Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Search Strategy and Selection Criteria
2.2. Study Selection and Data Extraction
2.3. Quality Assessment and Risk of Bias
2.4. Statistical Analysis
3. Results
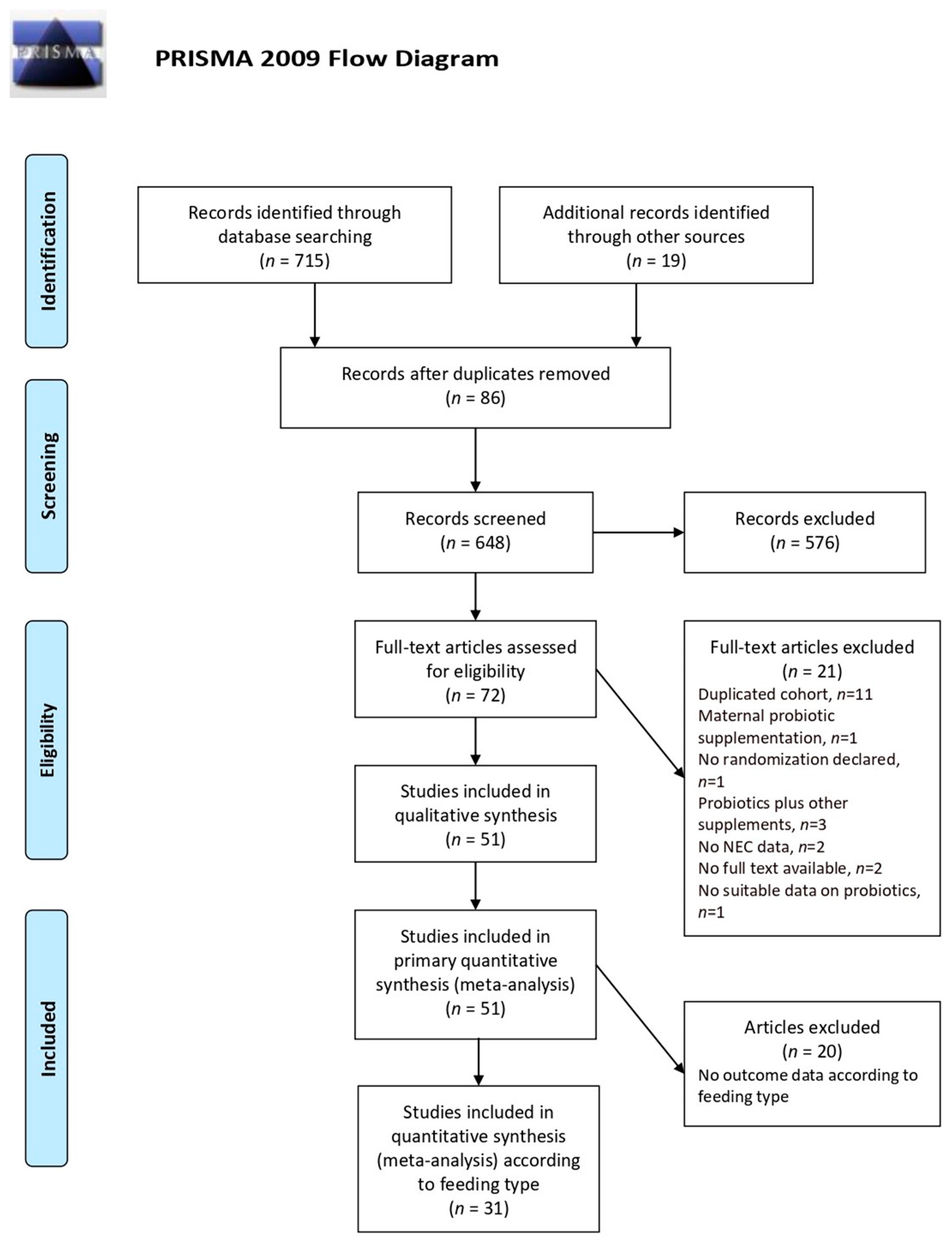
3.1. Search Results and Study Characteristics
3.2. Quality Assessment and Risk of Bias
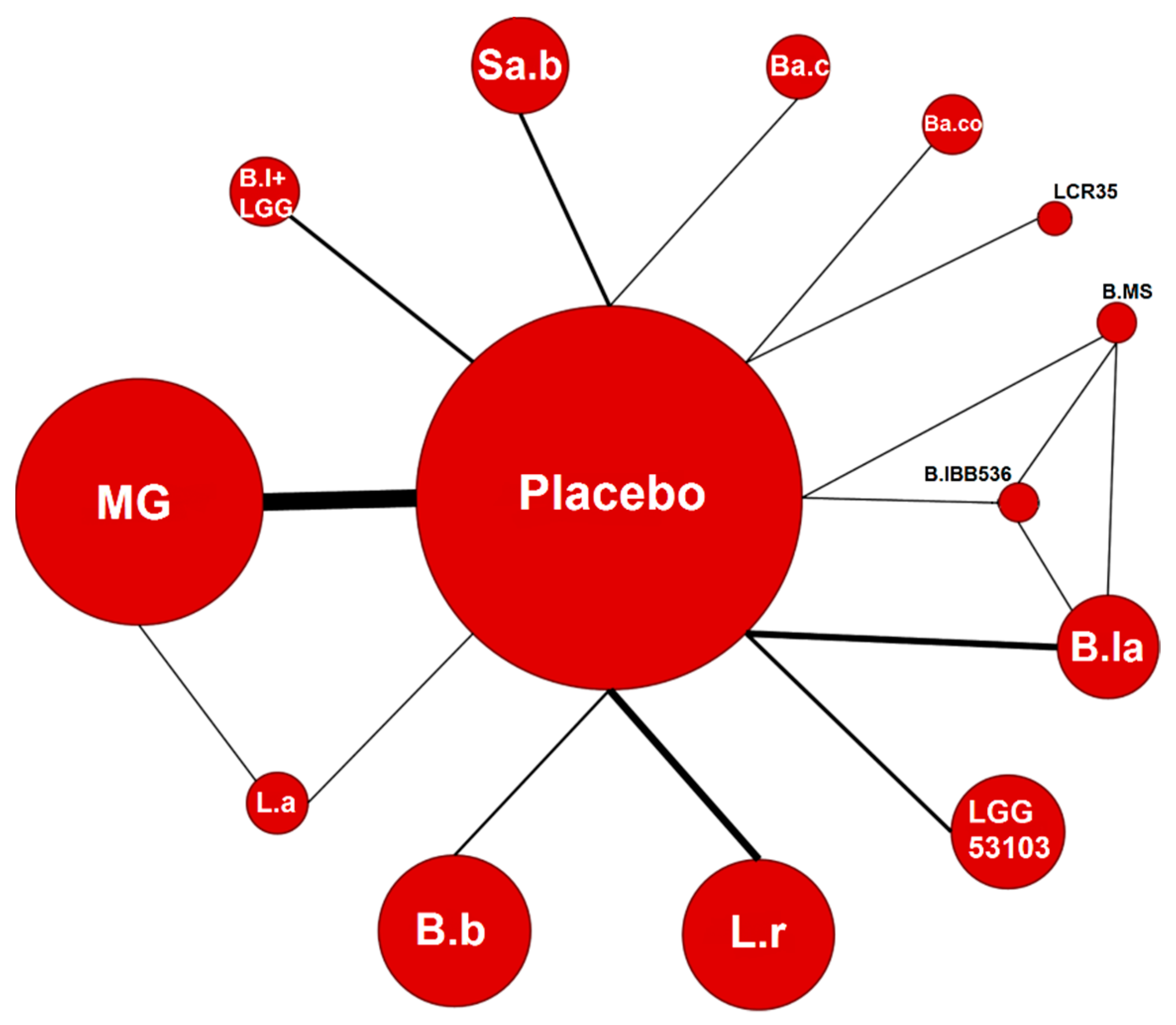
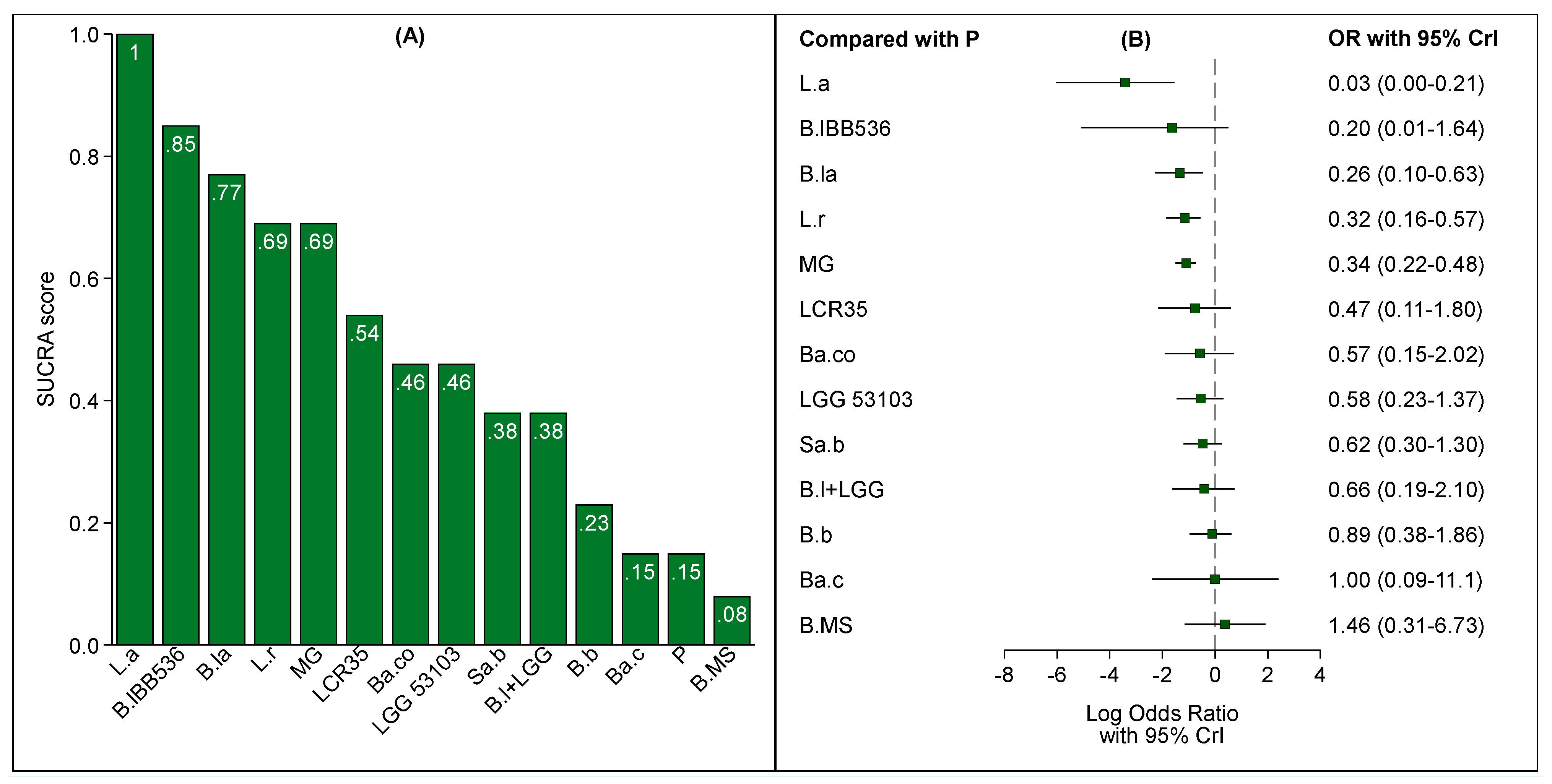
3.3. Probiotics and NEC: Overall Population
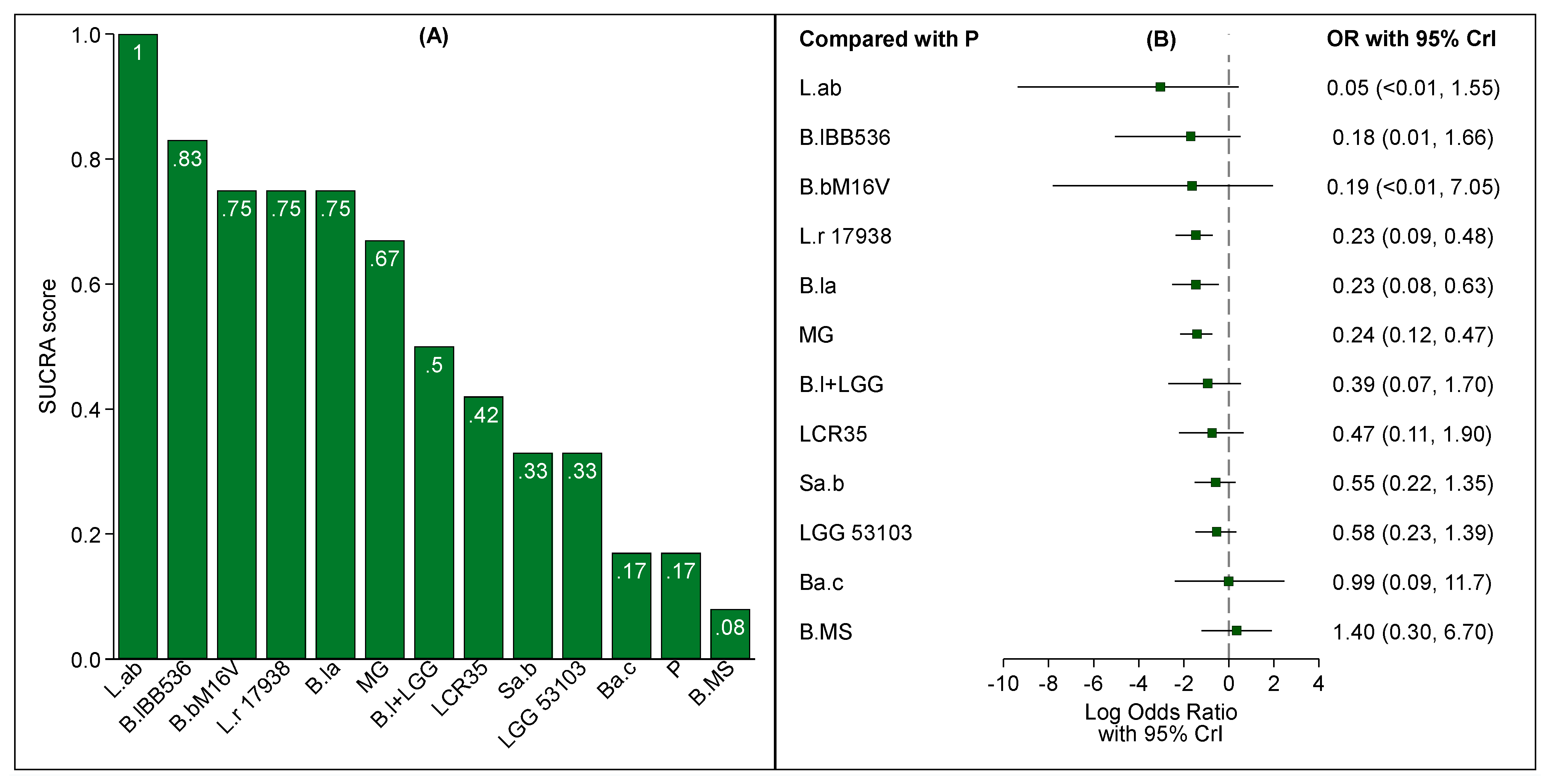
3.4. Probiotic and NEC According to Feeding Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supplementary Statistical Methods
References
- Alsaied, A.; Islam, N.; Thalib, L. Global incidence of Necrotizing Enterocolitis: A systematic review and Meta-analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Beghetti, I.; Martini, S.; Faldella, G.; Corvaglia, L. Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk. Oxid. Med. Cell. Longev. 2018, 2018, 7397659. [Google Scholar] [CrossRef] [PubMed]
- Shulhan, J.; Dicken, B.; Hartling, L.; Larsen, B.M. Current Knowledge of Necrotizing Enterocolitis in Preterm Infants and the Impact of Different Types of Enteral Nutrition Products. Adv. Nutr. 2017, 8, 80–91. [Google Scholar] [CrossRef]
- Altobelli, E.; Angeletti, P.M.; Verrotti, A.; Petrocelli, R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1322. [Google Scholar] [CrossRef]
- Chang, H.Y.; Chen, J.H.; Chang, J.H.; Lin, H.C.; Lin, C.Y.; Peng, C.C. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. PLoS ONE 2017, 12, e0171579. [Google Scholar] [CrossRef]
- Dermyshi, E.; Wang, Y.; Yan, C.; Hong, W.; Qiu, G.; Gong, X.; Zhang, T. The “golden Age” of Probiotics: A Systematic Review and Meta-Analysis of Randomized and Observational Studies in Preterm Infants. Neonatology 2017, 112, 9–23. [Google Scholar] [CrossRef]
- Hu, H.J.; Zhang, G.Q.; Zhang, Q.; Shakya, S.; Li, Z.Y. Probiotics Prevent Candida Colonization and Invasive Fungal Sepsis in Preterm Neonates: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pediatr. Neonatol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic supplementation and late-onset sepsis in preterm infants: A meta-analysis. Pediatrics 2016, 137, e20153684. [Google Scholar] [CrossRef]
- Sawh, S.C.; Deshpande, S.; Jansen, S.; Reynaert, C.J.; Jones, P.M. Prevention of necrotizing enterocolitis with probiotics: A systematic review and meta-analysis. PeerJ 2016, 4, e2429. [Google Scholar] [CrossRef]
