Serum Vitamin D Affected Type 2 Diabetes though Altering Lipid Profile and Modified the Effects of Testosterone on Diabetes Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Laboratory Measurements
2.4. Ascertainment of Cases
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Associations of Serum 25(OH)D3, 25(OH)D2 and Total 25(OH)D with Glucose Metabolism
3.3. Sensitivity Analyses
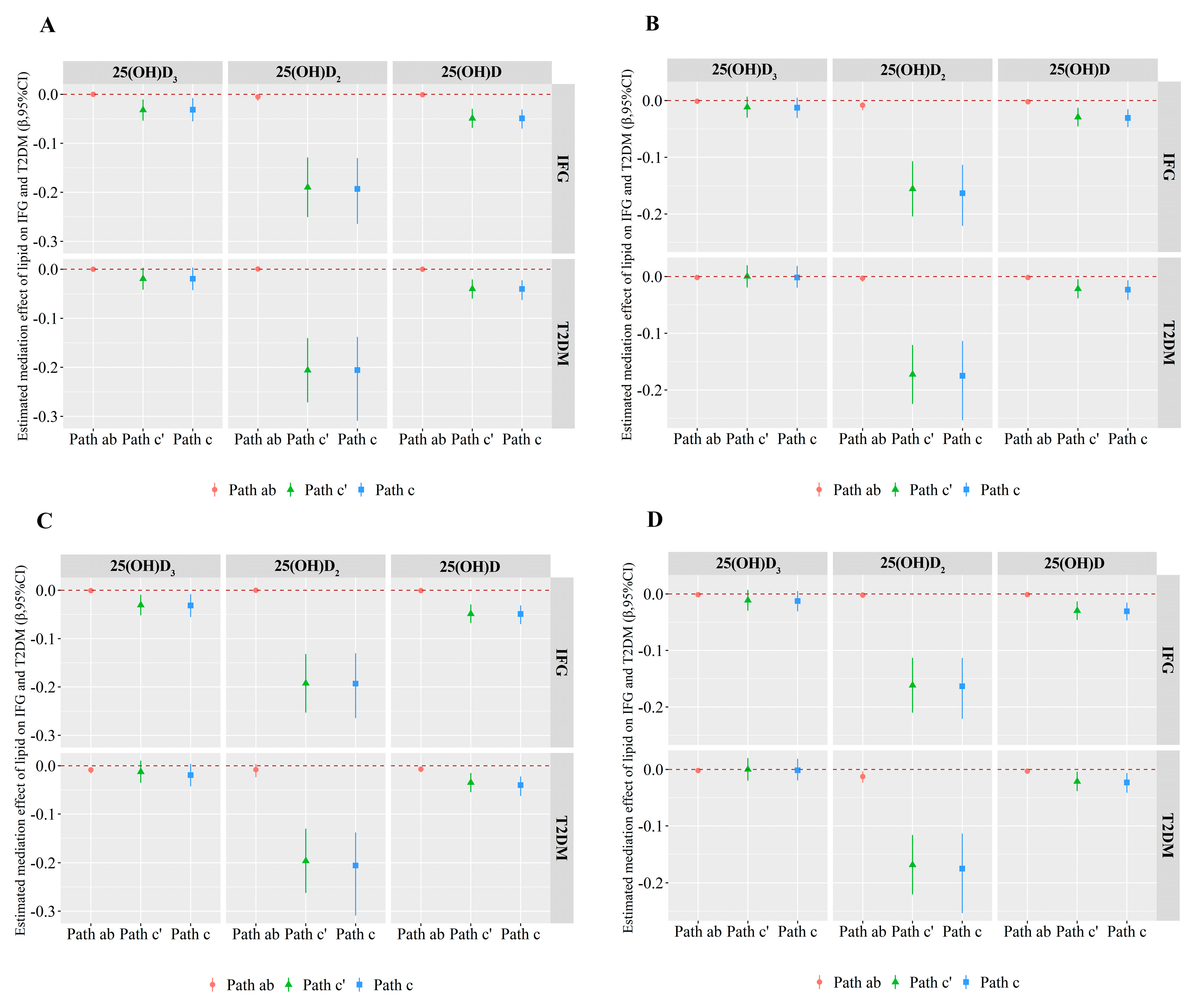
3.4. Mediation Effects
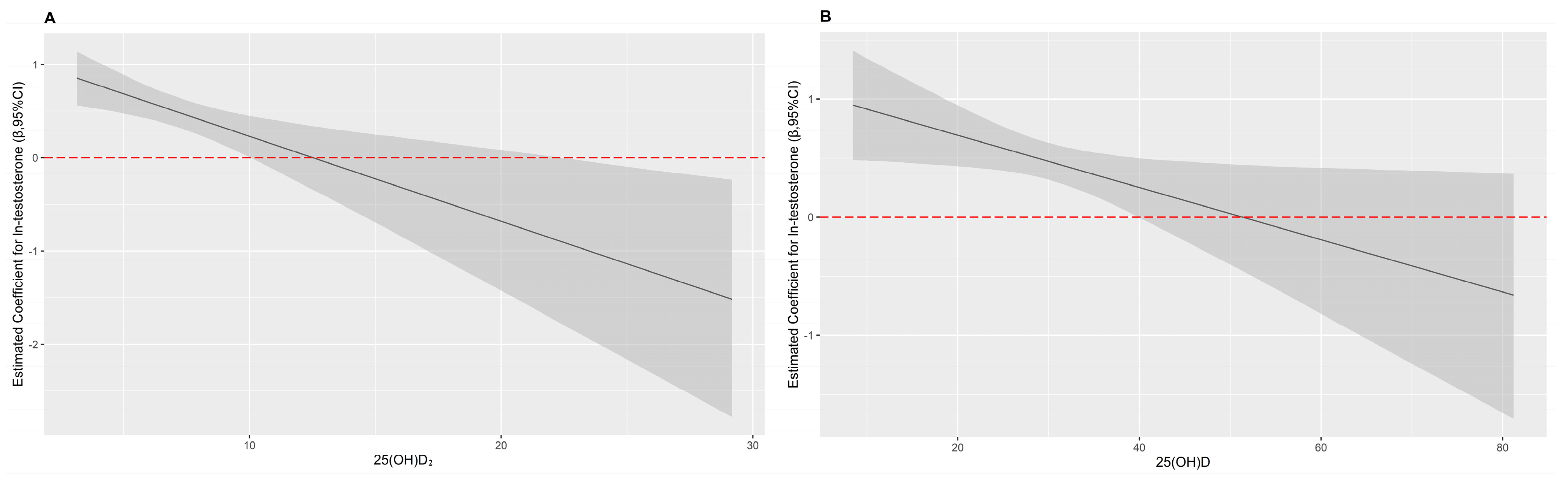
3.5. Interaction Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Li, L.; Zhang, L.; Ren, Y.; Zhou, H.; Cui, L.; Mao, Z.; Hu, D.; Wang, C. Prevalence, awareness, treatment, control of type 2 diabetes mellitus and risk factors in Chinese rural population: The RuralDiab study. Sci. Rep. 2016, 6, 31426. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 482–487. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wei, H.; Xue, H.; Zhang, J.; Chen, M.; Gong, Y.; Cheng, G. Higher serum 25(OH)D level is associated with decreased risk of impairment of glucose homeostasis: Data from Southwest China. BMC Endocr. Disord. 2018, 18, 25. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zheng, T.; Ran, X.; Ren, Y.; Chen, T.; Zhong, L.; Yan, D.; Yan, F.; Wu, Q.; Tian, H. Vitamin D and Incidence of Prediabetes or Type 2 Diabetes: A Four-Year Follow-Up Community-Based Study. Dis. Markers 2018, 2018, 1926308. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Lucas, T.S.; Duncan, A.M.; Rabasa-Lhoret, R.; Vieth, R.; Gibbs, A.L.; Badawi, A.; Wolever, T.M. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes. Metab. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and metaanalysis. Clin. Chem. 2013, 59, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Vitamin D Status, Calcium Intake and Risk of Developing Type 2 Diabetes: An Unresolved Issue. Nutrients 2019, 11, 642. [Google Scholar] [CrossRef] [Green Version]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Dastorani, M.; Aghadavod, E.; Mirhosseini, N.; Foroozanfard, F.; Zadeh Modarres, S.; Amiri Siavashani, M.; Asemi, Z. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod. Biol. Endocrinol. 2018, 16, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Lovegrove, J.A.; Givens, D.I. Vitamin D intake and risk of CVD and all-cause mortality: Evidence from the Caerphilly Prospective Cohort Study. Public Health Nutr. 2017, 20, 2744–2753. [Google Scholar] [CrossRef] [Green Version]
