Postoperative Ileus after Stimulation with Probiotics before Ileostomy Closure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Simple Size
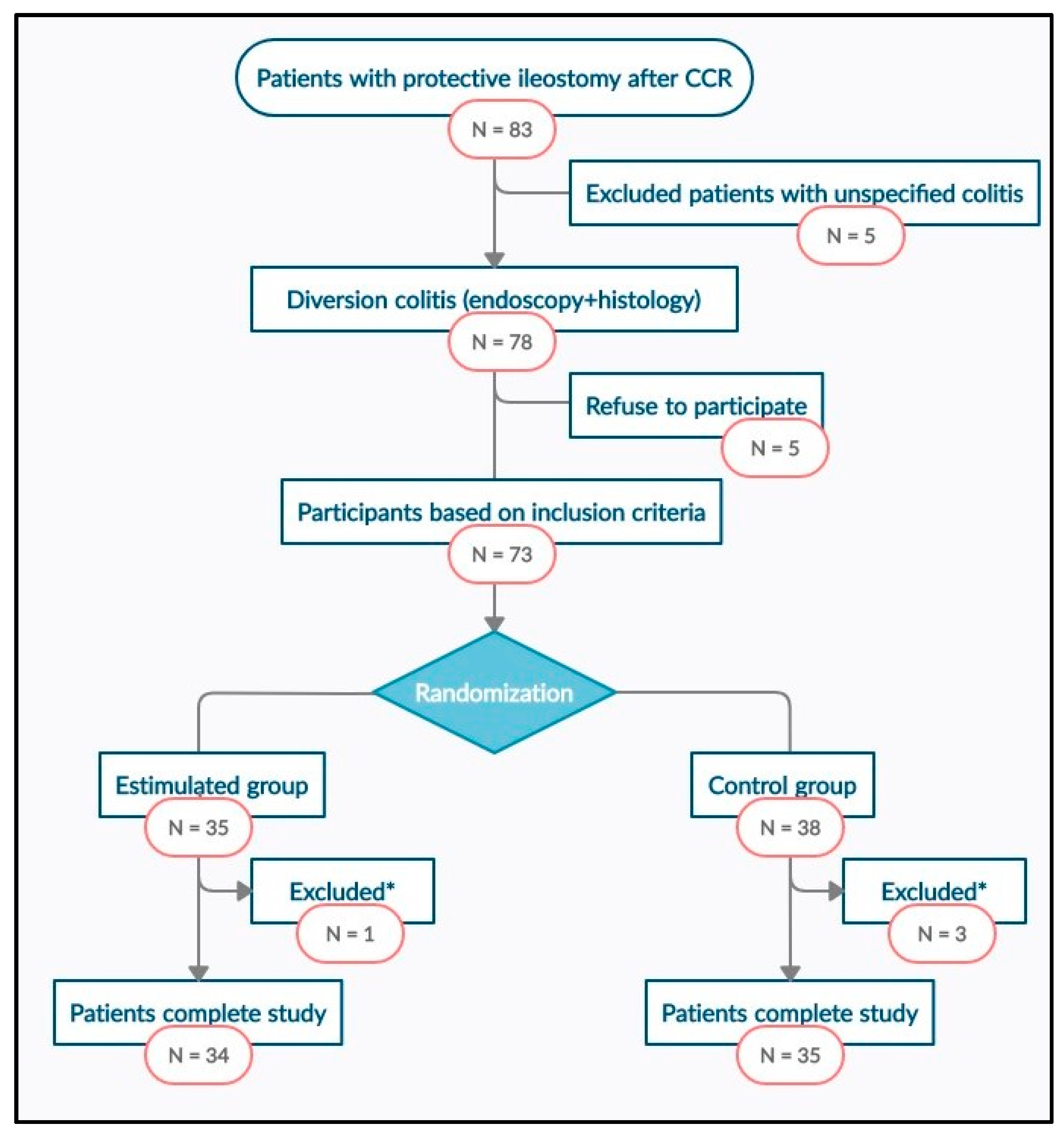
2.3. Selection of Patients
2.4. Randomization and Intervention
- Stimulation group (SG): preoperative stimulation of the distal limb of the ileostomy loop with probiotics was performed during the 20 days prior to surgery every second day. During the process and after it, the patient himself registers the appearance of symptoms after each stimulation session: abdominal pain, emission of gas and stool. A sterile Foley catheter No.14 Ch connected to an infusion set was introduced through the defunctioned bowel to allow the slow infusion, for 20–30 min, of a solution with 4.5 mg of probiotics diluted in 250 mL of 0.9% physiological saline. Each preparation was made under sterile conditions and maintaining the cold chain. Vivomixx® lyophilized live bacteria, count by SIGMA-TAU PHARMACEUTICALS and manufactured in Spain by GRIFOLS S.A., contained 4.5 × 1011 of live bacteria in each preparation:
- ○
- Four strains of Lactobacillus:
- ■
- Lactobacillus acidophilus DSM 24735
- ■
- Lactobacillus plantarum DSM 24730
- ■
- Lactobacillus paracasei DSM 24733
- ■
- Lactobacillus delbrueckii subsp. bulgaricus DSM 24734.
- ○
- Three strains of Bifidobacterium:
- ■
- Bifidobacterium breve DSM 24732
- ■
- Bifidobacterium longum DSM 24736
- ■
- Bifidobacterium infantis DSM 24737
- ○
- One strain of Streptococcus
- ■
- Streptococcus thermophilus DSM 24731.
- Control group (CG): exactly the same procedure was carried out, but with the infusion set closed. During the process and after it, the patient himself registers the appearance of symptoms after each stimulation session: abdominal pain, emission of gas and stool.
2.5. Surgery and Follow-Up
2.6. Masking
2.7. Assessment Criteria
2.8. Statistical Analysis
2.9. Ethical Aspects
3. Results
3.1. Study Population
3.2. Main Assessment Criteria
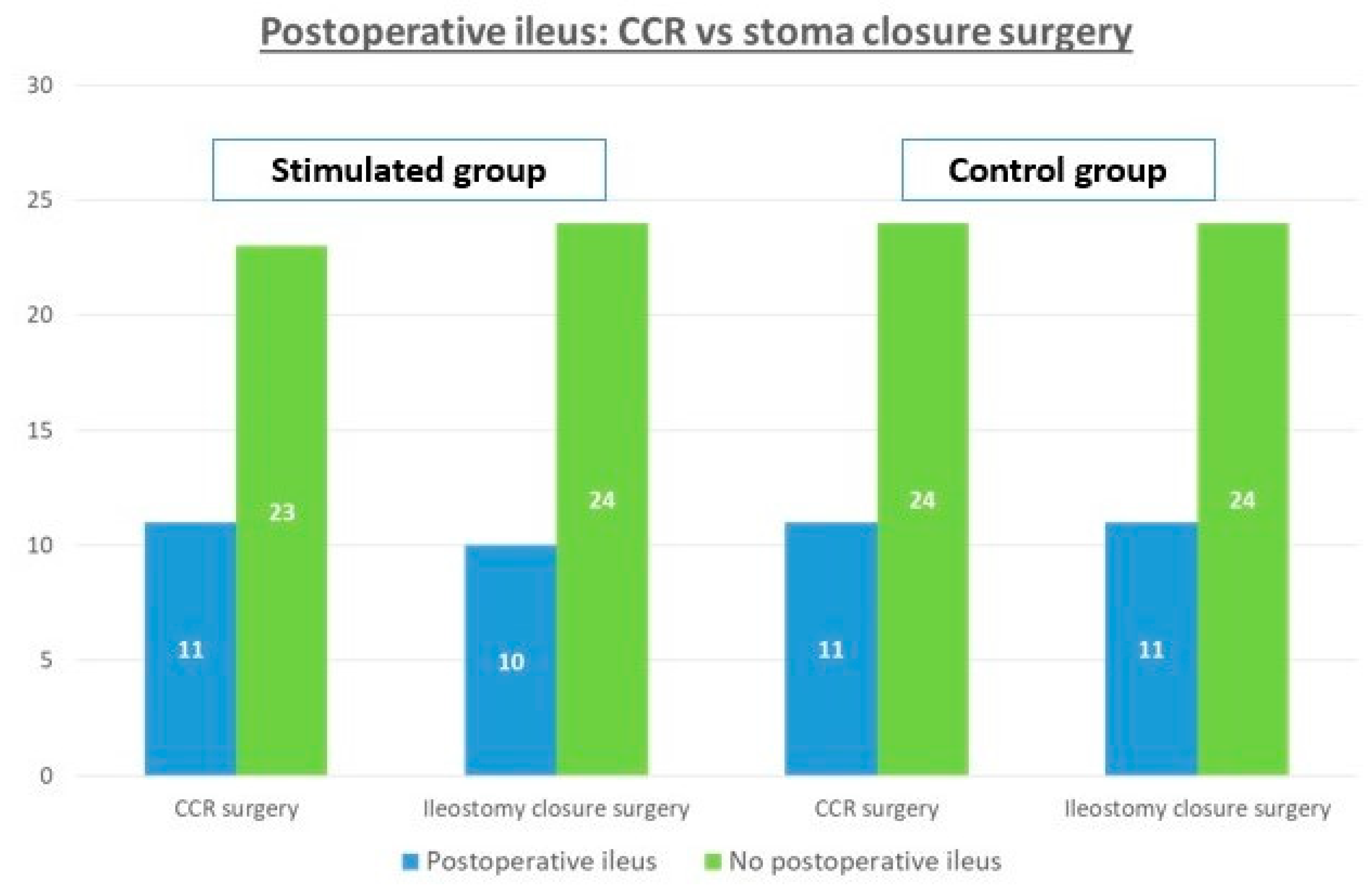
