Congenital, Intrapartum and Postnatal Maternal-Fetal-Neonatal SARS-CoV-2 Infections: A Narrative Review
Abstract
:1. Introduction
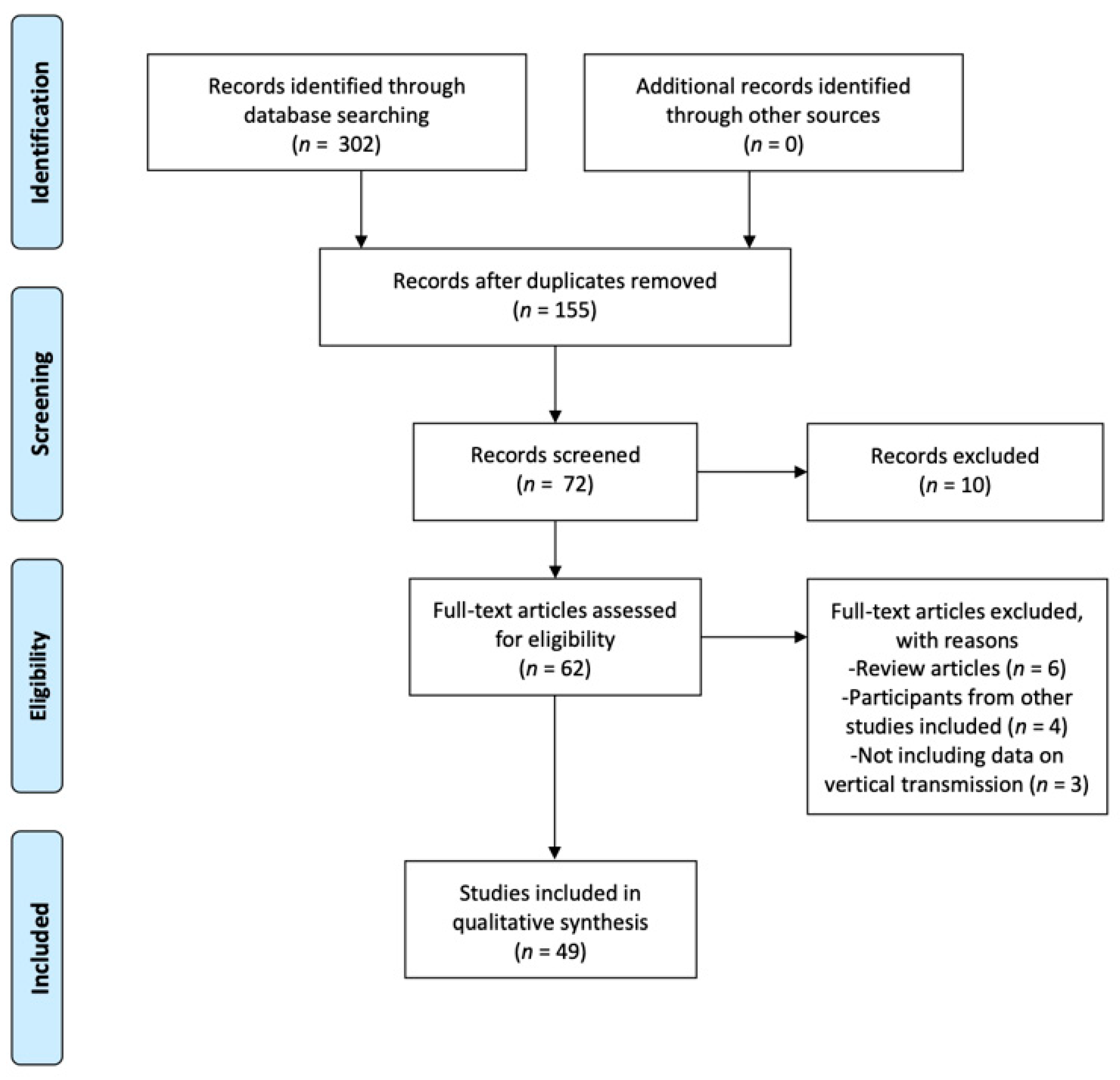
2. Materials and Methods
3. Results
3.1. Congenital, Intrapartum, and Postnatal Maternal-Fetal-Neonatal SARS-CoV-2 Infection
3.1.1. Amniotic Fluid
3.1.2. Umbilical Cord Blood
3.1.3. Placenta
3.1.4. Cervical Secretion
3.1.5. Breast Milk
4. Discussion
4.1. Amniotic Fluid
4.2. Umbilical Cord Blood
4.3. Placenta
4.4. Breastfeeding
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez-Sánchez, E.; Ramírez-Vargas, G.; Avellaneda-López, Y.; Orellana-Pecino, J.I.; García-Marín, E.; Díaz-Jimenez, J. Eating Habits and Physical Activity of the Spanish Population during the COVID-19 Pandemic Period. Nutrients 2020, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Responding to Community Spread of COVID-19: Interim Guidance. 2020, p. 1. Available online: https://apps.who.int/iris/bitstream/handle/10665/331421/WHO-COVID-19-Community_Transmission-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 20 November 2020).
