacliu, C.; Neu, J. Necrotizing enterocolitis: Long term complications. Curr. Pediatr. Rev. 2019, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Bhatia, A.M.; Kane, A.F.; Patel, R.M.; Denning, P.W. Pathogenesis of NEC: Role of the innate and adaptive immune response. Semin. Perinatol. 2017, 41, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Neu, J.; Pammi, M. Pathogenesis of NEC: Impact of an altered intestinal microbiome. Semin. Perinatol. 2017, 41, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Rouse, C.A. Docosahexaenoic acid and the preterm infant. Matern. Health Neonatol. Perinatol. 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.R.; Dasilva, D.A.; Cluette-Brown, J.E.; Dimonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011, 159, 743–749.e1–2. [Google Scholar] [CrossRef] [Green Version]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Moltó-Puigmartí, C.; Castellote, A.I.; Carbonell-Estrany, X.; López-Sabater, M.C. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin. Nutr. 2011, 30, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Bergmann, K.; Brenna, J.T.; Calder, P.C.; Campoy, C.; Clandinin, M.T.; Colombo, J.; Daly, M.; Decsi, T.; Demmelmair, H.; et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 2020, 111, 10–16. [Google Scholar] [CrossRef]
- Frost, B.L.; Caplan, M.S. Can fish oil reduce the incidence of necrotizing enterocolitis by altering the inflammatory response? Clin. Perinatol. 2019, 46, 65–75. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation of the General Law of Health in maters of Research for Health [REGLAMENTO de la Ley General de Salud en Materia de Investigación para la Salud. Diario Oficial de la Federación]. Published 7 February 1984. Available online: http://www.salud.gob.mx/unidades/cdi/nom/compi/rlgsmis.html (accessed on 13 January 2021).
- Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 2004, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Bernabe-Garcia, M.; Villegas-Silva, R.; Villavicencio-Torres, A.; Calder, P.C.; Rodriguez-Cruz, M.; Maldonado-Hernandez, J.; Macias-Loaiza, D.; Lopez-Alarcon, M.; Inda-Icaza, P.; Cruz-Reynoso, L. Enteral docosahexaenoic acid and retinopathy of prematurity: A randomized clinical trial. J. Parent. Enter. Nutr. 2019, 43, 874–882. [Google Scholar] [CrossRef] [Green Version]
- Bernabe-Garcia, M.; Lopez-Alarcon, M.; Villegas-Silva, R.; Mancilla-Ramirez, J.; Rodriguez-Cruz, M.; Maldonado-Hernandez, J.; Chavez-Rueda, K.A.; Blanco-Favela, F.; Espinoza-Garcia, L.; Lagunes-Salazar, S. Beneficial effects of enteral docosahexaenoic acid on the markers of inflammation and clinical outcomes of neonates undergoing cardiovascular surgery: An intervention study. Ann. Nutr. Metab. 2016, 69, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kliegman, R.M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. N. Am. 1986, 33, 179–201. [Google Scholar] [CrossRef]