- Sun, J.; Marwah, G.; Westgarth, M.; Buys, N.; Ellwood, D.; Gray, P.H. Effects of Probiotics on Necrotizing Enterocolitis, Sepsis, Intraventricular Hemorrhage, Mortality, Length of Hospital Stay, and Weight Gain in Very Preterm Infants: A Meta-Analysis. Adv. Nutr. 2017, 8, 749–763. [Google Scholar] [CrossRef]
- Thomas, J.P.; Raine, T.; Reddy, S.; Belteki, G. Probiotics for the prevention of necrotising enterocolitis in very low-birth-weight infants: A meta-analysis and systematic review. Acta Paediatr. 2017, 106, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- AlFaleh, K.; Anabrees, J.; Bassler, D.; Al-Kharfi, T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, 9, 584–671. [Google Scholar] [CrossRef]
- Athalye-Jape, G.; Rao, S.; Patole, S. Effects of probiotics on experimental necrotizing enterocolitis: A systematic review and meta-analysis. Pediatr. Res. 2018, 83, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Beghetti, I.; Maggio, L.; Martini, S.; Faldella, G.; Corvaglia, L. Filling the gaps: Current research directions for a rational use of probiotics in preterm infants. Nutrients 2018, 10, 1472. [Google Scholar] [CrossRef]
- Van den Akker, C.H.P.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R. Probiotics for preterm infants: A strain-specific systematic review and network meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar] [CrossRef]
- Van den Akker, C.H.P.; van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Berni Canani, R.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Pr. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [CrossRef]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B.; Chang, Y.; Florez, I.D.; Foroutan, F.; Shahid, S.; Zeraatkar, D. Probiotics Reduce Mortality and Morbidity in Preterm, Low Birth Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef]
- Repa, A.; Thanhaeuser, M.; Endress, D.; Weber, M.; Kreissl, A.; Binder, C.; Berger, A.; Haiden, N. Probiotics (Lactobacillus acidophilus and Bifidobacterium bifidum) prevent NEC in VLBW infants fed breast milk but not formula. Pediatr. Res 2015, 77, 381–388. [Google Scholar] [CrossRef]
- Aceti, A.; Maggio, L.; Beghetti, I.; Gori, D.; Barone, G.; Callegari, M.L.; Fantini, M.P.; Indrio, F.; Meneghin, F.; Morelli, L.; et al. Probiotics prevent late-onset sepsis in human milk-fed, very low birth weight preterm infants: Systematic review and meta-analysis. Nutrients 2017, 9, 904. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Scott, K.; Klaenhammer, T.R.; Quigley, E.; Sanders, M.E. Probiotic nomenclature matters. Gut Microbes 2016, 7, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpstonm, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D. WinBUGS—A Bayesian modelling framework: Concepts, structure, and extensibility. Stat. Comput. 2000, 10, 325–337. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef]
