A Randomized Controlled Trial Testing the Effectiveness of Coping with Cancer in the Kitchen, a Nutrition Education Program for Cancer Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Program Overview
2.2. Study Design
2.3. Study Population
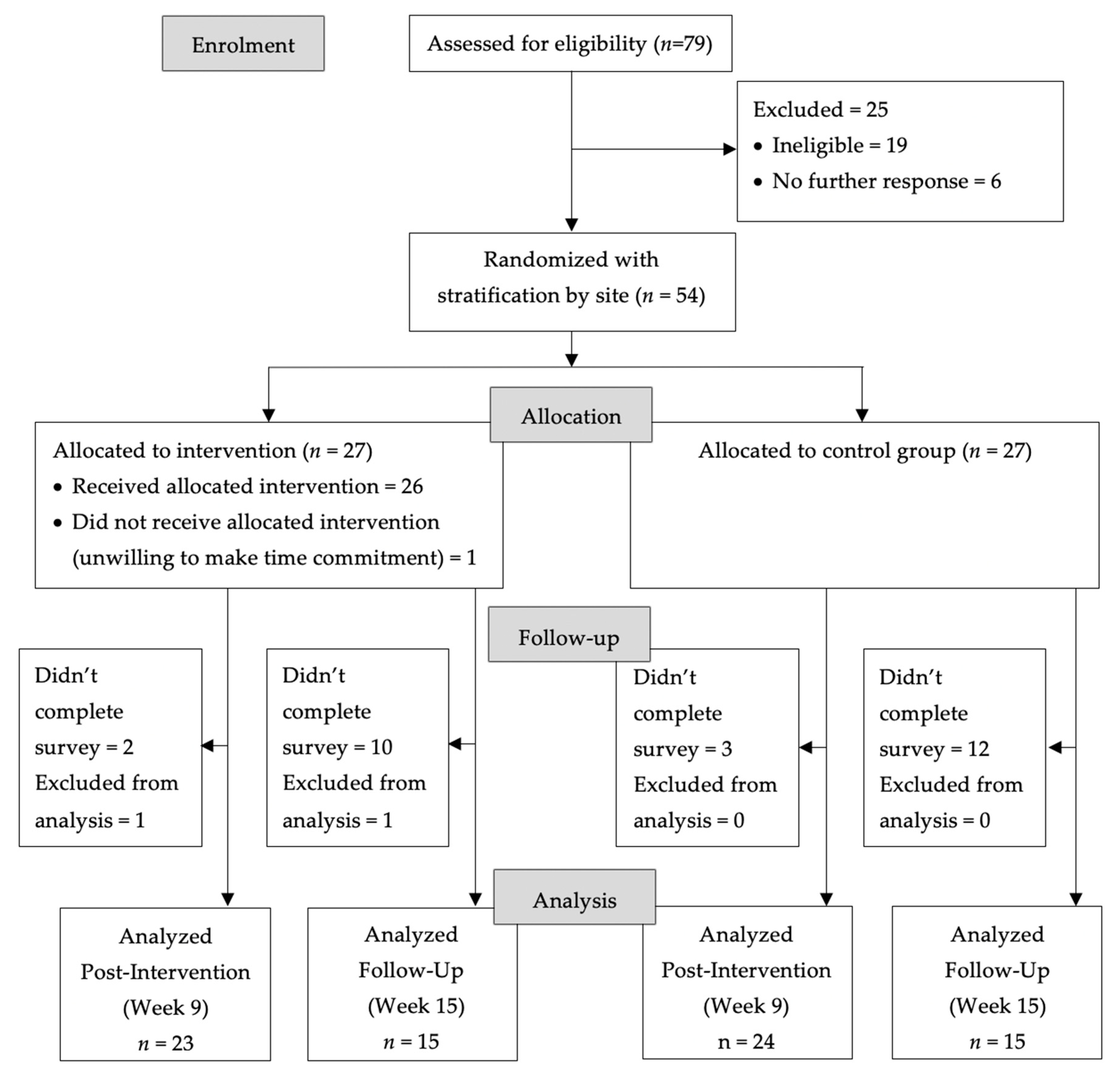
2.4. Randomization
2.5. CCK Onsite Teams
2.6. CCK Intervention Group
2.7. Printed Materials Control Group
2.8. Data Collection/Participant Survey
2.9. Baseline and Outcome Measures
2.10. Statistical Analysis
3. Results
4. Discussion
4.1. Primary Outcomes (Knowledge, Cooking Confidence, Skills) and Perceived Barriers
4.2. Dietary Intake
4.3. Quality of Life and Other Psychosocial Measures
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2019. CAA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- van Zutphen, M.; Boshuizen, H.C.; Kok, D.E.; van Baar, H.; Geijsen, A.J.M.R.; Wesselink, E.; Winkels, R.M.; van Halteren, H.K.; de Wilt, J.H.W.; Kampman, E.; et al. Colorectal Cancer Survivors Only Marginally Change Their Overall Lifestyle in the First 2 Years Following Diagnosis. J. Cancer Surviv. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Magbanua, M.J.M.; Weidner, G.; Weinberg, V.; Kemp, C.; Green, C.; Mattie, M.D.; Marlin, R.; Simko, J.; Shinohara, K.; et al. Changes in Prostate Gene Expression in Men Undergoing an Intensive Nutrition and Lifestyle Intervention. Proc. Natl. Acad. Sci. USA 2008, 105, 8369–8374. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.M.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased Telomerase Activity and Comprehensive Lifestyle Changes: A Pilot Study. Lancet Oncol. 2008, 9, 1048–1057. [Google Scholar] [CrossRef]
- Bodai, B.I.; Tuso, P. Breast Cancer Survivorship: A Comprehensive Review of Long-Term Medical Issues and Lifestyle Recommendations. Perm. J. 2015, 19, 48–79. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of Diet on Mortality and Cancer Recurrence among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report. 2018. Available online: https://www.wcrf.org/dietandcancer (accessed on 4 September 2020).
- Ferrini, K.; Ghelfi, F.; Mannucci, R.; Titta, L. Lifestyle, Nutrition and Breast Cancer: Facts and Presumptions for Consideration. Ecancermedicalscience 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR Guidelines for Cancer Prevention is Associated with Lower Mortality among Older Female Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, N.; Geelen, A.; Winkels, R.M.; Mwungura, B.; Fedirko, V.; Jenab, M.; Illner, A.K.; Brenner, H.; Ordonez-Mena, J.M.; De Jong, J.C.K.; et al. Adherence to the WCRF/AICR Dietary Recommendations for Cancer Prevention and Risk of Cancer in Elderly from Europe and the United States: A Meta-Analysis within the CHANCES Project. Cancer Epidemiol. Biomark. Prev. 2017, 26, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Chan, D.S.M.; Mitrou, P.; Norat, T.; Romaguera, D. A Systematic Review and Meta-Analysis of the 2007 WCRF/AICR Score in Relation to Cancer-Related Health Outcomes. Ann. Oncol. 2020, 31, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Ward, H.; Wark, P.A.; Vergnaud, A.C.; Peeters, P.H.; van Gils, C.H.; Ferrari, P.; Fedirko, V.; Jenab, M.; Boutron-Ruault, M.C.; et al. Pre-Diagnostic Concordance with the WCRF/AICR Guidelines and Survival in European Colorectal Cancer Patients: A Cohort Study. BMC Med. 2015, 13, 1–12. [Google Scholar] [CrossRef]