- Caparros-Gonzalez, R.A. Maternal and neonatal consequences of coronavirus COVID-19 infection during pregnancy: A scoping review. Rev. Esp. Salud Publica 2020, 94, e202004033. [Google Scholar] [PubMed]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, M.; Zhu, Z.; Liu, Y. Coronavirus disease 2019 (COVID-19) and pregnancy: A systematic review. J. Matern. Neonatal Med. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Sighaldeh, S.S.; Kalan, M.E. Care of newborns born to mothers with COVID-19 infection; a review of existing evidence. J. Matern. Neonatal Med. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Huo, S.; Ma, Y.; Ke, Y.; Wang, P.; Zhao, A. Emotional Eating in Pregnant Women during the COVID-19 Pandemic and Its Association with Dietary Intake and Gestational Weight Gain. Nutrients 2020, 12, 2250. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Lin, X.-G.; Liu, Y.-Y.; Wu, J.-L.; Sharifu, L.M.; Hu, X.-L.; Rong, Z.-H.; Liu, W.; Luo, X.-P.; et al. Experience of Clinical Management for Pregnant Women and Newborns with Novel Coronavirus Pneumonia in Tongji Hospital, China. Curr. Med. Sci. 2020, 40, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.S.; Diambomba, Y.; Acharya, G.; Morris, S.K.; Bitnun, A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. et Gynecol. Scand. 2020, 99, 565–568. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, R.; Zheng, S.; Chen, X.; Wang, J.; Sheng, X.; Zhou, J.; Cai, H.; Fang, Q.; Yu, F.; et al. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg. Infect. Dis. 2020, 26, 1335–1336. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Li, W.; Zhou, Z.; Liu, S.; Rong, Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med. 2020, 14, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Wang, J.; Mo, Y.; Duan, W.; Xiang, G.; Yi, M.; Bao, L.; Shi, Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: A case report. J. Infect. Public Heal. 2020, 13, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Stonoga, E.T.S.; Lanzoni, L.D.A.; Rebutini, P.Z.; De Oliveira, A.L.P.; Chiste, J.A.; Fugaça, C.A.; Prá, D.M.M.; Percicote, A.P.; Rossoni, A.; Nogueira, M.B.; et al. Intrauterine Transmission of SARS-CoV-2. Emerg. Infect. Dis. 2021, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Z.; Zhang, J.; Zhu, F.; Tang, Y.; Shen, X. A Case of 2019 Novel Coronavirus in a Pregnant Woman with Preterm Delivery. Clin. Infect. Dis. 2020, 71, 844–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Wei, H.; Zhang, Z.; Chang, J.; Ma, X.; Gao, X.; Chen, Q.; Pang, Q. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J. Med. Virol. 2020, 92, 1657–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Wang, X.; Liu, P.; Wei, C.; He, B.; Zheng, J.; Zhao, D. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J. Clin. Virol. 2020, 127, 104356. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, W.; Kang, Q.; Zeng, W.; Feng, L.; Wu, J. No SARS-CoV-2 detected in amniotic fluid in mid-pregnancy. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Zamaniyan, M.; Ebadi, A.; Aghajanpoor, M.S.; Rahmani, Z.; Haghshenas, M.; Azizi, S. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat. Diagn. 2020. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Cao, J.D.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Zambrano, L.I.; Fuentes-Barahona, I.C.; Bejarano-Torres, D.A.; Bustillo, C.; Gonzales, G.; Vallecillo-Chinchilla, G.; Sanchez-Martínez, F.E.; Valle-Reconco, J.A.; Sierra, M.; Bonilla-Aldana, D.K.; et al. A pregnant woman with COVID-19 in Central America. Travel Med. Infect. Dis. 2020, 36, 101639. [Google Scholar] [CrossRef]