- Al-Hosni, M.; Duenas, M.; Hawk, M.; Stewart, L.A.; Borghese, R.A.; Cahoon, M.; Atwood, L.; Howard, D.; Ferrelli, K.; Soll, R. Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 2012, 32, 253–259. [Google Scholar] [CrossRef]
- Arora, S.; Khurana, M.S.; Saini, R. To study the role of probiotics in the prevention of necrotizing enterocolitis in preterm neonates. Int. J. Contemp. Pediatr. 2017, 4, 1792. [Google Scholar] [CrossRef]
- Awad, H.; Mokhtar, H.; Imam, S.S.; Gad, G.I.; Hafez, H.; Aboushady, N. Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pak. J. Biol. Sci. 2010, 13, 253–262. [Google Scholar] [CrossRef]
- Bin-Nun, A.; Bromiker, R.; Wilschanski, M.; Kaplan, M.; Rudensky, B.; Caplan, M.; Hammerman, C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J. Pediatr. 2005, 147, 192–196. [Google Scholar] [CrossRef]
- Braga, T.D.; da Silva, G.A.P.; de Lira, P.I.C.; de Carvalho Lima, M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: A double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Ali, M.M.; Hossain, M.M.; Singh, J.; Yousuf, A.N.M.; Yasmin, F.; Chowdhury, F.R. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: A double-blind randomized controlled trial. J. Coll. Physicians Surg. Pak. 2016, 26, 770–774. [Google Scholar] [PubMed]
- Costalos, C.; Skouteri, V.; Gounaris, A.; Sevastiadou, S.; Triandafilidou, A.; Ekonomidou, C.; Kontaxaki, F.; Petrochilou, V. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum. Dev. 2003, 74, 89–96. [Google Scholar] [CrossRef]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2015, 387, 649–660. [Google Scholar] [CrossRef]
- Cui, X.; Shi, Y.; Gao, S.; Xue, X.; Fu, J. Effects of Lactobacillus reuteri DSM 17938 in preterm infants: A double-blinded randomized controlled study. Ital. J. Pediatr. 2019, 45, 1–7. [Google Scholar] [CrossRef]
- Dani, C.; Biadaioli, R.; Bertini, G.; Martelli, E.; Rubaltelli, F.F. Probiotics Feeding in Prevention of Urinary Tract and Necrotizing Enterocolitis in Preterm Infants. Biol. Neonate 2002, 82, 103–108. [Google Scholar] [CrossRef]
- Demirel, G.; Erdeve, O.; Celik, I.H.; Dilmen, U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: A randomized, controlled study. Acta Paediatr. Int. J. Paediatr. 2013, 102, 560–565. [Google Scholar] [CrossRef]