- Tollosa, D.N.; Tavener, M.; Hure, A.; James, E.L. Adherence to Multiple Health Behaviours in Cancer Survivors: A Systematic Review and Meta-Analysis. J. Cancer Surviv. 2019, 13, 327–343. [Google Scholar] [CrossRef]
- Buzaglo, J.S.; Zaleta, A.K.; McManus, S.; Golant, M.; Miller, M.F. Cancer Support Source (R): Validation of a Revised Multi-Dimensional Distress Screening Program for Cancer Patients and Survivors. Supportive Care Cancer 2019. [Google Scholar] [CrossRef]
- Miller, M.F.; Mullins, C.D.; Onukwugha, E.; Golant, M.; Buzaglo, J.S. Discriminatory Power of a 25-Item Distress Screening Tool: A Cross-Sectional Survey of 251 Cancer Survivors. Qual. Life Res. 2014, 23, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Koshimoto, S.; Arimoto, M.; Saitou, K.; Uchibori, M.; Hashizume, A.; Honda, A.; Amano, K.; Nakajima, Y.; Uetake, H.; Matsushima, E. Need and Demand for Nutritional Counselling and Their Association with Quality of Life, Nutritional Status and Eating-Related Distress among Patients with Cancer Receiving Outpatient Chemotherapy: A Cross-Sectional Study. Supportive Care Cancer 2019, 27, 3385–3394. [Google Scholar] [CrossRef]
- Kotronoulas, G.; Papadopoulou, C.; Burns-Cunningham., K.; Simpson, M.; Maguire, R. A systematic review of the supportive care needs of people living with and beyond cancer of the colon and/or rectum. Eur. J. Oncol. Nurs. 2017, 29, 60–70. [Google Scholar] [CrossRef]
- Beeken, R.J.; Williams, K.; Wardle, J.; Croker, H. “What about Diet?” A Qualitative Study of Cancer Survivors’ Views on Diet and Cancer and Their Sources of Information. Eur. J. Cancer Care Engl. 2016, 25, 774–783. [Google Scholar] [CrossRef]
- Campbell, M.K.; Carr, C.; Devellis, B.; Switzer, B.; Biddle, A.; Amamoo, M.A.; Walsh, J.; Zhou, B.; Sandler, R. A Randomized Trial of Tailoring and Motivational Interviewing to Promote Fruit and Vegetable Consumption for Cancer Prevention and Control. Ann. Behav. Med. 2009, 38, 71–85. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the Crest of the Teachable Moment: Promoting Long-Term Health after the Diagnosis of Cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [PubMed]
- Contento, I.R. Nutrition Education: Linking Research, Theory, and Practice. Asia Pac. J. Clin. Nutr. 2008, 17, 176–179. [Google Scholar] [PubMed]
- Coa, K.I.; Smith, K.C.; Klassen, A.C.; Caulfield, L.E.; Helzlsouer, K.; Peairs, K.; Shockney, L. Capitalizing on the “Teachable Moment” to Promote Healthy Dietary Changes among Cancer Survivors: The Perspectives of Health Care Providers. Supportive Care Cancer 2015, 23, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.F.; Bender, A.G.; Feeney, C.; Kaplan, J.; McGinley-Gieser, D.; Petrucci, J.; Santangelo, A.; Saxton, C.; Soult, B.; Sutton, A.; et al. Evaluation of a Community-Based Experiential Nutrition and Cooking Education Program for Cancer Survivors. Ann. Behav. Med. 2018, 52, S497. [Google Scholar]
- Bandura, A. Self-Efficacy: Toward a Unifying Theory of Behavioral Change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Velicer, W.F. The Transtheoretical Model of Health Behavior Change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Graffigna, G.; Barello, S. Spotlight on the Patient Health Engagement Model (PHE Model): A Psychosocial Theory to Understand People’s Meaningful Engagement in Their Own Health Care. Patient Prefer. Adherence 2018, 12, 1261–1271. [Google Scholar] [CrossRef]