- The International Neonatal Network. The CRIB (clinical risk index for babies) score: A tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The International Neonatal Network. Lancet 1993, 342, 193–198. [Google Scholar] [CrossRef]
- Evershed, R. Gas chromatography of lipids. In Lipid Analysis. A Practical Approach; Hamilton, S., Hamilton, R.J., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 113–151. [Google Scholar]
- Lu, J.; Jilling, T.; Li, D.; Caplan, M.S. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 2007, 61, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuka, Y.; Okada, K.; Yamakawa, Y.; Ikuse, T.; Baba, Y.; Inage, E.; Fujii, T.; Izumi, H.; Oshida, K.; Nagata, S.; et al. Omega-3 fatty acids attenuate mucosal inflammation in premature rat pups. J. Pediatr. Surg. 2011, 46, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Brenna, J.T.; Wang, D.H.; Zhu, W.; Meng, D.; Ganguli, K.; Kothapalli, K.S.; Requena, P.; Innis, S.; Walker, W.A. Long-chain polyunsaturated fatty acids attenuate the IL-1beta-induced proinflammatory response in human fetal intestinal epithelial cells. Pediatr. Res. 2015, 78, 626–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, S.E.; Montalto, M.B.; Ponder, D.L.; Werkman, S.H.; Korones, S.B. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr. Res. 1998, 44, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Adamkin, D.H.; Hall, R.T.; Kalhan, S.C.; Lair, C.; Lim, M.; Stevens, D.C.; Twist, P.F.; Diersen-Schade, D.A.; Harris, C.L.; et al. Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. J. Pediatr. 2002, 140, 547–554. [Google Scholar] [CrossRef]
- Smithers, L.G.; Gibson, R.A.; McPhee, A.; Makrides, M. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2008, 87, 912–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvag, A.K.; Ronnestad, A.; Gronn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.T.; Makrides, M.; McPhee, A.J.; Sullivan, T.R.; Davis, P.G.; Thio, M.; Simmer, K.; Rajadurai, V.S.; Travadi, J.; Berry, M.J.; et al. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N. Engl. J. Med. 2017, 376, 1245–1255. [Google Scholar] [CrossRef]
- Parra-Cabrera, S.; Moreno-Macias, H.; Mendez-Ramirez, I.; Schnaas, L.; Romieu, I. Maternal dietary omega fatty acid intake and auditory brainstem-evoked potentials in Mexican infants born at term: Cluster analysis. Early Hum. Dev. 2008, 84, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Farahnak, Z.; Yuan, Y.; Vanstone, C.A.; Weiler, H.A. Maternal and neonatal red blood cell n-3 polyunsaturated fatty acids inversely associate with infant whole-body fat mass assessed by dual-energy X-ray absorptiometry. Appl. Physiol. Nutr. Metab. 2020, 45, 318–326. [Google Scholar] [CrossRef]
- Hackam, D.J.; Afrazi, A.; Good, M.; Sodhi, C.P. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin. Dev. Immunol. 2013, 2013, 475415. [Google Scholar] [CrossRef] [Green Version]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef] [Green Version]