- Dilli, D.; Aydin, B.; Fettah, N.; Özyazıcı, E.; Beken, S.; Zenciroğlu, A.; Okumuş, N.; Özyurt, B.; İpek, M.; Akdağ, A.; et al. The ProPre-Save Study: Effects of Probiotics and Prebiotics Alone or Combined on Necrotizing Enterocolitis in Very Low Birth Weight Infants. J. Pediatr. 2015, 28, 1537–1541. [Google Scholar] [CrossRef]
- Singh Dongol, S.; Klobassa, D.S.; Resch, B.; Urlesberger, B.; Shrestha, R.P.B. Placebo controlled introduction of prophylactic supplementation of probiotics to decrease the incidence of necrotizing enterocolitis at Dhulikhel Hospital in Nepal. Kathmandu Univ. Med. J. 2017, 15, 319–323. [Google Scholar]
- Dutta, S.; Ray, P.; Narang, A. Comparison of Stool Colonization in Premature Infants by Three Dose Regimes of a Probiotic Combination: A Randomized Controlled Trial. Am. J. Perinatol. 2015, 32, 733–740. [Google Scholar] [CrossRef]
- Fernández-Carrocera, L.A.; Solis-Herrera, A.; Cabanillas-Ayón, M.; Gallardo-Sarmiento, R.B.; García-Pérez, C.S.; Montaño-Rodríguez, R.; Echániz-Aviles, M.O.L. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F5. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Ohtsuka, Y.; Lee, T.; Kudo, T.; Shoji, H.; Sato, H.; Nagata, S.; Shimizu, T.; Yamashiro, Y. Bifidobacterium breve enhances transforming growth factor β1 signaling by regulating Smad7 expression in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gómez–Rodríguez, G.; Amador-Licona, N.; Daza-Benítez, L.; Barbosa-Sabanero, G.; Carballo-Magdaleno, D.; Aguilar-Padilla, R.; González-Ramirez, E. Single strain versus multispecies probiotic on necrotizing enterocolitis and faecal IgA levels in very low birth weight preterm neonates: A randomized clinical trial. Pediatr. Neonatol. 2019, 60, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.; Jacquot, A.; Gauthier, H.; Kempf, C.; Beissel, A.; Pidoux, O.; Jumas-Bilak, E.; Decullier, E.; Lachambre, E.; Beck, L.; et al. Probiotics and growth in preterm infants: A randomized controlled trial, PREMAPRO study. Clin. Nutr. 2015, 35, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Enríquez, N.P.; Rosas-Sumano, A.B.; Monzoy-Ventre, M.A.; Galicia-Flores, L. Lactobacillus reuteri DSM 17938 en la prevención de enterocolitis necrosante en recién nacidos prematuros. Estudio piloto de eficacia y seguridad. Rev. Mex. Pediatr. 2016, 83, 37–43. [Google Scholar]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M. Probiotic Effects on Late-onset Sepsis in Very Preterm Infants: A Randomized Controlled Trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef]