3.3. Secondary Assessment Criteria: Postoperative Ileus and Colorectal Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthiessen, P.; Hallböök, O.; Rutegård, J.; Simert, G.; Sjödahl, R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: A randomized multicenter trial. Ann. Surg. 2007, 246, 207–214. [Google Scholar] [CrossRef]
- Sharma, A.; Deeb, A.-P.; Rickles, A.S.; Iannuzzi, J.C.; Monson, J.R.T.; Fleming, F.J. Closure of defunctioning loop ileostomy is associated with considerable morbidity. Colorectal Dis. 2013, 15, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Peacock, O.; Tierney, G.M.; Tou, S.; Hurst, N.G.; Speake, W.J.; Williams, J.P.; Lund, J.N. Day-case closure of ileostomy: Feasible, safe and efficient. Colorectal Dis. 2015, 17, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, R.; Savage, P.; Boutros, M.; Landry, T.; Reynier, P.; Morin, N.; Vasilevsky, C.-A.; Filion, K.B. Incidence and predictors of postoperative ileus after loop ileostomy closure: A systematic review and meta-analysis. Surg. Endosc. 2019, 33, 2430–2443. [Google Scholar] [CrossRef]
- Danielsen, A.K.; Park, J.; Jansen, J.E.; Bock, D.; Skullman, S.; Wedin, A.; Marinez, A.C.; Haglind, E.; Angenete, E.; Rosenberg, J. Early closure of a temporary ileostomy in patients with rectal cancer: A multicenter randomized controlled trial. Ann. Surg. 2017, 265, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beamish, E.L.; Johnson, J.; Shaw, E.J.; Scott, N.A.; Bhowmick, A.; Rigby, R.J. Loop ileostomy-mediated fecal stream diversion is associated with microbial dysbiosis. Gut Microbes 2017, 8, 467–478. [Google Scholar] [CrossRef]
- Williams, L.; Armstrong, M.J.; Finan, P.; Sagar, P.; Burke, D. The effect of faecal diversion on human ileum. Gut 2007, 56, 796–801. [Google Scholar] [CrossRef] [Green Version]