- Golant, M.; Thiboldeaux, K. The Wellness Community’s Integrative Model of Evidence-Based Psychosocial Programs, Services, and Interventions. Psycho-Oncology 2010. [Google Scholar] [CrossRef]
- Golant, M.; Zaleta, A.; Ash-Lee, S.; Buzaglo, J.; Stein, K.; Saxton, C.; Donzinger, M.; Thiboldeaux, K.; House, L. The Engaged Patient: The Cancer Support Community’s Comprehensive Model of Psychosocial Programs, Services and Research. In PsychoOncology, 4th ed.; Breitbart, W.S., Butow, P.N., Jacobsen, P.B., Lam, W., Lazenby, M., Loscalzo, M.J., Eds.; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Leszcz, M.; Goodwin, P.J. The Rationale and Foundations of Group Psychotherapy for Women with Metastatic Breast Cancer. Int. J. Group Psychother. 1998, 48, 245–273. [Google Scholar] [CrossRef]
- Borek, A.J.; Abraham, C. How Do Small Groups Promote Behaviour Change? An Integrative Conceptual Review of Explanatory Mechanisms. Appl. Psychol. Health Well-Being 2018, 10, 30–61. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Clipp, E.C.; Mcbride, C.; Lobach, D.F.; Lipkus, I.; Peterson, B.; Clutter Snyder, D.; Sloane, R.; Arbanas, J.; Kraus, W.E. Design of FRESH START: A Randomized Trial of Exercise and Diet among Cancer Survivors. Med. Sci. Sport. Exerc. 2003, 35. [Google Scholar] [CrossRef] [PubMed]
- Vijan, S.; Stuart, N.S.; Fitzgerald, J.T.; Ronis, D.L.; Hayward, R.A.; Slater, S.; Hofer, T.P. Barriers to Following Dietary Recommendations in Type 2 Diabetes. Diabet. Med. 2005, 22, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Midthune, D.; Kahle, L.; Dodd, K.W. Development and Evaluation of the National Cancer Institute’s Dietary Screener Questionnaire Scoring Algorithms. J. Nutr. 2017, 147, 1226–1233. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy Scale: Development and Validation of the General Measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Yanez, B.; Pearman, T.; Lis, C.G.; Beaumont, J.L.; Cella, D. The FACT-G7: A Rapid Version of the Functional Assessment of Cancer Therapy-General (FACT-G) for Monitoring Symptoms and Concerns in Oncology Practice and Research. Ann. Oncol. 2013, 24, 1073–1078. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Löwe, B. An Ultra-Brief Screening Scale for Anxiety and Depression: The PHQ-4. Psychosomatics 2009, 50, 613–621. [Google Scholar] [CrossRef]
- Hann, D.M.; Jacobsen, P.B.; Azzarello, L.M.; Martin, S.C.; Curran, S.L.; Fields, K.K.; Greenberg, H.; Lyman, G. Measurement of Fatigue in Cancer Patients: Development and Validation of the Fatigue Symptom Inventory. Qual. Life Res. 1998, 7, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Cyranowski, J.M.; Zill, N.; Bode, R.; Butt, Z.; Kelly, M.A.R.; Pilkonis, P.A.; Salsman, J.M.; Cella, D. Assessing Social Support, Companionship, and Distress: National Institute of Health (NIH) Toolbox Adult Social Relationship Scales. Health Psychol. 2013, 32, 293–301. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-805-80283-2. [Google Scholar]
- Machin, D.; Fayer, P.M. Quality of Life: The Assessment, Analysis and Interpretation of Patient-Reported Outcomes; John Wiley and Sons: Chichester, UK, 2013; ISBN 978-1-118-69945-4. [Google Scholar]