- Kaban, R.K.; Wardhana; Hegar, B.; Rohsiswatmo, R.; Handryastuti, S.; Amelia, N.; Muktiarti, D.; Indrio, F.; Vandenplas, Y. Lactobacillus reuteri DSM 17938 improves feeding intolerance in preterm infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 545–553. [Google Scholar] [CrossRef]
- Kanic, Z.; Micetic Turk, D.; Burja, S.; Kanic, V.; Dinevski, D. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien. Klin. Wochenschr. 2015, 127, 210–215. [Google Scholar] [CrossRef]
- Kitajima, H.; Sumida, Y.; Tanaka, R.; Yuki, N.; Takayama, H.; Fujimura, M. Early administration of Bifidobacterium breve to preterm infants: Randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 76, 101–108. [Google Scholar] [CrossRef]
- Lin, H.-C.; Su, B.-H.; Chen, A.-C.; Lin, T.-W.; Tsai, C.-H.; Yeh, T.-F.; Oh, W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005, 115, 1–4. [Google Scholar] [CrossRef]
- Lin, H.C.; Hsu, C.H.; Chen, H.L.; Chung, M.Y.; Hsu, J.F.; Lien, R.I.; Tsao, L.Y.; Chen, C.H.; Su, B.H. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A Multicenter, Randomized, Controlled trial. Pediatrics 2008, 122, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Mostert, M.; Leonessa, M.L.; Priolo, C.; Farina, D.; Monetti, C.; Latino, M.A.; Gomirato, G. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: A randomized study. Clin. Infect. Dis. 2006, 42, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Vossbeck, S.; Eikmanns, B.; Hoegel, J.; Pohlandt, F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: A randomized controlled trial. Neonatology 2010, 98, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Koebnick, C.; Schildt, J.; Schmidt, S.; Mueller, M.; Possner, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: A double-blind, placebo-controlled, randomized study. J. Clin. Microbiol. 2006, 44, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.Y.; Sari, F.N.; Arayici, S.; Guzoglu, N.; Erdeve, O.; Uras, N.; Oguz, S.S.; Dilmen, U. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F110–F115. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, T.; Nagata, S.; Wang, C.; Takahashi, T.; Tsuji, H.; Asahara, T.; Nomoto, K.; Takei, H.; Nittono, H.; Yamashiro, Y. Bifidobacterium Supplementation of Colostrum and Breast Milk Enhances Weight Gain and Metabolic Responses Associated with Microbiota Establishment in Very-Preterm Infants. Biomed. Hub 2019, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates—A randomised double blind placebo controlled trial. PLoS ONE 2014, 9, e89511. [Google Scholar] [CrossRef]
