Mediterranean Personalized Diet Combined with Physical Activity Therapy for the Prevention of Cardiovascular Diseases in Italian Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Body Composition Assessmen
2.2.1. Anthropometric Assessment
2.2.2. Bioelectrical Impedance Analysis (BIA)
2.2.3. Dual-Energy X-ray Absorptiometry (DXA)
2.3. Laboratory Tests, Cardiovascular and Inflammatory Risk Indexes
- (1)
- (2)
- Lipoproteins cholesterol (cLDL/cHDL) ratio was calculated according to the formula [15]:with normal values < 2.cLDL/cHDL ratio = cLDL (mg/dL)/cHDL (mg/dL),
- (3)
- Triglycerides (TG) /cHDL ratio was calculated according to the formula [14]:with normal values < 1.TG/cHDL ratio = TG (mg/dL)/cHDL (mg/dL),
- (4)
- (5)
- The Fatty Liver Index (FLI) was calculated according to the formula [42]:with absence of steatosis for values < 30.FLI = (e0.953 × loge(triglycerides) + 0.139 × BMI + 0.718 × loge(ggt) + 0.053 × waistcircumference − 15.745)/(1 + e0.953 × loge(triglycerides) + 0.139 × BMI + 0.718 × loge(ggt) + 0.053 × waistcircumference − 15.745) × 100,
- (6)
- ASCVD Risk Algorithm was calculated using the ACC/AHA calculator [23].
- (7)
- LAP was calculated according to the formula [17]:LAP = (WC-65) × TG for men and (WC-58) × TG for women.
- (8)
- BARD score consists of the weighted sum of three variables: body mass index ≥ 28 represents 1 point, the Aspartate Aminotransferase (AST)/Alanine Aminotransferase (ALT) ratio ≥ 0.8 represents 2 points, and diabetes mellitus represents 1 point. A score of 2–4 had an odds ratio of 17 (confidence interval: 9.2–31.9) to determine advanced fibrosis and a negative predictive value of 96% [43].