- Keane, C.; Park, J.; Öberg, S.; Wedin, A.; Bock, D.; O’Grady, G.; Bissett, I.; Rosenberg, J.; Angenete, E. Functional outcomes from a randomized trial of early closure of temporary ileostomy after rectal excision for cancer. Br. J. Surg. 2019, 106, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombey, T.; Panagiotopoulou, I.G.; Hind, D.; Fearnhead, N.S. Preoperative bowel stimulation prior to ileostomy closure to restore bowel function more quickly and improve postoperative outcomes: A systematic review. Colorectal Dis. 2019, 21, 994–1003. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, T.L.; McEvoy, M.D.; Mythen, M.G.; Bergamaschi, R.; Gupta, R.; Holubar, S.D.; Senagore, A.J.; Gan, T.J.; Shaw, A.D.; Thacker, J.K.M.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Gastrointestinal Dysfunction Within an Enhanced Recovery Pathway for Elective Colorectal Surgery. Anesth. Analg. 2018, 126, 1896–1907. [Google Scholar] [CrossRef]
- Alsharqawi, N.; Alhashemi, M.; Kaneva, P.; Baldini, G.; Fiore, J.F.; Feldman, L.S.; Lee, L. Validity of the I-FEED score for postoperative gastrointestinal function in patients undergoing colorectal surgery. Surg. Endosc. 2020, 34, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, R.; Trabulsi, N.; Morin, N.; Phang, T.; Liberman, S.; Feldman, L.; Fried, G.; Boutros, M. Study protocol evaluating the use of bowel stimulation before loop ileostomy closure to reduce postoperative ileus: A multicenter randomized controlled trial. Colorectal Dis. 2017, 19, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Abrisqueta, J.; Abellan, I.; Luján, J.; Hernández, Q.; Parrilla, P. Stimulation of the efferent limb before ileostomy closure: A randomized clinical trial. Dis. Colon Rectum 2014, 57, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Menendez, P.; Garcia, A.; Lozano, E.; Pelaez, R. Effectiveness of afferent loop stimulation prior to ileostomy closure. Cir. Esp. 2013, 91, 547–548. [Google Scholar] [CrossRef]
- Vázquez-Melero, A.; Totoricaguena, A.L.; García Alonso, P. Stimulation of the Efferent Loop before Loop Ileostomy Closure; National Library of Medicine: Bethesda, MD, USA, 2018. [Google Scholar]