- Rojas, M.A.; Lozano, J.M.; Rojas, M.X.; Rodriguez, V.A.; Rondon, M.A.; Bastidas, J.A.; Perez, L.A.; Rojas, C.; Ovalle, O.; Garcia-Harker, J.E.; et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012, 130, e1113. [Google Scholar] [CrossRef]
- Romeo, M.G.; Romeo, D.M.; Trovato, L.; Oliveri, S.; Palermo, F.; Cota, F.; Betta, P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2011, 31, 63–69. [Google Scholar] [CrossRef]
- Rougé, C.; Piloquet, H.; Butel, M.-J.; Berger, B.; Rochat, F.; Ferraris, L.; Des Robert, C.; Legrand, A.; de la Cochetiere, M.-F.; N’Guyen, J.-M.; et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009, 89, 1828–1835. [Google Scholar] [CrossRef]
- Roy, A.; Chaudhuri, J.; Sarkar, D.; Ghosh, P.; Chakraborty, S. Role of enteric supplementation of probiotic on late-onset sepsisi by candida species in preterm low-boirth weight neonates: A randomized double blind, placebo-controlled trial. N. Am. J. Med. Sci. 2014, 6, 50–57. [Google Scholar] [PubMed]
- Saengtawesin, V.; Tangpolkaiwalsak, R.; Kanjanapattankul, W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J. Med. Assoc. Thai. 2014, 97 (Suppl. 6), S20–S25. [Google Scholar]
- Samanta, M.; Sarkar, M.; Ghosh, P.; Ghosh, J.K.; Sinha, M.K.; Chatterjee, S. Prophylactic probiotics for prevention of necrotizing enetrocolitis in very low birth weight newborns. J. Trop. Pediatr. 2008, 55, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.N.; Dizdar, E.A.; Oguz, S.; Erdeve, O.; Uras, N.; Dilmen, U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: A randomized, controlled trial. Eur. J. Clin. Nutr. 2011, 65, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Serce, O.; Benzer, D.; Gursoy, T.; Karatekin, G.; Ovali, F. Efficacy of saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: A randomised controlled trial. Early Hum. Dev. 2013, 89, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Shadkam, M.N.; Jalalizadeh, F.; Nasiriani, K. Effects of probiotic lactobacillus reuteri (DSM 17938) on the incidence of necrotizing enterocolitis in very low birth weight premature infants. Iran. J. Neonatol. 2015, 6, 15–20. [Google Scholar] [CrossRef]
- Shashidhar, A.; Suman Rao, P.N.; Nesargi, S.; Bhat, S.; Chandrakala, B.S. Probiotics for promoting feed tolerance in very low birth weight neonates—A randomized controlled trial. Indian Pediatr. 2017, 54, 363–367. [Google Scholar] [CrossRef]
- Stratiki, Z.; Costalos, C.; Sevastiadou, S.; Kastanidou, O.; Skouroliakou, M.; Giakoumatou, A.; Petrohilou, V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 2007, 83, 575–579. [Google Scholar] [CrossRef]