- (9)
- NLR is easily calculated by dividing the absolute neutrophil count by the absolute lymphocyte count from a complete blood count with differential [22]. The values for Low risk < 1.6, Medium risk 1.6–2.4 and High risk > 2.4.
- (10)
- PLR is calculated by dividing the platelet count by the lymphocytes [44]. The cut off is < 150.
- (11)
- CRP and ESR were used to evaluate inflammatory risk [45].
2.4. Personalized Diet Therapy
2.5. Planned Physical Activity
2.6. Statistical Analysis
2.7. Ethics Approval
3. Results
3.1. Subjects
3.2. Anthropometry and Body Composition
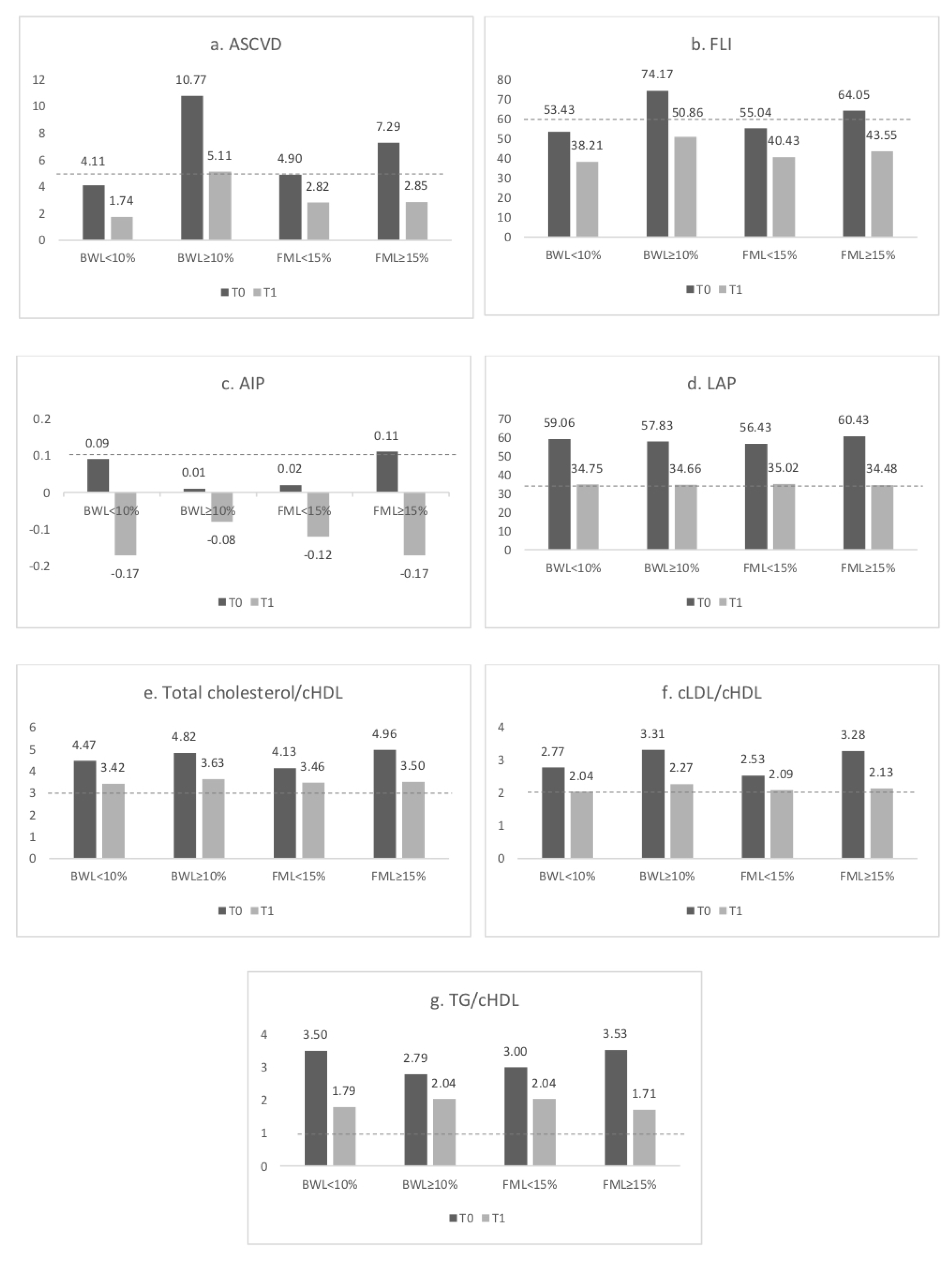
3.3. Cardiovascular Risk Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO|Diet. Nutrition and the Prevention of Chronic Diseases. Available online: https://www.who.int/dietphysicalactivity/publications/trs916/en/ (accessed on 4 November 2020).
- Shaping the Future; The world health report; Weltgesundheitsorganisation: Geneva, Switzerland, 2003; ISBN 978-92-4-156243-0.
- De Lorenzo, A.; Gratteri, S.; Gualtieri, P.; Cammarano, A.; Bertucci, P.; Di Renzo, L. Why primary obesity is a disease? J. Transl. Med. 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Available online: http://www.who.int/entity/nutrition/publications/obesity/WHO_TRS_894/en/index.html (accessed on 8 September 2020).