- Greenlee, H.; Santiago-Torres, M.; McMillen, K.K.; Ueland, K.; Haase, A.M. Helping Patients Eat Better During and Beyond Cancer Treatment. Cancer J. 2019, 25, 320–328. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, S.Y.; Choi, G.S. Facilitators and Barriers to Adoption of a Healthy Diet in Survivors of Colorectal Cancer. J. Nurs. Scholarsh. 2019, 51, 509–517. [Google Scholar] [CrossRef]
- Corbett, T.; Cheetham, T.; Müller, A.M.; Slodkowska-Barabasz, J.; Wilde, L.; Krusche, A.; Richardson, A.; Foster, C.; Watson, E.; Little, P.; et al. Exploring Cancer Survivors’ Views of Health Behaviour Change: “Where Do You Start, Where Do You Stop with Everything?”. Psychooncology 2018, 27, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, L.; Koch, P.A.; Liu, A.; Galitzdorfer, L.; Costa, A.; Utter, J. Experiential Features of Culinary Nutrition Education That Drive Behavior Change: Frameworks for Research and Practice. Health Promot. Pract. 2020, 704, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; Stricker, C.T.; Brown, J.C.; Berardi, J.M.; Vaughn, D.; Domchek, S.; Filseth, S.; Branas, A.; Weiss-Trainor, E.; Schmitz, K.H.; et al. Evaluation of a Web-Based Weight Loss Intervention in Overweight Cancer Survivors Aged 50 Years and Younger. Obes. Sci. Pract. 2017, 3, 83–94. [Google Scholar] [CrossRef]
- Greenlee, H.; Gaffney, A.O.; Aycinena, A.C.; Koch, P.; Contento, I.; Karmally, W.; Richardson, J.M.; Shi, Z.; Lim, E.; Tsai, W.Y.; et al. Long-Term Diet and Biomarker Changes after a Short-Term Intervention among Hispanic Breast Cancer Survivors: The Cocinar Para Su Salud! Randomized Controlled Trial. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Aycinena, A.C.; Jennings, K.A.; Gaffney, A.O.; Koch, P.A.; Contento, I.R.; Gonzalez, M.; Guidon, E.; Karmally, W.; Hershman, D.; Greenlee, H. Cocinar Para Su Salud! Development of a Culturally Based Nutrition Education Curriculum for Hispanic Breast Cancer Survivors Using a Theory-Driven Procedural Model. Health Educ. Behav. 2017, 44, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Cases, M.G.; Cantor, A.B.; Frugé, A.D.; Smith, K.P.; Locher, J.; Cohen, H.J.; Tsuruta, Y.; Daniel, M.; Kala, R.; et al. Pilot Randomized Controlled Trial of a Home Vegetable Gardening Intervention among Older Cancer Survivors Shows Feasibility, Satisfaction, and Promise in Improving Vegetable and Fruit Consumption, Reassurance of Worth, and the Trajectory of Central Adiposity. J. Acad. Nutr. Diet. 2018, 118, 689–704. [Google Scholar] [CrossRef]
- Funnell, M.M.; Anderson, R.M.; Arnold, M.S.; Barr, P.A.; Donnelly, M.; Johnson, P.D.; Taylor-Moon, D.; White, N.H. Empowerment: An Idea Whose Time Has Come in Diabetes Education. Diabetes Educ. 1991, 17, 37–41. [Google Scholar] [CrossRef]
- Bravo, P.; Edwards, A.; Barr, P.J.; Scholl, I.; Elwyn, G.; McAllister, M. Conceptualising Patient Empowerment: A Mixed Methods Study. BMC Health Serv. Res. 2015, 15. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Alfano, C.M.; Maitin-Shepard, M.; Thomson, C.A.; Schmitz, K.H.; Pinto, B.M.; Stein, K.; Zucker, D.S.; Syrjala, K.L.; Fallon, E.; et al. Agenda for Translating Physical Activity, Nutrition, and Weight Management Interventions for Cancer Survivors into Clinical and Community Practice. Obesity 2017, 25, S9–S22. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; Merluzzi, T.; Alivernini, F.; De Laurentiis, M.; Botti, G.; Giordano, A. A Meta-Analytic Review of the Relationship of Cancer Coping Selfefficacy with Distress and Quality of Life. Oncotarget 2017, 8, 36800–36811. [Google Scholar] [CrossRef]