- Al-Ajlan, A.; Krishnaswamy, S.; Alokail, M.S.; Aljohani, N.J.; Al-Serehi, A.; Sheshah, E.; Alshingetti, N.M.; Fouda, M.; Turkistani, I.Z.; Al-Daghri, N.M. Vitamin D deficiency and dyslipidemia in early pregnancy. BMC Pregnancy Childbirth 2015, 15, 314. [Google Scholar] [CrossRef] [Green Version]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef]
- Jensen, M.B. Vitamin D and male reproduction. Nat. Rev. Endocrinol. 2014, 10, 175–186. [Google Scholar] [CrossRef]
- Chen, C.; Zhai, H.; Cheng, J.; Weng, P.; Chen, Y.; Li, Q.; Wang, C.; Xia, F.; Wang, N.; Lu, Y. Causal Link Between Vitamin D and Total Testosterone in Men: A Mendelian Randomization Analysis. J. Clin. Endocrinol. Metab. 2019, 104, 3148–3156. [Google Scholar] [CrossRef]
- Wang, N.; Han, B.; Li, Q.; Chen, Y.; Chen, Y.; Xia, F.; Lin, D.; Jensen, M.D.; Lu, Y. Vitamin D is associated with testosterone and hypogonadism in Chinese men: Results from a cross-sectional SPECT-China study. Reprod. Biol. Endocrinol. 2015, 13, 74. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Sforza, A.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Type 2 diabetes mellitus and testosterone: A meta-analysis study. Int. J. Androl. 2011, 34, 528–540. [Google Scholar] [CrossRef]
- Grossmann, M. Testosterone and glucose metabolism in men: Current concepts and controversies. J. Endocrinol. 2014, 220, R37–R55. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, J.; Liu, X.; Luo, Z.; Wang, Y.; Dong, X.; Wei, D.; Li, R.; Wang, Y.; Huo, W.; et al. Association of serum testosterone with different classes of glucose metabolism and the mediation effect of obesity: The Henan Rural Cohort Study. Diabetes/Metab. Res. Rev. 2019, 35, e3133. [Google Scholar] [CrossRef]
- Liu, X.; Mao, Z.; Li, Y.; Wu, W.; Zhang, X.; Huo, W.; Yu, S.; Shen, L.; Li, L.; Tu, R.; et al. Cohort Profile: The Henan Rural Cohort: A prospective study of chronic non-communicable diseases. Int. J. Epidemiol. 2019, 48, 1756–1756j. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [Green Version]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahwan, M.J.; Jairoun, A.A.; Farajallah, A.; Shanabli, S. Prevalence of dyslipidemia and factors affecting lipid profile in patients with type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.K.; Garland, C.F.; Gorham, E.D.; BuDoff, L.; Barrett-Connor, E. Plasma 25-hydroxyvitamin D concentration and risk of type 2 diabetes and pre-diabetes: 12-year cohort study. PLoS ONE 2018, 13, e0193070. [Google Scholar] [CrossRef] [Green Version]
- Kositsawat, J.; Freeman, V.L.; Gerber, B.S.; Geraci, S. Association of A1C levels with vitamin D status in U.S. adults: Data from the National Health and Nutrition Examination Survey. Diabetes Care 2010, 33, 1236–1238. [Google Scholar] [CrossRef] [Green Version]