- Khan, S.; Peng, L.; Siddique, R.; Nabi, G.; Xue, M.; Liu, J.; Han, G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control Hosp. Epidemiol. 2020, 41, 748–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, N.; Li, W.; Kang, Q.; Xiong, Z.; Wang, S.; Lin, X.; Liu, Y.; Xiao, J.; Liu, H.; Deng, D.; et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020, 20, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. JAMA 2020, 323, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Rajput, U.; Dawre, R.; Valvi, C.; Nagpal, R.; Magdum, N.; Vankar, H.; Sonkawade, N.; Das, A.; Vartak, S.; et al. Early-onset symptomatic neonatal COVID-19 infection with high probability of vertical transmission. Infection 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Wang, S.; Guo, L.; Chen, L.; Liu, W.; Cao, Y.; Zhang, J.; Feng, L. A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin. Infect. Dis. 2020, 71, 853–857. [Google Scholar] [CrossRef]
- Yang, H.; Sun, G.; Tang, F.; Peng, M.; Gao, Y.; Peng, J.; Xie, H.; Zhao, Y.; Jin, Z. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J. Infect. 2020, 81, e40–e44. [Google Scholar] [CrossRef]
- Patanè, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2020, 2, 100145. [Google Scholar] [CrossRef]
- Chen, S.; Huang, B.; Luo, D.J.; Li, X.; Yang, F.; Zhao, Y.; Nie, X.; Huang, B.X. Pregnancy with new coronavirus infection: A clinical characteristics and placental pathological analysis of three cases. Chin. J. Pathol. 2020, 49, 418–423. [Google Scholar] [CrossRef]
- Chen, S.; Liao, E.; Cao, D.; Gao, Y.; Sun, G.; Shao, Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J. Med. Virol. 2020, 92, 1556–1561. [Google Scholar] [CrossRef] [Green Version]
- Penfield, C.A.; Brubaker, S.G.; Limaye, M.A.; Lighter, J.; Ratner, A.J.; Thomas, K.M.; Meyer, J.A.; Roman, A.S. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM 2020, 2, 100133. [Google Scholar] [CrossRef]
- Kirtsman, M.; Diambomba, Y.; Poutanen, S.M.; Malinowski, A.K.; Vlachodimitropoulou, E.; Parks, W.T.; Erdman, L.; Morris, S.K.; Shah, P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. Can. Med Assoc. J. 2020, 192, E647–E650. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Hsu, A.L.; Guan, M.; Do, E.J.; Stephens, A.J.; Khaleel, N.; Kagan, N.; Tuhlei, B.C.; Wan, X. Placental SARS-CoV-2 in a Pregnant Woman with Mild COVID-19 Disease. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, F.; Bugatti, M.; Drera, E.; Tripodo, C.; Sartori, E.; Cancila, V.; Papaccio, M.; Castellani, R.; Casola, S.; Boniotti, M.B.; et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. Ebiomedicine 2020, 59, 102951. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS–CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 275–278. [Google Scholar] [CrossRef]
- Grob, R.; Conzelmann, C.; Müller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Münch, J. Detection of SARS-CoV-2 in Human Breast Milk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef]
- Tam, P.C.K.; Ly, K.M.; Kernich, M.L.; Spurrier, N.; Lawrence, D.; Gordon, D.L.; Tucker, E.C. Detectable Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Human Breast Milk of a Mildly Symptomatic Patient With Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Bastug, A.; Hanifehnezhad, A.; Tayman, C.; Ozkul, A.; Ozbay, O.; Kazancioglu, S.; Bodur, H. Virolactia in an Asymptomatic Mother with COVID-19. Breastfeed. Med. 2020, 15, 488–491. [Google Scholar] [CrossRef]
- Lugli, L.; Bedetti, L.; Lucaccioni, L.; Gennari, W.; Leone, C.; Ancora, G.; Berardi, A. An Uninfected Preterm Newborn Inadvertently Fed SARS-CoV-2–Positive Breast Milk. Pediatrics 2020, 146, e2020004960. [Google Scholar] [CrossRef]
- Chambers, C.; Krogstad, P.; Bertrand, K.; Contreras, D.; Tobin, N.H.; Bode, L.; Aldrovandi, G. Evaluation for SARS-CoV-2 in Breast Milk from 18 Infected Women. JAMA 2020, 324, 1347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, W.; Su, H.; Li, S.; Shereen, M.A.; Lv, Z.; Niu, Z.; Li, D.; Liu, F.; Luo, Z.; et al. Breastfeeding Risk from Detectable Severe Acute Respiratory Syndrome Coronavirus 2 in Breastmilk. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Y.; Hu, Y.; Li, B.; Xu, J. Breastfed 13 month-old infant of a mother with COVID-19 pneumonia: A case report. Int. Breastfeed. J. 2020, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chi, X.; Hai, H.; Sun, L.; Zhang, M.; Xie, W.-F.; Chen, W. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg. Microbes Infect. 2020, 9, 1467–1469. [Google Scholar] [CrossRef]