- Dockham, B.; Schafenacker, A.; Yoon, H.; Ronis, D.L.; Kershaw, T.; Titler, M.; Northouse, L. Implementation of a Psychoeducational Program for Cancer Survivors and Family Caregivers at a Cancer Support Community Affiliate: A Pilot Effectiveness Study. Cancer Nurs. 2016, 39, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.E.; Lipkus, I.; Sloane, R.; Snyder, D.C.; Lobach, D.F.; Demark-Wahnefried, W. Long-Term Outcomes of the FRESH START Trial: Exploring the Role of Self-Efficacy in Cancer Survivors’ Maintenance of Dietary Practices and Physical Activity. Psychooncology 2013, 22, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Yang, Q.; Wang, A.N.; Zhang, J.P. Psychometric Properties and Performance of Existing Self-Efficacy Instruments in Cancer Populations: A Systematic Review. Health Qual. Life Outcomes 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Náfrádi, L.; Nakamoto, K.; Schulz, P.J. Is Patient Empowerment the Key to Promote Adherence? A Systematic Review of the Relationship between Self-Efficacy, Health Locus of Control and Medication Adherence. PLoS ONE 2017, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, E.; Fumagalli, L.P.; Radaelli, G.; Bertele, P.; Vogt, J.; Hammerschmidt, R.; Lara, J.L.; Carriazo, A.; Masella, C. Empowering Patients through EHealth: A Case Report of a Pan-European Poject Health Policy, Reform, Governance and Law. BMC Health Serv. Res. 2015, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Pomerleau, J.; Lock, K.; Knai, C.; McKee, M. Interventions Designed to Increase Adult Fruit and Vegetable Intake Can Be Effective: A Systematic Review of the Literature. J. Nutr. 2005, 135, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of Changes in Health-Related Quality of Life: The Remarkable Universality of Half a Standard Deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef]
- Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 2017, 9, 1003. [Google Scholar] [CrossRef]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of Nutrition and Physical Exercise Intervention in Palliative Cancer Patients: A Randomized Controlled Trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef]
- Grassi, L.; Caruso, R.; Mitchell, A.J.; Sabato, S.; Nanni, M.G. Screening for Emotional Disorders in Patients with Cancer Using the Brief Symptom Inventory (BSI) and the BSI-18 versus a Standardized Psychiatric Interview (the World Health Organization Composite International Diagnostic Interview). Cancer 2018, 124, 2415–2426. [Google Scholar] [CrossRef]
- Kuhnt, S.; Brähler, E.; Faller, H.; Härter, M.; Keller, M.; Schulz, H.; Wegscheider, K.; Weis, J.; Boehncke, A.; Hund, B.; et al. Twelve-Month and Lifetime Prevalence of Mental Disorders in Cancer Patients. Psychother. Psychosom. 2016, 85, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of Depression, Anxiety, and Adjustment Disorder in Oncological, Haematological, and Palliative-Care Settings: A Meta-Analysis of 94 Interview-Based Studies. Lancet. Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Mehnert, A.; Hartung, T.J.; Friedrich, M.; Vehling, S.; Brähler, E.; Härter, M.; Keller, M.; Schulz, H.; Wegscheider, K.; Weis, J.; et al. One in Two Cancer Patients is Significantly Distressed: Prevalence and Indicators of Distress. Psychooncology 2018, 27, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in Cancer: The Many Biobehavioral Pathways Driving Tumor Progression. Cancer Treat. Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef]