- Halpern, M.D.; Clark, J.A.; Saunders, T.A.; Doelle, S.M.; Hosseini, D.M.; Stagner, A.M.; Dvorak, B. Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G757–G764. [Google Scholar] [CrossRef] [Green Version]
- Chheda, S.; Palkowetz, K.H.; Garofalo, R.; Rassin, D.K.; Goldman, A.S. Decreased interleukin-10 production by neonatal monocytes and T cells: Relationship to decreased production and expression of tumor necrosis factor-alpha and its receptors. Pediatr. Res. 1996, 40, 475–483. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 PUFA and inflammation: From membrane to nucleus and from bench to bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef]
- Llanos, A.R.; Moss, M.E.; Pinzon, M.C.; Dye, T.; Sinkin, R.A.; Kendig, J.W. Epidemiology of neonatal necrotising enterocolitis: A population-based study. Paediatr. Perinat. Epidemiol. 2002, 16, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.L.; Kim, J.H. Human milk and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 34–38. [Google Scholar] [CrossRef]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Xiong, T.; Maheshwari, A.; Neu, J.; Ei-Saie, A.; Pammi, M. An Overview of systematic reviews of randomized-controlled trials for preventing necrotizing enterocolitis in preterm infants. Neonatology 2020, 117, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Spinner, J.A.; Morris, S.A.; Nandi, D.; Costarino, A.T.; Marino, B.S.; Rossano, J.W.; Shamszad, P. Necrotizing enterocolitis and associated mortality in neonates with congenital heart disease: A multi-institutional study. Pediatr. Crit. Care Med. 2020, 21, 228–234. [Google Scholar] [CrossRef]
- Patel, R.M.; Knezevic, A.; Shenvi, N.; Hinkes, M.; Keene, S.; Roback, J.D.; Easley, K.A.; Josephson, C.D. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 2016, 315, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Butler, M.; Dechelotte, P.; Playford, R.J.; Ghosh, S. Comparison of cytokine modulation by natural peroxisome proliferator-activated receptor gamma ligands with synthetic ligands in intestinal-like Caco-2 cells and human dendritic cells--potential for dietary modulation of peroxisome proliferator-activated receptor gamma in intestinal inflammation. Am. J. Clin. Nutr. 2008, 87, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Comparative anti-inflammatory effects of plant- and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158662. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | GROUP | |
|---|---|---|
| DHA Intervention (Algal Oil) | Control (High-Oleic Sunflower Oil) | |
| Capric | 1.0 | 0.0 |
| Lauric | 4.3 | 0.0 |
| Myristic | 14.0 | 0.1 |
| Palmitic | 12.2 | 5.2 |
| Palmitoleic | 1.9 | 0.1 |
| Stearic | 0.7 | 3.8 |
| Oleic | 18.9 | 58.9 |
| Linoleic | 1.3 | 29.2 |
| Gamma-Linolenic | 0.4 | 0.3 |
| Alpha-Linolenic | <0.1 | 1.2 |
| Eicosenoic | <0.1 | 0.3 |
| Arachidonic | <0.1 | 0.0 |