- Tewari, V.V.; Dubey, S.K.; Gupta, G. Bacillus clausii for Prevention of Late-onset Sepsis in Preterm Infants: A Randomized Controlled Trial. J. Trop. Pediatr. 2015, 61, 377–385. [Google Scholar] [CrossRef]
- Totsu, S.; Yamasaki, C.; Terahara, M.; Uchiyama, A.; Kusuda, S. Bifidobacterium and enteral feeding in preterm infants: Cluster-randomized trial. Pediatr. Int. 2014, 56, 714–719. [Google Scholar] [CrossRef]
- Hikaru, U.; Koichi, S.; Yayoi, S.; Hiromichi, S.; Hiroaki, S.; Yoshikazu, O.; Seigo, S.; Nagata, S.; Toshiaki, S.; Yamashiro, Y. Bifidobacteria prevents preterm infants from developing infection and sepsis. Int. J. Probiotics Prebiotics 2010, 5, 33–36. [Google Scholar]
- Usman, S.; Zareen, A.; Ali, M.; Sarwar, H.A.; Azhar, J.; Jamal, S. Probiotic prophylaxis in prevention of necrotizing enterocolitis—A case control study. Pakistan J. Med. Heal. Sci. 2018, 12, 1623–1626. [Google Scholar]
- Van Niekerk, E.; Nel, D.G.; Blaauw, R.; Kirsten, G.F. Probiotics reduce necrotizing enterocolitis severity in HIV-exposed premature infants. J. Trop. Pediatr. 2015, 61, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shoji, H.; Sato, H.; Nagata, S.; Ohtsuka, Y.; Shimizu, T.; Yamashiro, Y. Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Wejryd, E.; Marchini, G.; Frimmel, V.; Jonsson, B.; Abrahamsson, T. Probiotics promoted head growth in extremely low birth weight infants in a double-blind placebo-controlled trial. Acta Paediatr. 2019, 108, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.; Wang, Y.; Fu, J.; Sun, M.; Mao, Z.; Vandenplas, Y. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J. Pediatr. (Rio. J.) 2016, 92, 296–301. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Kulecka, M.; Ambrozkiewicz, F.; Paziewska, A.; Lechowicz, M.; Konopka, E.; Majewska, U.; Borszewska-Kornacka, M.; Mikula, M.; Cukrowska, B.; et al. Effect of saccharomyces boulardii and mode of delivery on the early development of the gut microbial community in preterm infants. PLoS ONE 2016, 11, e0150306. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Salzman, N.H.; Bennett, S.H.; Barman, M.; Mills, D.A.; Marcobal, A.; Tancredi, D.J.; Bevins, C.L.; Sherman, M.P. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: Impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 216–225. [Google Scholar] [CrossRef]