- McGill, A.T.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhatatbeh, M.J.; Abdul-Razzak, K.K. Association between serum 25-hydroxyvitamin D, hemoglobin A1c and fasting blood glucose levels in adults with diabetes mellitus. Biomed. Rep. 2018, 9, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, A.K.; Williamson, E.J.; Hodge, A.M.; Ebeling, P.R.; Eyles, D.W.; Kvaskoff, D.; O’Dea, K.; Giles, G.G.; English, D.R. Vitamin D status and the risk of type 2 diabetes: The Melbourne Collaborative Cohort Study. Diabetes Res. Clin. Pract. 2019, 149, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.A.; Tosh, A.K.; Belenchia, A.M. Vitamin D insufficiency and insulin resistance in obese adolescents. Ther. Adv. Endocrinol. Metab. 2014, 5, 166–189. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Misra, A.; Pandey, R.M.; Upadhyay, A.D.; Gulati, S.; Singh, N. Vitamin D Supplementation in Overweight/obese Asian Indian Women with Prediabetes Reduces Glycemic Measures and Truncal Subcutaneous Fat: A 78 Weeks Randomized Placebo-Controlled Trial (PREVENT-WIN Trial). Sci. Rep. 2020, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Farrokhian, A.; Raygan, F.; Bahmani, F.; Talari, H.R.; Esfandiari, R.; Esmaillzadeh, A.; Asemi, Z. Long-Term Vitamin D Supplementation Affects Metabolic Status in Vitamin D-Deficient Type 2 Diabetic Patients with Coronary Artery Disease. J. Nutr. 2017, 147, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Buijsse, B.; Boeing, H.; Hirche, F.; Weikert, C.; Schulze, M.B.; Gottschald, M.; Kuhn, T.; Katzke, V.A.; Teucher, B.; Dierkes, J.; et al. Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident type 2 diabetes: A prospective case-cohort study. Eur. J. Epidemiol. 2013, 28, 743–752. [Google Scholar] [CrossRef]
- Zheng, J.S.; Imamura, F.; Sharp, S.J.; van der Schouw, Y.T.; Sluijs, I.; Gundersen, T.E.; Ardanaz, E.; Boeing, H.; Bonet, C.; Gomez, J.H.; et al. Association of Plasma Vitamin D Metabolites With Incident Type 2 Diabetes: EPIC-InterAct Case-Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 1293–1303. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Menon, R.K.; Sharp, S.J.; Mannan, N.; Timms, P.M.; Martineau, A.R.; Rickard, A.P.; Boucher, B.J.; Chowdhury, T.A.; Griffiths, C.J.; et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016, 18, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, A.; Naderpoor, N.; de Courten, M.P.; Teede, H.; Kellow, N.; Walker, K.; Scragg, R.; de Courten, B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2017, 105, 1372–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longenecker, C.T.; Hileman, C.O.; Carman, T.L.; Ross, A.C.; Seydafkan, S.; Brown, T.T.; Labbato, D.E.; Storer, N.; Tangpricha, V.; McComsey, G.A. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: A randomized placebo-controlled trial. Antivir. Ther. 2012, 17, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Grammatiki, M.; Rapti, E.; Karras, S.; Ajjan, R.A.; Kotsa, K. Vitamin D and diabetes mellitus: Causal or casual association? Rev. Endocr. Metab. Disord. 2017, 18, 227–241. [Google Scholar] [CrossRef]
- Liu, X.; Yu, S.; Mao, Z.; Li, Y.; Zhang, H.; Yang, K.; Zhang, H.; Liu, R.; Qian, X.; Li, L.; et al. Dyslipidemia prevalence, awareness, treatment, control, and risk factors in Chinese rural population: The Henan rural cohort study. Lipids Health Dis. 2018, 17, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemel, M.B. Regulation of adiposity and obesity risk by dietary calcium: Mechanisms and implications. J. Am. Coll. Nutr. 2002, 21, 146s–151s. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [Green Version]
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.J. 25-hydroxyvitamin D₃ and 1,25-dihydroxyvitamin D₃ promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Hadžija, M.P.; Siketić, Z.; Hadžija, M.; Barac, M.; Radović, I.B. Study of the diacylglycerol composition in the liver and serum of mice with prediabetes and diabetes using MeV TOF-SIMS. Diabetes Res. Clin. Pract. 2020, 159, 107986. [Google Scholar] [CrossRef] [Green Version]
- Sonnenberg, J.; Luine, V.N.; Krey, L.C.; Christakos, S. 1,25-Dihydroxyvitamin D3 Treatment Results in Increased Choline Acetyltransferase Activity in Specific Brain Nuclei. Endocrinology 1986, 118, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Frisch, S.; Koertke, H.; Kuhn, J.; Dreier, J.; Obermayer-Pietsch, B.; Wehr, E.; Zittermann, A. Effect of vitamin D supplementation on testosterone levels in men. Horm. Metab. Res. 2011, 43, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tian, Y.; Wu, T.; Cao, C.X.; Li, H.; Wang, K.J. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Asian J. Androl. 2014, 16, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Gianatti, E.J.; Grossmann, M. Testosterone deficiency in men with Type 2 diabetes: Pathophysiology and treatment. Diabet. Med. 2020, 37, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.; Matsumoto, M.; Tamura, Y. Potential application of testosterone replacement therapy as treatment for obesity and type 2 diabetes in men. Steroids 2018, 138, 161–166. [Google Scholar] [CrossRef]