| Eicosapentaenoic | <0.1 | 0.0 |
| Behenic | 0.2 | 0.7 |
| Docosahexaenoic | 44.3 | 0.0 |
| Nervonic | 0.1 | 0.0 |
| GROUP | p | ||
|---|---|---|---|
| DHA n = 100 | Control n = 100 | ||
| At Birth | |||
| Born by cesarean section, n (%) | 96 (96) | 99 (99) | 0.391 |
| Received antenatal steroid, n (%) | 48 (48) | 42 (42) | 0.601 |
| Gestational age, weeks | 30 (29, 32) | 31 (30, 32) | 0.513 |
| Male sex, n (%) | 52 (52) | 47 (47) | 0.572 |
| Apgar score at minute 5 ≥ 7, n (%) | 96 (96) | 95 (95) | 1.000 |
| At Baseline | |||
| Corrected gestational age, weeks | 31.0 (29.8, 32.3) | 31.1 (28.0, 32.5) | 0.306 |
| GROUP | p | |||
|---|---|---|---|---|
| DHA n = 100 | Control n = 93 | NEC n = 7 | ||
| Birthweight 1000–1250 g, n (%) | 40 (40) | 44 (47) | 4 (57) | 0.460 |
| Birthweight 1251–1500 g, n (%) | 60 (60) | 49 (53) | 3 (43) | |
| Small for gestational age, n (%) | 14 (14) | 12 (13) | 1 (14) | 0.974 |
| Twins, n (%) | 23 (23) | 30 (32) | 2 (29) | 0.354 |
| Severe asphyxia at birth, n (%) | 9 (9) | 10 (11) | 0 | 0.627 |
| Severity of disease (CRIB) at baseline | 2.0 (1, 5.0) | 1.0 (1, 4.5) | 1.0 (0, 6.0) | 0.760 |
| Hemoglobin at baseline, g/dl | 15.7 (14.2, 17.7) | 16.5 (14.8, 18.0) | 14.1 (12.9, 16.8) | 0.150 |
| Respiratory distress syndrome, n (%) | 90 (90) | 84 (90) | 7 (100) | 0.681 |
| Suspected or confirmed sepsis, n (%) | 66 (66) | 57 (61) | 7 (100) | 0.112 |
| PDA, n (%) | 18 (18) | 21 (23) | 3 (43) | 0.259 |
| Apnea, n (%) | 36 (36) | 24 (26) | 4 (57) | 0.110 |
| Apnea events in week 1, n | 1 (1, 2) | 1 (1, 2) | 1 | 0.780 |
| Apnea events in week 2, n | 2 (1, 2) | 1.5 (1, 3) | 1 (1, 1.5) | 0.669 |
| Apnea events during hospitalization, n | 3.0 (2, 5) | 3.0 (1, 7) | 4.5 (1.3, 8) | 0.881 |
| Requirement for phase III ventilator support, n (%) | 65 (66) | 62 (67) | 6 (86) | 0.552 |
| Duration with SpO2 < 85% in week 1, h | 5.5 (2.0, 12.0) | 4 (2.0, 6.0) | 0 | 0.219 |
| Duration with SpO2 < 85% in week 2, h | 7.0 (5.0, 13.5) | 4 (3.5, 8.0) | 0 | 0.146 |
| Retinopathy of the prematurity, n (%) | 23 (23) | 23 (25) | 0* | 0.325 |
| Antibiotics used, n (%) | 97 (97) | 92 (99) | 7 (100) | 0.589 |
| Postnatal age at antibiotic start, h | 8 (4, 24) | 8 (4, 24) | 8 (4, 48) | 0.937 |
| Antibiotic duration, days | 26 (17, 42) | 27 (19, 42) | 28 (25, 38) | 0.950 |
| Postnatal steroid use, n (%) | 21 (21) | 21 (23) | 3 (43) | 0.408 |
| Ibuprofen use, n (%) | 9 (9) | 6 (7) | 1 (14) | 0.665 |
| Omeprazole during intervention period, n (%) | 43 (43) | 41 (46) | 2 (29) | 0.671 |
| Duration of omeprazole use during intervention period, days | 4 (1, 6) | 4 (2, 7) | 4.5 (3, 6) | 0.759 |
| Infants with RBC transfusion during intervention period, n (%) | 38 (38) | 32 (34) | 3 (43) | 0.821 |
| Number of RBC transfusions during intervention period, n | 1 (1, 1) | 1 (1, 1) | 1.5 (1, 2) | 0.467 |
| Length of NICU stay, days | 9.5 (2, 18) | 12.0 (3, 21) | 18.0 (9, 31) | 0.166 |