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; La Rosa, M. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol. 2020, 37, 861–865. [Google Scholar] [CrossRef] [Green Version]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. 2020, 2, 100118. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, H.; Wang, L.; Zhao, Y.; Zeng, L.; Gao, H.; Liu, Y. Infants Born to Mothers With a New Coronavirus (COVID-19). Front. Pediatr. 2020, 8, 104. [Google Scholar] [CrossRef]
- Romero, D.G.; Pérez, J.O.; Bautista, L.G.; Santana-Cabrera, L. Pronóstico perinatal y de la paciente embarazada con infección por COVID-19. Rev. Clínica Española 2020, 220, 533–534. [Google Scholar] [CrossRef]
- Karami, P.; Naghavi, M.; Feyzi, A.; Aghamohammadi, M.; Novin, M.S.; Mobaien, A.; Qorbanisani, M.; Karami, A.; Norooznezhad, A.H. WITHDRAWN: Mortality of a pregnant patient diagnosed with COVID-19: A case report with clinical, radiological, and histopathological findings. Travel Med. Infect. Dis. 2020, 101665. [Google Scholar] [CrossRef]
- Li, N.; Han, L.; Peng, M.; Lv, Y.; Ouyang, Y.; Liu, K.; Yue, L.; Li, Q.; Sun, G.; Chen, L.; et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: A case-control study 2020. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Li, L.; Wu, X.; Zheng, D.; Wang, J.; Yang, L.; Zheng, C. Pregnancy and Perinatal Outcomes of Women With Coronavirus Disease (COVID-19) Pneumonia: A Preliminary Analysis. Am. J. Roentgenol. 2020, 215, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Tang, K.; Guo, Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Sang, L.; Du, S.; Li, T.; Chang, Y.; Yang, X.-A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J. Med. Virol. 2020, 92, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, W.; Wu, X.; Zhang, T.; Zhao, Y.; Ren, W.; Xia, J. Clinical Manifestation and Laboratory Characteristics of SARS-CoV-2 Infection in Pregnant Women. Virol. Sin. 2020, 35, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Sun, R.; Chen, J.; Xie, Y.; Zhang, S.; Wang, X. Radiological findings and clinical characteristics of pregnant women with COVID -19 pneumonia. Int. J. Gynecol. Obstet. 2020, 150, 58–63. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Wei, M.; Cheng, B.H.; Zhou, X.C.; Li, J.; Tian, J.H.; Dong, L.; Hu, R.H. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 2020, 55, 166–171. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef]
- Caparros-Gonzalez, R.A.; Romero-Gonzalez, B.; Gonzalez-Perez, R.; Lara-Cinisomo, S.; Martin-Tortosa, P.L.; Oliver-Roig, A.; Peralta-Ramirez, M.I. Maternal and Neonatal Hair Cortisol Levels and Psychological Stress Are Associated With Onset of Secretory Activation of Human Milk Production. Adv. Neonatal Care 2019, 19, E11–E20. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Graham, A.L. Potential Maternal and Infant Outcomes from Coronavirus 2019-nCoV (SARS-CoV-2) Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Robertson, C.A.; Lowther, S.A.; Birch, T.; Tan, C.; Sorhage, F.; Stockman, L.; McDonald, L.C.; Lingappa, J.R.; Bresnitz, E. SARS and Pregnancy: A Case Report. Emerg. Infect. Dis. 2004, 10, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.; Duncan, D.; Reiken, M.; Perry, R.; Messick, J.; Sheedy, C.; Haase, J.; Gorab, J. SARS in Pregnancy. AWHONN Lifelines 2004, 8, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; De Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.-D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 2020, 9, e58716. [Google Scholar] [CrossRef] [PubMed]
- Adibi, J.J.; A Marques, E.T.; Cartus, A.; Beigi, R.H. Teratogenic effects of the Zika virus and the role of the placenta. Lancet 2016, 387, 1587–1590. [Google Scholar] [CrossRef] [Green Version]
- Meier, B.M.; Motlagh, M.; Rasanathan, K. The United Nations Children’s Fund; Oxford University Press (OUP): Oxford, UK, 2018; Available online: https://www.unicef.org/reports/annual-report-2018 (accessed on 20 November 2020).