- Salminen, M.; Vahlberg, T.; Raiha, I.; Niskanen, L.; Kivela, S.L.; Irjala, K. Sex hormones and the risk of type 2 diabetes mellitus: A 9-year follow up among elderly men in Finland. Geriatr. Gerontol. Int. 2015, 15, 559–564. [Google Scholar] [CrossRef]
- Selva, D.M.; Hirsch-Reinshagen, V.; Burgess, B.; Zhou, S.; Chan, J.; McIsaac, S.; Hayden, M.R.; Hammond, G.L.; Vogl, A.W.; Wellington, C.L. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J. Lipid Res. 2004, 45, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Parikh, G.; Varadinova, M.; Suwandhi, P.; Araki, T.; Rosenwaks, Z.; Poretsky, L.; Seto-Young, D. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm. Metab. Res. 2010, 42, 754–757. [Google Scholar] [CrossRef] [Green Version]
- Yu, I.C.; Lin, H.Y.; Liu, N.C.; Sparks, J.D.; Yeh, S.; Fang, L.Y.; Chen, L.; Chang, C. Neuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-kappaB-mediated PTP1B expression. Diabetes 2013, 62, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens Decrease Plasma Adiponectin, an Insulin-Sensitizing Adipocyte-Derived Protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef] [Green Version]

| Variables | NGT | IFG | T2DM | p Value | |
|---|---|---|---|---|---|
| IFG vs. NGT | T2DM vs. NGT | ||||
| Subjects, n | 897 | 913 | 849 | ||
| Age, (years) | 61 (54, 66) | 61 (54, 66) | 61 (54, 66) | 0.961 | 0.970 |
| Male, n (%) | 344 (38.35) | 352 (38.55) | 324 (38.16) | 0.929 | 0.936 |
| BMI, (kg/m2) | 23.42 (21.18, 25.75) | 24.22 (22.12, 26.53) | 25.43 (23.48, 27.68) | <0.001 | <0.001 |
| Smoking status, n (%) | 0.700 | 0.038 | |||
| Never smoking | 667 (74.36) | 692 (75.79) | 625 (73.62) | ||
| Previous smoker | 57 (6.35) | 59 (6.46) | 80 (9.42) | ||
| Current smoker | 173 (19.29) | 162 (17.74) | 144 (16.96) | ||
| Alcohol intake, n (%) | 0.506 | 0.298 | |||
| Never drinking | 737 (82.16) | 766 (83.90) | 675 (79.51) | ||
| Previous drinker | 48 (5.35) | 49 (5.37) | 58 (6.83) | ||
| Current drinker | 112 (12.49) | 98 (10.73) | 116 (13.66) | ||
| Physical activity, n (%) | 0.520 | 0.040 | |||
| Low | 213 (23.75) | 238 (26.07) | 245 (28.86) | ||
| Mediate | 453 (50.50) | 446 (48.85) | 388 (45.70) | ||
| High | 231 (25.75) | 229 (25.08) | 216 (25.44) | ||
| Average monthly individual income, n (%) | 0.228 | 0.309 | |||
| <500, CNY | 352 (39.24) | 381 (41.73) | 345 (40.64) | ||
| 500~, CNY | 279 (31.10) | 294 (32.20) | 236 (27.80) | ||
| 1000~, CNY | 266 (29.65) | 238 (26.07) | 268 (31.57) | ||
| Level of education, n (%) | 0.749 | 0.515 | |||