- Bays, H. Adiposopathy, metabolic syndrome, quantum physics, general relativity, chaos and the Theory of Everything. Expert Rev. Cardiovasc. Ther. 2005, 3, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.; Huxley, R.; Jackson, C.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- De Lorenzo, A.; Bianchi, A.; Maroni, P.; Iannarelli, A.; Di Daniele, N.; Iacopino, L.; Di Renzo, L. Adiposity rather than BMI determines metabolic risk. Int. J. Cardiol. 2013, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E. Adiposopathy is “sick fat” a cardiovascular disease? J. Am. Coll. Cardiol. 2011, 57, 2461–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bays, H.E.; González-Campoy, J.M.; Bray, G.A.; Kitabchi, A.E.; Bergman, D.A.; Schorr, A.B.; Rodbard, H.W.; Henry, R.R. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev. Cardiovasc. Ther. 2008, 6, 343–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef]
- Castelli, W.P.; Abbott, R.D.; McNamara, P.M. Summary estimates of cholesterol used to predict coronary heart disease. Circulation 1983, 67, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Jayanthi, R.; Srinivasan, A.R.; Hanifah, M.; Maran, A.L. Associations among Insulin Resistance, Triacylglycerol/High Density Lipoprotein (TAG/HDL ratio) and Thyroid hormone levels-A study on Type 2 diabetes mellitus in obese and overweight subjects. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S121–S126. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar] [PubMed]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotter, I.; Rył, A.; Szylińska, A.; Pawlukowska, W.; Lubkowska, A.; Laszczyńska, M. Lipid Accumulation Product (LAP) as an Index of Metabolic and Hormonal Disorders in Aging Men. Exp. Clin. Endocrinol. Diabetes 2017, 125, 176–182. [Google Scholar] [CrossRef]
- Khang, A.R.; Lee, H.W.; Yi, D.; Kang, Y.H.; Son, S.M. The fatty liver index, a simple and useful predictor of metabolic syndrome: Analysis of the Korea National Health and Nutrition Examination Survey 2010–2011. Diabetes Metab. Syndr. Obes 2019, 12, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, I.; Hur, E.; Kokturk, F. Inflammatory Markers: C-Reactive Protein, Erythrocyte Sedimentation Rate, and Leukocyte Count in Vitamin D Deficient Patients with and without Chronic Kidney Disease. Int. J. Endocrinol. 2013, 2013, 802165. [Google Scholar] [CrossRef]
- Haybar, H.; Pezeshki, S.M.S.; Saki, N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Exp. Mol. Pathol. 2019, 110, 104267. [Google Scholar] [CrossRef]
- Bazzi, W.M.; Dejbakhsh, S.Z.; Bernstein, M.; Russo, P. Neutrophil-lymphocyte ratio in small renal masses. Int. Sch. Res. Not. 2014, 2014, 759253. [Google Scholar] [CrossRef]
- Arnett Donna, K.; Blumenthal Roger, S.; Albert Michelle, A.; Buroker Andrew, B.; Goldberger Zachary, D.; Hahn Ellen, J.; Himmelfarb Cheryl, D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Celiński, K.; Prozorow-Król, B.; Swatek, J.; Słomka, M.; Lach, T. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease. Med. Sci. Monit. 2012, 18, CR735–CR740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klisic, A.; Abenavoli, L.; Fagoonee, S.; Kavaric, N.; Kocic, G.; Ninić, A. Older age and HDL-cholesterol as independent predictors of liver fibrosis assessed by BARD score. Minerva Med. 2019, 110, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [Green Version]
- Martini, D. Health Benefits of Mediterranean Diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, L.; Cioccoloni, G.; Falco, S.; Abenavoli, L.; Moia, A.; Sinibaldi Salimei, P.; De Lorenzo, A. Influence of FTO rs9939609 and Mediterranean diet on body composition and weight loss: A randomized clinical trial. J. Transl. Med. 2018, 16, 308. [Google Scholar] [CrossRef]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Siscovick, D.S.; Psaty, B.M.; Longstreth, W.T.; Mozaffarian, D. Physical Activity and Risk of Coronary Heart Disease and Stroke in Older Adults: The Cardiovascular Health Study. Circulation 2016, 133, 147–155. [Google Scholar] [CrossRef]
- Pasanisi, F.; Contaldo, F.; de Simone, G.; Mancini, M. Benefits of sustained moderate weight loss in obesity. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 401–406. [Google Scholar]
- De Lorenzo, A.; Siclari, M.; Gratteri, S.; Romano, L.; Gualtieri, P.; Marchetti, M.; Merra, G.; Colica, C. Developing and cross-validation of new equations to estimate fat mass in Italian population. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2513–2524. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. (Eds.) Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988; ISBN 978-0-87322-121-4. [Google Scholar]
- Cao, Q.; Yu, S.; Xiong, W.; Li, Y.; Li, H.; Li, J.; Li, F. Waist-hip ratio as a predictor of myocardial infarction risk: A systematic review and meta-analysis. Medicine 2018, 97, e11639. [Google Scholar] [CrossRef]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxidative Med. Cell Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef] [PubMed]
- Encyclopedia of Human Nutrition—3rd Edition. Available online: https://www.elsevier.com/books/encyclopedia-of-human-nutrition/unknown/978-0-12-375083-9 (accessed on 9 September 2020).