- World Health Organization; UNICEF. Planning Guide for National Implementation of the Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2007; Available online: https://www.who.int/nutrition/publications/infantfeeding/gs_iycf_planning_guide.pdf (accessed on 20 November 2020).
- Chen, D.; Yang, H.; Cao, Y.; Cheng, W.; Duan, T.; Fan, C.; Fan, S.; Feng, L.; Gao, Y.; He, F.; et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int. J. Gynecol. Obstet. 2020, 149, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi-Zarchi, M.; Neamatzadeh, H.; Dastgheib, S.A.; Abbasi, H.; Mirjalili, S.R.; Behforouz, A.; Ferdosian, F.; Bahrami, R. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr. Pathol. 2020, 39, 246–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Patients under Investigation for 2019-nCoV in Healthcare Settings. Pregnancy and Breastfeeding. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html (accessed on 1 May 2020).
- Walker, G.J.; Clifford, V.; Bansal, N.; Stella, A.O.; Turville, S.; Stelzer-Braid, S.; Klein, L.D.; Rawlinson, W.D. SARS-CoV-2 in human milk is inactivated by Holder pasteurisation but not cold storage. J. Paediatr. Child Heal. 2020, 1–3. [Google Scholar] [CrossRef]
| Reference | City and Country | Study Design | Number of Pregnant Women | Mean Maternal Age (Years) | Number of Fetuses | Trimester of COVID-19 Diagnosis | Maternal Positive Throat Swab for COVID-19 | Mean Gestational Age at Birth (Weeks) | Neonatal Positive Throat Swab for COVID-19 | Potential Sources of Transmission Studied | Confirmed Source of Transmission | Neonatal Feeding Method | Vaginal Delivery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [47] | Lima (Peru) | Case report | 1 | 41 | 1 | 3 | 1 | 34 | 1 | Not assessed | None | Formula | 0 |
| [48] | New York (USA) | Case series | 43 | 26.9 | 18 | 3 | 43 | 37 | 0 | Not reported | Not reported | Not reported | 10 |
| [11] | Wuhan (China) | Case series | 9 | 29.8 | 9 | 3 | 9 | 37.2 | 0 | Amniotic fluid, cord blood, and breast milk | None | Not reported | 0 |
| [30] | Wuhan (China) | Case series | 3 | 29.7 | 3 | 3 | 3 | 36.6 | 0 | Placenta | None | Not reported | Not reported |
| [31] | Wuhan (China) | Case series | 5 | 28.8 | 5 | 3 | 5 | 39.5 | 0 | Placenta | None | Formula | 3 |
| [49] | Wuhan (China) | Case series | 4 | 29 | 4 | 3 | 4 | 37.1 | 0 | None | None | Formula | 1 |
| [50] | Las Palmas Gran Canaria (Spain) | Case report | 1 | 44 | 1 | 3 | 1 | 29 | 0 | Not reported | Not reported | Not reported | 0 |
| [51] | Zanjan (Iran) | Case report | 1 | 27 | 1 | 3 | 1 | 30 | Not reported | Not reported | None | Not reported | 1 |
| [23] | Wuhan (China) | Case report | 3 | 29.3 | 3 | 3 | 3 | 37 | 0 | Umbilical cord blood | None | Not reported | 3 |
| [52] | Wuhan (China) | Case-Control | 16 | 30.3 | 17 | 3 | 16 | 38 | 0 | Not reported | Not reported | Not reported | 2 |
| [12] | Wuhan (China) | Case report | 1 | 30 | 1 | 3 | 1 | 35 | 0 | Serum, urine, feces, amniotic fluid, umbilical cord blood, placenta, and breast milk | None | Not reported | 0 |
| [53] | Wuhan (China) | Case series | 15 | 32.5 | 15 | 1, 2 and 3 | 15 | 37.1 | 0 | Not reported | Not reported | Not reported | 1 |