| Never attended school | 238 (26.53) | 228 (24.97) | 207 (24.38) | ||
| Primary school | 271 (30.21) | 281 (30.78) | 255 (30.03) | ||
| Junior secondary and above | 388 (43.26) | 404 (44.25) | 387 (45.58) | ||
| Family history of T2DM, n (%) | 15 (1.67) | 14 (1.53) | 45 (5.30) | 0.814 | <0.001 |
| SBP, (mmHg) | 118.00 (108.00, 131.00) | 121.00 (110.00, 134.00) | 125.00 (114.00, 138.00) | <0.001 | <0.001 |
| PP, (mmHg) | 45.00 (39.00, 53.00) | 49.00 (42.00, 57.00) | 47.00 (39.00, 56.00) | 0.022 | <0.001 |
| TC, (mmol/L) | 4.60 (0.80) | 4.83 (4.25, 5.44) | 4.79, (4.21, 5.50) | <0.001 | <0.001 |
| TG, (mmol/L) | 1.41 (1.00, 1.95) | 1.64 (1.11, 2.31) | 1.92 (1.34, 2.94) | <0.001 | <0.001 |
| HDL-C, (mmol/L) | 1.37 (1.16, 1.62) | 1.32 (1.12, 1.57) | 1.23 (1.02, 1.47) | 0.010 | <0.001 |
| LDL-C, (mmol/L) | 2.82 (0.71) | 3.00 (2.47, 3.50) | 2.83 (0.95) | <0.001 | 0.583 |
| FPG, (mmol/L) | 4.94 (0.46) | 5.30 (4.89, 5.82) | 7.94 (7.00, 10.21) | <0.001 | <0.001 |
| HbA1c, (%) | 5.30 (5.10, 5.50) | 5.90 (5.70, 6.00) | 7.40 (6.60, 8.90) | <0.001 | <0.001 |
| INS, (uIU/mL) | 11.94 (9.31, 15.20) | 12.59 (9.86, 16.33) | 14.12 (11.40, 18.52) | 0.001 | <0.001 |
| Testosterone, (ng/mL) | |||||
| Male | 2.30 (1.70, 3.00) | 2.00 (1.40, 2.70) | 1.80 (1.30, 2.50) | <0.001 | <0.001 |
| Female | 0.10 (0.02, 0.10) | 0.10 (0.02, 0.10) | 0.10 (0.10, 0.10) | 0.053 | <0.001 |
| 25(OH)D3, (ng/mL) | 23.07 (19.02, 28.00) | 22.07 (18.09, 27.03) | 22.87 (18.30, 28.04) | <0.001 | 0.182 |
| 25(OH)D2, (ng/mL) | 7.60 (6.04, 9.84) | 6.46 (5.43, 7.85) | 6.30 (5.23, 7.80) | <0.001 | <0.001 |
| 25(OH)D, (ng/mL) | 31.12 (26.29, 37.50) | 29.26 (24.61, 34.23) | 29.77 (24.84, 35.51) | <0.001 | <0.001 |
| Variables | Male ORs (95% CIs) | Female ORs (95% CIs) | ||||
|---|---|---|---|---|---|---|
| Vitamin D | Ln-Testosterone | p for Interaction | Vitamin D | Ln-Testosterone | p for Interaction | |
| IFG | ||||||
| 25(OH)D3 | 0.97 (0.95, 0.99) * | - | - | 0.99 (0.98, 1.01) | - | - |
| 25(OH)D2 | 0.83 (0.78, 0.88) * | - | - | 0.86 (0.82, 0.90) * | - | - |
| 25(OH)D | 0.95 (0.93, 0.97) * | - | - | 0.98 (0.96, 0.99) * | - | - |
| Ln-testosterone | - | 0.82 (0.63, 1.10) | - | - | 1.12 (0.99, 1.28) | - |
| 25(OH)D3 + Ln-testosterone | 0.97 (0.95, 0.99) * | 0.87 (0.66, 1.13) | - | 1.00 (0.98, 1.01) | 1.12 (0.99, 1.28) | - |
| 25(OH)D2 + Ln-testosterone | 0.83 (0.78, 0.88) * | 0.90 (0.69, 1.17) | - | 0.86 (0.81, 0.90) * | 1.15 (1.01, 1.31) * | - |
| 25(OH)D + Ln-testosterone | 0.95 (0.94, 0.97) * | 0.92 (0.70, 1.20) | - | 0.98 (0.96, 0.99) * | 1.12 (0.98, 1.28) | - |