- López, F.F.; López, J.G.; Novo, M.P.; González, M.J.L.; Cotoré, J.P. Estimulación preoperatoria del asa eferente de la ileostomía con ácidos grasos de cadena corta. Cir. Esp. 2019, 97, 54–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Guo, C.; Mu, D.; Feng, B.; Zuo, X.; Li, Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: A meta-analysis. BMC Gastroenterol. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redman, M.; Ward, E.; Phillips, R. The efficacy and safety of probiotics in people with cancer: A systematic review. Ann. Oncol. 2014, 25, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.J.; Oh, H.; Lee, J.; Cho, J.R.; Kim, M.J.; Kim, D.; Kang, S. Effects of probiotics on bowel function restoration following ileostomy closure in rectal cancer patients: A randomized controlled trial. Colorectal Dis. 2020. [Google Scholar] [CrossRef]
- Wolthuis, A.; Bislenghi, G.; Fieuws, S.; van Overstraeten, A.d.B.; Boeckxstaens, G.; D’Hoore, A. Incidence of prolonged postoperative ileus after colorectal surgery: A systematic review and meta-analysis. Colorectal Dis. 2016, 18, O1–O9. [Google Scholar] [CrossRef]
- Sugawara, K.; Kawaguchi, Y.; Nomura, Y.; Suka, Y.; Kawasaki, K.; Uemura, Y.; Koike, D.; Nagai, M.; Furuya, T.; Tanaka, N. Perioperative factors predicting prolonged postoperative ileus after major abdominal surgery. J. Gastrointest. Surg. 2017, 22, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Rybakov, E.G.; Zarodniuk, I.V.; A Shelygin, Y.; A Khomyakov, E. Risk factors for postoperative ileus after colorectal cancer surgery. Colorectal Dis. 2018, 20, 189–194. [Google Scholar] [CrossRef] [PubMed]
| Stimulated Group (n = 34) | No Stimulated Group (n = 35) | p | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 65 (45–81) | 68 (41–80) | 0.130 |
| Sex ratio (Male:Female) | 23:11 | 25:10 | 0.733 |
| BMI (kg/m2) | 23.5 (21.6–32.6) | 27.6 (18.8–40.2) | 0.091 |
| ASA | 0.483 | ||
| ASA I-II | 31 | 30 | |
| ASA III | 3 | 5 | |
| Smoker/nonsmoker | 20/14 | 23/12 | 0.826 |
| Colorectal surgery | |||
| Surgical procedure: | 0.129 | ||
| LAR | 25 | 25 | |
| uLAR | 9 | 10 | |
| Surgical approach: | 0.551 | ||