- Zhang, A.M.; Sun, Z.Q.; Zhang, L.M. Mosapride combined with probiotics on gastrointestinal function and growth in premature infants. Exp. Ther. Med. 2017, 13, 2675–2680. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Hart, W.; Albarracín, D.; Eagly, A.H.; Brechan, I.; Lindberg, M.J.; Merrill, L. Feeling Validated Versus Being Correct: A Meta-Analysis of Selective Exposure to Information. Psychol. Bull. 2009, 135, 555–588. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; Davey, J.; Clarke, M.J.; Thompson, S.G.; Higgins, J.P. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int. J. Epidemiol. 2012, 41, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Efthimiou, O. Practical guide to the meta-analysis of rare events. Evid. Based Ment. Health 2018, 21, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, M.J.; Sutton, A.J.; Lambert, P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 2004, 23, 1351–1375. [Google Scholar] [CrossRef]
- Brooks, S.; Gelman, A. Alternative methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar]
- Rücker, G.; Schwarzer, G.; Carpenter, J.; Olkin, I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat. Med. 2009, 28, 721–738. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Ades, A. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials; National Institute for Health and Care Excellence (NICE): London, UK, 2014. [Google Scholar]
- Veroniki, A.A.; Mavridis, D.; Higgins, J.P.; Salanti, G. Characteristics of a loop of evidence that affect detection and estimation of inconsistency: A simulation study. BMC Med. Res. Methodol. 2014, 14, 106. [Google Scholar] [CrossRef]
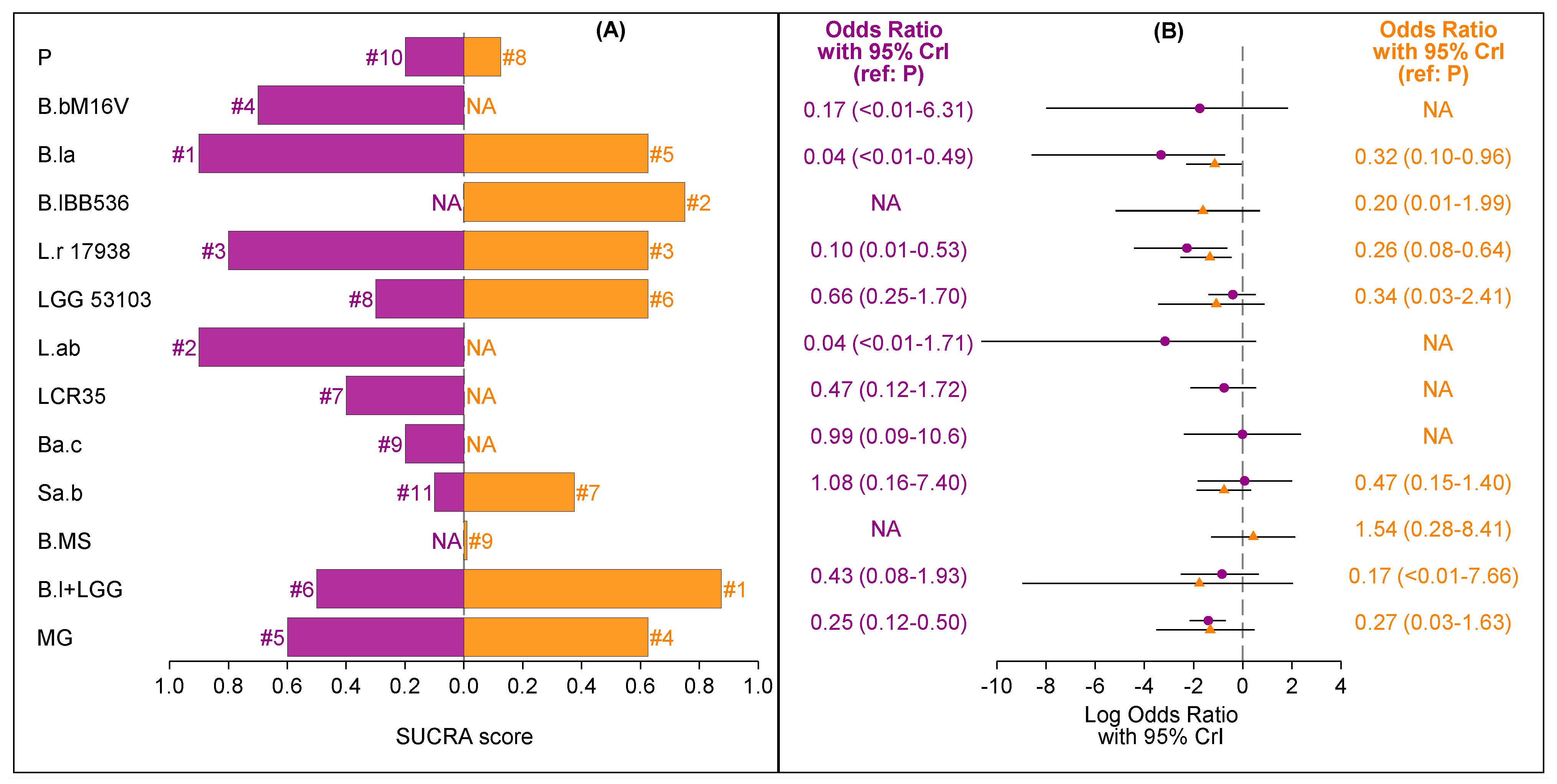
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beghetti, I.; Panizza, D.; Lenzi, J.; Gori, D.; Martini, S.; Corvaglia, L.; Aceti, A. Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Network Meta-Analysis. Nutrients 2021, 13, 192. https://doi.org/10.3390/nu13010192
Beghetti I, Panizza D, Lenzi J, Gori D, Martini S, Corvaglia L, Aceti A. Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Network Meta-Analysis. Nutrients. 2021; 13(1):192. https://doi.org/10.3390/nu13010192
Chicago/Turabian StyleBeghetti, Isadora, Davide Panizza, Jacopo Lenzi, Davide Gori, Silvia Martini, Luigi Corvaglia, and Arianna Aceti. 2021. "Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Network Meta-Analysis" Nutrients 13, no. 1: 192. https://doi.org/10.3390/nu13010192
APA StyleBeghetti, I., Panizza, D., Lenzi, J., Gori, D., Martini, S., Corvaglia, L., & Aceti, A. (2021). Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Network Meta-Analysis. Nutrients, 13(1), 192. https://doi.org/10.3390/nu13010192