| 25(OH)D3 + Ln-testosterone + 25(OH)D3 × Ln-testosterone | 0.98 (0.95, 1.02) | 1.10 (0.47, 2.58) | 0.559 | 0.95 (0.90, 1.01) | 1.57 (1.02, 2.42) | 0.109 |
| 25(OH)D2 + Ln-testosterone + 25(OH)D2 × Ln-testosterone | 0.82 (0.74, 0.90) * | 0.77 (0.29, 2.01) | 0.739 | 0.91 (0.79, 1.06) | 0.97 (0.66, 1.44) | 0.370 |
| 25(OH)D + Ln-testosterone + 25(OH)D × Ln-testosterone | 0.96 (0.92, 0.99) * | 0.98 (0.33, 2.95) | 0.906 | 0.95 (0.90, 1.00)* | 1.51 (0.91, 2.50) | 0.224 |
| T2DM | ||||||
| 25(OH)D3 | 0.98 (0.96, 1.01) | - | - | 1.00 (0.98, 1.02) | - | - |
| 25(OH)D2 | 0.82 (0.77, 0.88) * | - | - | 0.86 (0.81, 0.90) * | - | - |
| 25(OH)D | 0.96 (0.94, 0.98) * | - | - | 0.98 (0.97, 1.00) * | - | - |
| Ln-testosterone | - | 0.76 (0.58, 1.00) | - | - | 1.61 (1.38, 1.87) * | - |
| 25(OH)D3 + Ln-testosterone | 0.99 (0.96, 1.01) | 0.78 (0.59, 1.03) | - | 1.01 (0.99, 1.03) | 1.61 (1.38, 1.88) * | - |
| 25(OH)D2 + Ln-testosterone | 0.83 (0.77, 0.88) * | 0.82 (0.62, 1.09) | - | 0.85 (0.81, 0.90) * | 1.62 (1.38, 1.89) * | - |
| 25(OH)D + Ln-testosterone | 0.97 (0.95, 0.99) * | 0.82 (0.62, 1.09) | - | 0.99 (0.97, 1.00) | 1.60 (1.37, 1.86) * | - |
| 25(OH)D3 + Ln-testosterone + 25(OH)D3 × Ln-testosterone | 0.99 (0.95, 1.02) | 0.85 (0.37, 1.98) | 0.838 | 0.96 (0.90, 1.02) | 2.51 (1.45, 4.37) * | 0.099 |
| 25(OH)D2 + Ln-testosterone + 25(OH)D2 × Ln-testosterone | 0.81 (0.73, 0.90) * | 0.64 (0.25, 1.65) | 0.582 | 0.66 (0.55, 0.79) * | 3.14 (1.99, 4.96) * | 0.002 |
| 25(OH)D + Ln-testosterone + 25(OH)D × Ln-testosterone | 0.96 (0.93, 0.99) * | 0.68 (0.24, 1.95) | 1.006 | 0.93 (0.88, 0.98) * | 3.14 (1.68, 5.88) * | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, X.; Hou, J.; Wei, D.; Liu, P.; Fan, K.; Zhang, L.; Nie, L.; Li, X.; Huo, W.; et al. Serum Vitamin D Affected Type 2 Diabetes though Altering Lipid Profile and Modified the Effects of Testosterone on Diabetes Status. Nutrients 2021, 13, 90. https://doi.org/10.3390/nu13010090
Wang L, Liu X, Hou J, Wei D, Liu P, Fan K, Zhang L, Nie L, Li X, Huo W, et al. Serum Vitamin D Affected Type 2 Diabetes though Altering Lipid Profile and Modified the Effects of Testosterone on Diabetes Status. Nutrients. 2021; 13(1):90. https://doi.org/10.3390/nu13010090
Chicago/Turabian StyleWang, Lulu, Xue Liu, Jian Hou, Dandan Wei, Pengling Liu, Keliang Fan, Li Zhang, Luting Nie, Xing Li, Wenqian Huo, and et al. 2021. "Serum Vitamin D Affected Type 2 Diabetes though Altering Lipid Profile and Modified the Effects of Testosterone on Diabetes Status" Nutrients 13, no. 1: 90. https://doi.org/10.3390/nu13010090