- Arnold, E.M. The Cessation of Cancer Treatment as a Crisis. Soc Work Health Care. 2008, 1389, 37–41. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A Randomised Controlled Trial of Dietary Improvement for Adults with Major Depression (the “SMILES” Trial). BMC Med. 2017, 15, 1–13. [Google Scholar] [CrossRef]
- Giese-Davis, J.; Brandelli, Y.; Kronenwetter, C.; Golant, M.; Cordova, M.; Twirbutt, S.; Chang, V.; Kraemer, H.C.; Spiegel, D. Illustrating the Multi-Faceted Dimensions of Group Therapy and Support for Cancer Patients. Healthcare 2016, 4, 48. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Treatment & Survivorship: Facts & Figures 2019–2021; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
| Measure/Component | Description |
|---|---|
| Intervention Components | |
| Eight, in-person, 90-min group meetings convened weekly at community-based organizational facilities | |
| (1) Nutrition Education | Registered dietitians educated participants using slide presentations about the American Institute for Cancer Research’s (AICR’s) Recommendations for Cancer Prevention, “New American Plate®”, and “Foods That Fight Cancer (FTFC)™” using 8 modules. Module 1: AICR Recommendations for Cancer Prevention Module 2: AICR’s New American Plate and One-Pot Meals Module 3: Beans and Whole Grains Module 4: Breakfast and Snacks Module 5: Comfort Foods Module 6: Veggies Module 7: Building a FTFC Kitchen Module 8: Sharing and Caring Potluck |
| (2) Structured Group Learning and Support | Licensed social workers provided support through a structured and empowering group learning environment to address the complex, important (and, unfortunately, often rarely openly discussed) psychosocial barriers to nutrition behavior change in the context of cancer prevention, treatment, and survivorship. |
| (3) Cooking Demonstration | Culinary experts demonstrated convenient, easy, and tasty ways to prepare and cook FTFC and invited recipe tasting. |
| Sharing and Caring Potluck | The last in-person meeting of the program included a time to review the overall experience and engage in discussion. It was intended to explore milestones achieved, recognize precipitous moments of comprehension, connect to feelings related to the program ending, identify ongoing obstacles and/or challenges, identify changes and successes along the way, share ideas and hopes for continued success, and discuss take-aways from the group experience. |
| Recipe Cards | Each week participants received 2–3 printed recipe cards for foods exhibited and tasted during the culinary demonstration. Examples of recipes included Quinoa Salad, Everyday Green Smoothie, Southwestern Bean Salad, Buckwheat Cocoa-Chip Overnight Oats, Chili, and Whole Wheat Greek Pasta Salad. |
| Workbook | Pocket folders included written materials about dietary choices and recipe cards. |