| [54] | Guangdong (China) | Case series | 13 | 29 | 13 | 2 and 3 | 13 | 32 | None | Not reported | None | Not reported | 0 |
| [13] | Wuhan (China) | Case series | 19 | 31 | 19 | 3 | 19 | 36.3 | 0 | Breast milk, amniotic fluid, and cord blood | None | Formula | 1 |
| [55] | Chengde (China) | Case report | 1 | 22 | 1 | 3 | 1 | 38 | 0 | Serum | None | Formula | 0 |
| [14] | Wanzhou (China) | Case report | 1 | 25 | 1 | 3 | 1 | 35.3 | 0 | Amniotic fluid, cord blood, placenta, vaginal secretion, serum, anal sample, breast milk | None | Not reported | 0 |
| [15] | Paraná (Brazil) | Case report | 1 | 42 | 1 | 2 | 1 | 28 | 0 | Placenta, amniotic fluid, umbilical cord blood | Placenta, umbilical cord blood | None | 0 |
| [27] | Wuhan (China) | Case report | 1 | 34 | 1 | 3 | 1 | 40 | 0 | Breast milk | None | Formula | 0 |
| [16] | Suzhou (China) | Case report | 1 | 28 | 1 | 3 | 1 | 30 | 0 | Amniotic fluid, placenta, cord blood | None | Formula | 0 |
| [56] | Wuhan (China) | Case series | 8 | 29.8 | 8 | 3 | 8 | 37.7 | Not reported | Not reported | Not reported | Not reported | 2 |
| [57] | Wuhan (China) | Case series | 23 | 29 | 21 | 1 and 2 | 19 | 37 | 0 | Not reported | Not reported | Not reported | 2 |
| [17] | Beijing (China) | Case report | 1 | 25 | 1 | 3 | 1 | 38.4 | 0 | Maternal cervical secretion, maternal rectal, breast milk, amniotic fluid, and placenta | None | Not reported | 1 |
| [28] | Wuhan (China) | Case series | 13 | 30.2 | 13 | 3 | 13 | 38.2 | 0 | Not assessed | None | Not reported | 4 |
| [18] | Wuhan (China) | Case series | 7 | Not reported | 7 | 3 | 7 | 36.5 | 0 | Amniotic fluid, cord blood | None | Not reported | 0 |
| [24] | Wuhan (China) | Case series | 7 | 32 | 7 | 3 | 7 | 39 | 1 | Placenta, cord blood | None | Not reported | 0 |
| [19] | Wuhan (China) | Case series | 2 | 30.5 | 2 | 1 | 2 | Not reported | Not reported | Amniotic fluid | None | Not reported | Not reported |
| [22] | Tegucigalpa (Honduras) | Case report | 1 | 41 | 1 | 3 | 1 | 32 | 0 | Not assessed | None | Not reported | 0 |
| [20] | Sari (Iran) | Case report | 1 | 22 | 1 | 3 | 1 | 33 | 1 | Amniotic fluid, cord blood | Amniotic fluid | Formula | 0 |
| [58] | Wuhan (China) | Case series | 15 | 30 | 15 | 3 | 15 | 38 | 0 | Not reported | Not reported | Not reported | 0 |
| [59] | Wuhan (China) | Case series | 9 | 27 | 10 | 2 and 3 | 9 | 35 | 0 | Not assessed | Not assessed | Formula | 2 |
| [32] | New York (USA) | Case series | 11 | 31.6 | 11 | 3 | 11 | 34.4 | 0 | Placenta | Placenta | Not reported | 7 |
| [29] | Bergamo (Italy) | Case series | 22 | Not reported | 22 | 3 | 22 | 36 | 2 | Placenta | Placenta | Breastfeeding | 0 |
| [25] | Lausanne (Switzerland) | Case report | 1 | 28 | 1 | 2 | 1 | 19 | 1 | Placenta, cord blood, Maternal cervical secretion | Placenta | None (miscarriage) | 0 |
| [33] | Toronto (Canada) | Case report | 1 | 40 | 1 | 3 | 1 | 35 | 1 | Placenta, breast milk | Placenta, breast milk | Breastfeeding | 0 |
| [34] | Boston (USA) | Case series | 19 | 30 | 19 | 3 | 19 | 38 | 0 | Placenta | Placenta | Not reported | 7 |
| [35] | Missouri (USA) | Case report | 1 | 29 | 1 | 3 | 1 | 40 | 0 | Placenta | Placenta | Breastfeeding | 1 |