| Length of hospital stay, days | 45 (35, 55) | 46 (38, 53) | 47 (26, 66) * | 0.888 |
| Nutritional intake variable | GROUP | p | ||
|---|---|---|---|---|
| DHA n = 100 | Control n = 93 | NEC n = 7 | ||
| Week One of Postnatal Age | ||||
| Total fluid volume, mL/kg/day | 100 (88, 108) | 97 (88, 105) | 89 (75, 104) | 0.437 |
| Infants receiving TPN before EF, n (%) | 50 (57) | 48 (59) | 6 (86) | 0.327 |
| Postnatal age at starting TPN, days | 2.0 (1.7, 2.4) | 2.7 (1.0, 2.4) | 3.0 (1.8, 4.0) | 0.851 |
| Lipid supply by TPN, g/kg/day | 2.0 (0.6, 3.8) | 2.1 (0.4, 2.9) | 2.0 (1.4, 2.4) | 0.877 |
| Postnatal age at starting EF, days | 4.0 (2.0, 6.5) | 4.3 (2.5, 6.2) | 7.0 (4.1, 9.1) | 0.176 |
| Lipid intake-EFF, g/kg/day | 1.1 (0.5, 2.3) | 1.2 (0.6, 2.0) | 0.5 (0.2, 1.2) | 0.151 |
| Fatty Acid Intake from EFF, mg/kg/day | ||||
| Linoleic acid | 69 (32, 231) | 100 (31, 217) | 38 (11, 107) | 0.282 |
| Alpha-linolenic acid | 26 (10, 44) | 23 (10, 39) | 9 (4, 22) | 0.154 |
| Arachidonic acid | 5 (2, 9) | 5 (2, 8) | 2 (0.8, 5) | 0.171 |
| Docosahexaenoic acid | 5 (2, 9) | 5 (2, 8) | 2 (0.8, 5) | 0.161 |
| Week Two of Postnatal Age | ||||
| Total fluid volume, mL/kg/day | 136 (125, 147) | 138 (123, 147) | 127 (99, 131) | 0.081 |
| Lipid supply by TPN, g/kg/day | 1.8 (0.8, 2.4) | 1.8 (0.8, 2.3) | 2.1 (1.4, 2.5) | 0.717 |
| Lipid intake-EFF, g/kg/day | 4.5 (2.5, 5.8) | 4.2 (2.9, 5.7) | 1.2 (0.4, 3.9) | 0.059 |
| Fatty Acid Intake from EFF, mg/kg/day | ||||
| Linoleic acid | 576 (209, 880) | 506 (119, 833) | 82 (21, 505) | 0.106 |
| Alpha-linolenic acid | 86 (48, 111) | 79 (35, 108) | 23 (8, 74) | 0.064 |
| Arachidonic acid | 17 (10, 22) | 16 (7, 22) | 7 (1.4, 15) | 0.088 |
| Docosahexaenoic acid | 17 (10, 22) | 16 (7, 22) | 7 (1.4, 15) | 0.088 |
| Human Milk Intake | ||||
| Infants fed any volume of human milk, n (%) | 18 (18) | 13 (14) | 0 | 0.382 |
| Infants fed human milk during first week post-enteral feeding, n (%) | 11 (11) | 4 (4.2) | 0 | 0.157 |
| Intake during first week post-enteral feeding, mL/kg/day | 3.9 (2.1, 10.3) a | 4.9 (0.7, 9.9) b | 0 c | 0.003 |
| Infants fed human milk during second week post-enteral feeding, n (%) | 11 (11) | 6 (6.5) | 0 | 0.376 |
| Intake during second week post-enteral feeding, mL/kg/day | 8.3 (2.4, 17.2) a | 5.4 (1.8, 20.3) b | 0 c | 0.052 |
| Infants fed human milk during third week post-EF, n (%) | 9 (9) | 7 (7.5) | 0 | 0.679 |
| Intake during third week post-enteral feeding, mL/kg/day | 6.7 (2.6, 23.1) a | 7.9 (4.1, 13.6) a | 0 b | 0.029 |
| Infants fed human milk during fourth week post-EF, mL/kg/day, n (%) | 12 (12) | 7 (7.5) | 0 | 0.390 |
| Intake during fourth week post-enteral feeding, mL/kg/day | 5.6 (2.6, 12.7) a | 4.3 (1.5, 6.2) b | 0 c | 0.043 |
| Intake during hospital stay, mL/kg/day | 14 (7, 37) | 12 (3, 60) | 0 | 0.448 |
| Time required to reach full enteral feeding, days | 15 (12, 22) | 17 (12, 22) | 20 (16, 46) | 0.161 |