| S.M.A.R.T Goal-Setting Worksheets | Each week participants completed a one-page worksheet that prompted them to identify one to three specific, measurable, actionable, relevant and time-bound goals that were revisited at the next session. |
| Control Group Components | |
| Printed Educational Materials | Participants in the control group received seven comprehensive summaries from Coping with Cancer in the Kitchen weekly module content and 14 recipe cards (two from each of the 7 weeks of culinary demonstrations). These were mailed to participants in one package upon completion of the baseline survey. |
| Pre–Post Outcome Measures | |
| Knowledge about a Plant-Based Diet | Participants rated their agreement (1 = Strongly disagree; 5 = Strongly agree) with six custom items developed by the research team, e.g., “I understand the benefits of consuming whole grains versus processed grains”. A composite score was calculated as the average of the 6 ratings (range 1–5; Cronbach’s alpha = 0.80). |
| Confidence Preparing a Variety of Plant Foods | Participants indicated “How sure are you that you could prepare the foods listed below in a tasty way?” (1 = Very unsure; 5 = Very sure). The 14-item scale included 4 whole grains; 4 beans, seeds and legumes; 3 green leafy vegetables; and 3 mixed foods, e.g., healthy one-pot meals. A composite score was calculated as the average of the 14 items (range 1–5; Cronbach’s alpha = 0.75). |
| Skills to Practice a Plant-Based Diet | Participants rated their agreement (1 = Strongly disagree; 5 = Strongly agree) with five custom items developed by the research team, e.g., “I am confident that I can create a kitchen environment that makes it easier to store, prepare, and consume fruits, vegetables, whole grains, and beans.”; the average of the five ratings was calculated to create a skills composite score (range 1–5; Cronbach’s alpha = 0.88). |
| Barriers to Eating More Fruits and Vegetables and Whole Grains | We adapted items from an existing barriers instrument [33,34] to measure perceived barriers to eating more fruits and vegetables (F&V) (average score of 15 items; Cronbach’s alpha = 0.89) and whole grains (average score of 14 items; Cronbach’s alpha = 0.83). Participants were asked the general question, “Listed below are some common reasons why people don’t eat more servings of vegetables and fruits each day. Indicate whether or not this is a reason for you by marking how much you agree or disagree.” (1 = Strongly disagree; 5 = Strongly agree). In addition, using the same list of possible reasons (excluding spoil too quickly), participants indicated whether it was a common reason they did not eat more servings of whole grains. Example reasons included take too much time to prepare; my family doesn’t like them; hard to find a variety of good ones. |
| Dietary Intake [Dietary Screener Questionnaire (DSQ) in the NHANES 2009-10] https://epi.grants.cancer.gov/nhanes/dietscreen/ | A 26-item dietary screener developed by the National Cancer Institute [35], which we shortened to include 17 questions that ask about the frequency of intake in the past month of F&V, whole grains, and processed and red meats. Scoring algorithms convert screener responses to estimates of daily intake of cup equivalents of F&V, including legumes and excluding French fries, and whole grains (ounce equivalents). Frequency responses to the processed meat question is converted to times per day. The DSQ provides a less burdensome alternative to 24-h recall when interest is in a limited set of dietary factors. We piloted use of the DSQ during the 2017 single-arm pilot study; intake was comparable to those participating in the National Health and Nutrition Examination Survey 2009–2010 [35]. |