| [26] | Pune (India) | Case report | 1 | 24 | 1 | 3 | 1 | 38 | 1 | Umbilical cord stump, placenta | Umbilical cord stump, placenta | Formula | 1 |
| [36] | Palermo (Italy) | Case series | 15 | 29 | 1 | 3 | 1 | 37 | 1 | Placenta | Placenta | Not reported | 1 |
| [21] | Paris (France) | Case report | 1 | 23 | 1 | 3 | 1 | 35 | 1 | Vaginal secretion, placenta, amniotic fluid | Placenta and vaginal secretion | Formula | 0 |
| [37] | Connecticut (USA) | Case report | 1 | 35 | 1 | 2 | 1 | 22 | 0 | Placenta | Placenta | Not provided | 0 * |
| [38] | New York (USA) | Case report | 1 | 40 | 1 | 2 | 1 | 28 | 0 | Placenta | Placenta | Not provided | 0 |
| [39] | Heidenheim (Germany) | Case series | 2 | Not reported | 2 | Not reported | 2 | Not reported | 1 | Breast milk | Breast milk | Breastfeeding | Not reported |
| [40] | Adelaide (Australia) | Case report | 1 | 40 | 1 | Postpartum | 1 | 8 months (postpartum) | 1 | Breast milk | Breast milk | Breastfeeding | Not reported |
| [41] | Ankara (Turkey) | Case report | 1 | 20 | 1 | 3 | 1 | 38 | 1 | Breast milk | Breast milk | Breastfeeding | 1 |
| [45] | Zhejiang (China) | Case report | 1 | 32 | 1 | Postpartum | 1 | 13 months (postpartum) | 1 | Breast milk, feces, maternal serum | Breast milk, maternal serum | Breastfeeding | Not reported |
| [42] | Modena (Italy) | Case report | 1 | 33 | 1 | 3 | 1 | 32 | 1 | Breast milk | Breast milk | Breastfeeding | 0 |
| [43] | Los Angeles (USA) | Case series | 18 | 34.4 | 18 | 3 and postpartum | 18 | Not reported | Not reported | Breast milk | Breast milk | Not reported | Not reported |
| [46] | Shangai (China) | Case report | 1 | 33 | 1 | 3 | 1 | 38 | 0 | Vaginal secretion, maternal serum, breast milk | None | Not reported | 1 |
| [44] | Wuhan (China) | Case series | 5 | 33 | 5 | Postpartum | 5 | 36 | 0 | Vaginal secretion, serum, breast milk | Breast milk | Not reported | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caparros-Gonzalez, R.A.; Pérez-Morente, M.A.; Hueso-Montoro, C.; Álvarez-Serrano, M.A.; de la Torre-Luque, A. Congenital, Intrapartum and Postnatal Maternal-Fetal-Neonatal SARS-CoV-2 Infections: A Narrative Review. Nutrients 2020, 12, 3570. https://doi.org/10.3390/nu12113570
Caparros-Gonzalez RA, Pérez-Morente MA, Hueso-Montoro C, Álvarez-Serrano MA, de la Torre-Luque A. Congenital, Intrapartum and Postnatal Maternal-Fetal-Neonatal SARS-CoV-2 Infections: A Narrative Review. Nutrients. 2020; 12(11):3570. https://doi.org/10.3390/nu12113570
Chicago/Turabian StyleCaparros-Gonzalez, Rafael A., María Angeles Pérez-Morente, Cesar Hueso-Montoro, María Adelaida Álvarez-Serrano, and Alejandro de la Torre-Luque. 2020. "Congenital, Intrapartum and Postnatal Maternal-Fetal-Neonatal SARS-CoV-2 Infections: A Narrative Review" Nutrients 12, no. 11: 3570. https://doi.org/10.3390/nu12113570
APA StyleCaparros-Gonzalez, R. A., Pérez-Morente, M. A., Hueso-Montoro, C., Álvarez-Serrano, M. A., & de la Torre-Luque, A. (2020). Congenital, Intrapartum and Postnatal Maternal-Fetal-Neonatal SARS-CoV-2 Infections: A Narrative Review. Nutrients, 12(11), 3570. https://doi.org/10.3390/nu12113570