- Di Renzo, L.; Sarlo, F.; Petramala, L.; Iacopino, L.; Monteleone, G.; Colica, C.; De Lorenzo, A. Association between -308 G/A TNF-α polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis. Markers 2013, 35, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lorenzo, A.; Soldati, L.; Sarlo, F.; Calvani, M.; Di Lorenzo, N.; Di Renzo, L. New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. 2016, 22, 681–703. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran 2015, 29, 240. [Google Scholar]
- Kanthe, P.S.; Patil, B.S.; Bagali, S.; Deshpande, A.; Shaikh, B.; Aithala, M. Atherogenic Index as a Predictor of Cardiovascular Risk among Women with Different Grades of Obesity. Public Health 2012, 4, 8. [Google Scholar]
- Kantartzis, K.; Rettig, I.; Staiger, H.; Machann, J.; Schick, F.; Scheja, L.; Gastaldelli, A.; Bugianesi, E.; Peter, A.; Schulze, M.B.; et al. An extended fatty liver index to predict non-alcoholic fatty liver disease. Diabetes Metab. 2017, 43, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Stål, P. Liver fibrosis in non-alcoholic fatty liver disease—Diagnostic challenge with prognostic significance. World J. Gastroenterol. 2015, 21, 11077–11087. [Google Scholar] [CrossRef]
- Erdal, E.; İnanir, M. Platelet-to-lymphocyte ratio (PLR) and Plateletcrit (PCT) in young patients with morbid obesity. Rev. Assoc. Med. Bras. 2019, 65, 1182–1187. [Google Scholar] [CrossRef]
- Lapić, I.; Padoan, A.; Bozzato, D.; Plebani, M. Erythrocyte Sedimentation Rate and C-Reactive Protein in Acute Inflammation. Am. J. Clin. Pathol. 2020, 153, 14–29. [Google Scholar] [CrossRef]
- Block, G. Human dietary assessment: Methods and issues. Prev. Med. 1989, 18, 653–660. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Di Renzo, L.; Morini, P.; de Miranda, R.C.; Romano, L.; Colica, C. New equations to estimate resting energy expenditure in obese adults from body composition. Acta Diabetol. 2018, 55, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Avolio, E.; Bollero, P.; Costa de Miranda, R.; Ferraro, S.; Sinibaldi Salimei, P.; De Lorenzo, A.; Di Renzo, L. Evidences of a New Psychobiotic Formulation on Body Composition and Anxiety. Mediat. Inflamm. 2017, 2017, 5650627. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Merra, G.; Gasbarrini, A.; De Lorenzo, A.; Cioccoloni, G.; Gualtieri, P.; Perrone, M.A.; Bernardini, S.; Bernardo, V.; Di Renzo, L.; et al. Efficacy and safety of very-low-calorie ketogenic diet: A double blind randomized crossover study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2274–2289. [Google Scholar] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports Medicine; et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, N.J.; Law, M.R. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003, 326, 1419. [Google Scholar] [CrossRef] [Green Version]