| Fatty Acid | DHA n = 100 | Control n = 93 | NEC n = 7 | p | DHA n = 100 | Control n = 93 | NEC n = 7 |
|---|---|---|---|---|---|---|---|
| In Baseline Erythrocyte Membranes | In Human Milk during Hospital Stay | ||||||
| Lauric | 0.34 (0.15, 0.67) | 0.38 (0.22, 0.79) | 0.36 (0.15, 0.67) | 0.534 | 7.6 (7.0, 9.6) | 6.6 (6.6, 7.2) | - * |
| Myristic | 0.71 (0.57, 1.06) | 0.80 (0.61, 1.24) | 1.02 (0.71, 1.06) | 0.252 | 9.0 (7.7, 9.5) | 8.8 (8.2, 9.4) | - * |
| Palmitic | 32.5 (29.9, 40.7) | 33.4 (32.2, 42.3) | 31.7 (30.3, 48.0) | 0.463 | 22.0 (20.1, 26.9) | 24.7 (24.2, 25.2) | - * |
| Palmitoleic | 1.12 (0.78, 1.42) | 1.18 (0.80, 1.48) | 1.47 (1.0, 2.50) | 0.055 | 2.3 (2.1, 2.6) | 2.3 (2.2, 2.4) | - * |
| Stearic | 17.4 (15.9, 18.9) | 16.9 (4.3, 23.6) | 17.1 (15.8, 19.0) | 0.485 | 6.6 (6.1, 6.7) | 7.0 (6.6, 7.3) | - * |
| Oleic | 16.7 (15.2, 18.8) | 16.6 (14.6, 19.3) | 16.7 (16.0, 18.8) | 0.894 | 33.7 (31.6, 34.9) | 32.0 (31.9, 32.9) | - * |
| Linoleic | 5.1 (4.0, 7.4) | 5.0 (4.1, 7.3) | 3.6 (2.2, 7.3) | 0.473 | 16.1 (15.9, 17.4) | 15.5 (14.4, 17.0) | - * |
| Alpha-linolenic | 0.12 a (0.09, 0.30) | 0.10 b (0.07, 0.17) | 0.07 b (0.07, 0.17) | 0.042 | 1.2 (1.1, 1.5) | 1.1 (1.0, 1.2) | - * |
| Arachidonic | 14.67 (5.07, 20.11) | 15.50 (5.51, 20.2) | 13.5 (4.3, 18.9) | 0.829 | 0.6 (0.5, 0.6) | 0.5 (0.5, 0.6) | - * |
| Eicosapentaenoic | 0.54 (0.24, 0.83) | 0.53 (0.27, 0.70) | 0.40 (0.31, 0.69) | 0.782 | 0.03 a (0.03, 0.06) | 0.10 b (0.09, 0.12) | - * |
| Nervonic | 2.82 (2.0, 3.4) | 2.62 (2.11, 3.4) | 2.90 (2.0, 3.1) | 0.896 | 0.03 (0.04, 0.05) | 0.03 (0.03, 0.04) | - * |
| Docosahexaenoic | 2.92 (1.2, 3.4) | 2.99 (0.95, 3.7) | 3.0 (2.0, 4.0) | 0.812 | 0.22 a (0.16, 0.27) | 0.35 b (0.33, 0.36) | - * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabe-García, M.; Calder, P.C.; Villegas-Silva, R.; Rodríguez-Cruz, M.; Chávez-Sánchez, L.; Cruz-Reynoso, L.; Mateos-Sánchez, L.; Lara-Flores, G.; Aguilera-Joaquín, A.R.; Sánchez-García, L. Efficacy of Docosahexaenoic Acid for the Prevention of Necrotizing Enterocolitis in Preterm Infants: A Randomized Clinical Trial. Nutrients 2021, 13, 648. https://doi.org/10.3390/nu13020648
Bernabe-García M, Calder PC, Villegas-Silva R, Rodríguez-Cruz M, Chávez-Sánchez L, Cruz-Reynoso L, Mateos-Sánchez L, Lara-Flores G, Aguilera-Joaquín AR, Sánchez-García L. Efficacy of Docosahexaenoic Acid for the Prevention of Necrotizing Enterocolitis in Preterm Infants: A Randomized Clinical Trial. Nutrients. 2021; 13(2):648. https://doi.org/10.3390/nu13020648
Chicago/Turabian StyleBernabe-García, Mariela, Philip C. Calder, Raúl Villegas-Silva, Maricela Rodríguez-Cruz, Luis Chávez-Sánchez, Leonardo Cruz-Reynoso, Leovigildo Mateos-Sánchez, Gabriel Lara-Flores, Augusto R. Aguilera-Joaquín, and Luisa Sánchez-García. 2021. "Efficacy of Docosahexaenoic Acid for the Prevention of Necrotizing Enterocolitis in Preterm Infants: A Randomized Clinical Trial" Nutrients 13, no. 2: 648. https://doi.org/10.3390/nu13020648