| Laparoscopic | 24 | 24 | |
| Open | 10 | 11 | |
| Type of anastomosis: | 0.430 | ||
| Stapled EEA | 31 | 33 | |
| Coloanal anastomosis | 3 | 2 | |
| Neoadjuvant therapy | 26 | 25 | 0.239 |
| Adjuvant treatment | 26 | 28 | 0.256 |
| Ileostomy closure | |||
| Time between surgeries (months) | 12 (8–37) | 9 (6–32) | 0.813 |
| Surgery: | 0.690 | ||
| Small bowel resection | 33 | 34 | |
| Ileocecal resection | 1 | 1 | |
| Surgical approach: | 0.291 | ||
| Peri-ileostomy | 30 | 28 | |
| Midline laparotomy | 4 | 7 | |
| Type of anastomosis: | 0.355 | ||
| Sewn | 29 | 29 | |
| Stapled | 5 | 6 | |
| Time (minutes) | 50 (30–70) | 65 (50–120) | 0.053 |
| Stimulated Group (n = 34) | No Stimulated Group (n = 35) | p | |
|---|---|---|---|
| Postoperative ileus, n (%) | 10 (29.4%) | 11 (31.4%) | 0.192 |
| Nasogastric tube, n (%) | 9 (26%) | 11 (31.4%) | 0.116 |
| Time to tolerating a diet, days-mean (range) | 2 (1–24) | 3 (2–50) | 0.619 |
| Start of the passage of flatus, days-mean (range) | 2 (1–20) | 2 (1–48) | 0.173 |
| Start of the passage of stool, days-mean (range) | 3 (1–21) | 3 (1–48) | 0.184 |
| Postoperative stay, days-mean (range) | 4 (4–26) | 5 (4–56) | 0.105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Padilla, Á.; Morales-Martín, G.; Pérez-Quintero, R.; Gómez-Salgado, J.; Balongo-García, R.; Ruiz-Frutos, C. Postoperative Ileus after Stimulation with Probiotics before Ileostomy Closure. Nutrients 2021, 13, 626. https://doi.org/10.3390/nu13020626
Rodríguez-Padilla Á, Morales-Martín G, Pérez-Quintero R, Gómez-Salgado J, Balongo-García R, Ruiz-Frutos C. Postoperative Ileus after Stimulation with Probiotics before Ileostomy Closure. Nutrients. 2021; 13(2):626. https://doi.org/10.3390/nu13020626
Chicago/Turabian StyleRodríguez-Padilla, Ángela, Germán Morales-Martín, Rocío Pérez-Quintero, Juan Gómez-Salgado, Rafael Balongo-García, and Carlos Ruiz-Frutos. 2021. "Postoperative Ileus after Stimulation with Probiotics before Ileostomy Closure" Nutrients 13, no. 2: 626. https://doi.org/10.3390/nu13020626