- Garies, S.; Hao, S.; McBrien, K.; Williamson, T.; Peng, M.; Khan, N.A.; Padwal, R.S.; Quan, H.; Leung, A.A. Prevalence of Hypertension, Treatment, and Blood Pressure Targets in Canada Associated With the 2017 American College of Cardiology and American Heart Association Blood Pressure Guidelines. JAMA Netw. Open 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Drosatos, K.; Buxton, J.L. Nutriepigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 252–259. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.; Ahmad, I.; Easton, G. Promoting physical activity: The general practice agenda. Br. J. Gen. Prac. 2016, 66, 454–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacombe, J.; Armstrong, M.E.G.; Wright, F.L.; Foster, C. The impact of physical activity and an additional behavioural risk factor on cardiovascular disease, cancer and all-cause mortality: A systematic review. BMC Public Health 2019, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Jones, D.M.; Huffman, M.D.; Karmali, K.N.; Sanghavi, D.M.; Wright, J.S.; Pelser, C.; Gulati, M.; Masoudi, F.A.; Goff, D.C. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2017, 69, 1617–1636. [Google Scholar] [CrossRef]
- Roriz, A.K.C.; Passos, L.C.S.; de Oliveira, C.C.; Eickemberg, M.; Moreira, P.d.A.; Sampaio, L.R. Evaluation of the accuracy of anthropometric clinical indicators of visceral fat in adults and elderly. PLoS ONE 2014, 9, e103499. [Google Scholar] [CrossRef]
- Taverna, M.J.; Martínez-Larrad, M.T.; Frechtel, G.D.; Serrano-Ríos, M. Lipid accumulation product: A powerful marker of metabolic syndrome in healthy population. Eur. J. Endocrinol. 2011, 164, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, G.; Wang, A.; Xu, T.; Tong, W.; Zhang, Y. Comparison of lipid accumulation product with body mass index as an indicator of hypertension risk among Mongolians in China. Obes. Res. Clin. Pract. 2013, 7, e308–e314. [Google Scholar] [CrossRef]
- Hosseinpanah, F.; Barzin, M.; Mirbolouk, M.; Abtahi, H.; Cheraghi, L.; Azizi, F. Lipid accumulation product and incident cardiovascular events in a normal weight population: Tehran Lipid and Glucose Study. Eur. J. Prev. Cardiol. 2016, 23, 187–193. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Atherogenic Index of Plasma (AIP): A Novel Predictive Indicator for the Coronary Artery Disease in Postmenopausal Women | Lipids in Health and Disease | Full Text. Available online: https://lipidworld.biomedcentral.com/articles/10.1186/s12944-018-0828-z (accessed on 5 November 2020).
- Al-Osami, M.H.; Awadh, N.I.; Khalid, K.B.; Awadh, A.I. Neutrophil/lymphocyte and platelet/lymphocyte ratios as potential markers of disease activity in patients with Ankylosing spondylitis: A case-control study. Adv. Rheumatol. 2020, 60, 13. [Google Scholar] [CrossRef]
- Early Inflammatory Status Related to Pediatric Obesity—Abstract—Europe PMC. Available online: https://europepmc.org/article/pmc/6591428 (accessed on 5 November 2020).
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Toselli, S.; Badicu, G.; Bragonzoni, L.; Spiga, F.; Mazzuca, P.; Campa, F. Comparison of the Effect of Different Resistance Training Frequencies on Phase Angle and Handgrip Strength in Obese Women: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| T0 | T1 | p-Value * | |
|---|---|---|---|
| Weight | 88.4 ± 24.9 | 79.7 ± 18.7 | <0.0001 |
| BMI | 32.3 ± 8.0 | 29.2 ± 6.0 | <0.0001 |
| Neck circumference | 38.8 ± 4.4 | 37.1 ± 3.9 | <0.0001 |
| Waist circumference | 98.6 ± 18.0 | 90.7 ± 13.6 | <0.0001 |
| Abdomen circumference | 109.3 ± 19.6 | 100.0 ± 13.2 | <0.0001 |
| Hip circumference | 113.3 ± 14.6 | 107.3 ± 11.1 | <0.0001 |
| WHR | 0.869 ± 0.098 | 0.846 ± 0.093 | 0.0002 |
| Rz | 470 ± 79 | 468 ± 76 | 0.85 |
| Xc | 50 ± 10 | 52 ± 10 | 0.21 |
| PA° | 6.17 ± 1.11 | 6.38 ± 1.07 | 0.1 |
| TBW (L) | 42.6 ± 9.3 | 41.5 ± 8.6 | 0.004 |
| TBW (%) | 49.3 ± 7.3 | 52.4 ± 6.7 | <0.0001 |
| ECW (L) | 19.2 ± 4.1 | 18.3 ± 3.7 | 0.003 |
| ECW (%) | 45.4 ± 5.0 | 44.5 ± 4.6 | 0.11 |
| ICW (L) | 23.4 ± 6.1 | 23.3 ± 5.8 | 0.8 |
| ICW (%) | 54.6 ± 5.0 | 55.5 ± 4.6 | 0.10 |
| FM (kg) | 36.75 ± 17.16 | 29.80 ± 12.24 | <0.0001 |
| FM (%) | 40.3 ± 9.1 | 36.5 ± 8.8 | <0.0001 |
| LM (kg) | 48.56 ± 10.71 | 47.42 ± 10.14 | <0.0001 |
| ASMMI | 8.32 ± 1.82 | 8.06 ± 1.71 | 0.003 |
| Cardiovascular Risk Indexes | T0 | T1 | p Value * |
|---|---|---|---|
| ASCVD risk | 6.27 ± 7.21 | 2.84 ± 3.44 | 0.0001 |
| NLR | 1.73 ± 0.74 | 1.84 ± 0.57 | 0.31 |
| PLR | 116.24 ± 37.58 | 125.78 ± 65.29 | 0.29 |
| Total cholesterol/cHDL | 4.64 ± 1.38 | 3.50 ± 0.87 | <0.0001 |
| cLDL/cHDL | 2.95 ± 1.09 | 2.12 ± 0.69 | <0.0001 |
| TG/cHDL | 3.38 ± 2.53 | 1.89 ± 1.07 | <0.0001 |
| AIP | 0.06 ± 0.27 | −0.14 ± 0.22 | <0.0001 |
| FLI | 59.74 ± 32.26 | 42.06 ± 30.73 | <0.0001 |
| LAP | 58.66 ± 42.79 | 34.72 ± 23.43 | <0.0001 |
| BARD | 3.42 ± 0.11 | 2.89 ± 0.23 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Renzo, L.; Cinelli, G.; Dri, M.; Gualtieri, P.; Attinà, A.; Leggeri, C.; Cenname, G.; Esposito, E.; Pujia, A.; Chiricolo, G.; et al. Mediterranean Personalized Diet Combined with Physical Activity Therapy for the Prevention of Cardiovascular Diseases in Italian Women. Nutrients 2020, 12, 3456. https://doi.org/10.3390/nu12113456
Di Renzo L, Cinelli G, Dri M, Gualtieri P, Attinà A, Leggeri C, Cenname G, Esposito E, Pujia A, Chiricolo G, et al. Mediterranean Personalized Diet Combined with Physical Activity Therapy for the Prevention of Cardiovascular Diseases in Italian Women. Nutrients. 2020; 12(11):3456. https://doi.org/10.3390/nu12113456
Chicago/Turabian StyleDi Renzo, Laura, Giulia Cinelli, Maria Dri, Paola Gualtieri, Alda Attinà, Claudia Leggeri, Giuseppe Cenname, Ernesto Esposito, Alberto Pujia, Gaetano Chiricolo, and et al. 2020. "Mediterranean Personalized Diet Combined with Physical Activity Therapy for the Prevention of Cardiovascular Diseases in Italian Women" Nutrients 12, no. 11: 3456. https://doi.org/10.3390/nu12113456