| General Quality of Life [a rapid version of the Functional Assessment of Cancer Therapy-General (FACT-G7)] | The Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire is a general quality of life instrument that can be used to assess top-rated symptoms and concerns in cancer patients [36]. The FACT-G7 is a brief 7-item adaptation [37]. Internal consistency and reliability in the present study was good (Cronbach’s alpha = 0.80). |
| Psychological Distress [4-item Patient Health Questionnaire for Depression and Anxiety (PHQ-4)] | The PHQ-4 is a brief 4-item validated screening scale for measuring core symptoms and signs of depression and anxiety [38]. |
| Fatigue | Participants were asked to rate their level of fatigue on the average in the last week [0 = Not at all fatigued; 10 = Fatigued as I could be]. This item comes from the Fatigue Symptom Inventory that assesses the frequency and severity of fatigue and its perceived interference [39]. |
| Emotional Support [NIH Toolbox® Emotional Support Fixed Form Age 18+ v 2.0, Short Form (SOC8)] | Participants completed the SOC8 which measures emotional support, or the perceived availability of someone to provide empathy or advice in times of need [40]. Higher scores represent more emotional support. Scores are converted to standardized T scores (mean = 50, standard deviation = 10); normative reference groups are the US general population. |
| Perceived Control over Course of Cancer | Participants were asked, “To what extent do you feel you have control over the course of your cancer (that is, whether your cancer will come back, get worse, or you will develop a different type of cancer)?” (0 = No control at all; 4 = Complete control). |
| Total Sample (N = 53) | Coping with Cancer in the Kitchen Intervention (n = 26) | Printed Materials Control (n = 27) | p-Value a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | No. of Participants | % | No. of Participants | % | No. of Participants | % | ||||
| Sociodemographic Characteristics | ||||||||||
| Age, years | 0.25 | |||||||||
| Mean | 61.2 | 59.5 | 62.8 | |||||||
| SD | 10.5 | 9.7 | 11.1 | |||||||
| Range | 37–80 | 37–74 | 41–80 | |||||||
| Gender | 0.99 | |||||||||
| Female | 49 | 92 | 24 | 92 | 25 | 93 | ||||
| Male | 4 | 8 | 2 | 8 | 2 | 7 | ||||
| Race | 0.43 | |||||||||
| Non-Hispanic white | 41 | 77 | 18 | 69 | 23 | 85 | ||||
| Non-Hispanic black | 4 | 8 | 2 | 8 | 2 | 7 | ||||
| Non-Hispanic, other | 6 | 11 | 4 | 15 | 2 | 7 | ||||
| Hispanic or Latino | 2 | 4 | 2 | 8 | 0 | 0 | ||||
| Education | 0.30 | |||||||||
| Did not graduate college | 14 | 26 | 6 | 23 | 8 | 30 | ||||
| College graduate | 16 | 30 | 6 | 23 | 10 | 37 | ||||
| Postgraduate | 23 | 43 | 14 | 54 | 9 | 33 | ||||
| Geographic region | 0.53 | |||||||||
| Urban | 21 | 40 | 11 | 44 | 10 | 37 | ||||
| Suburban | 27 | 52 | 11 | 44 | 16 | 59 | ||||
| Rural | 3 | 6 | 2 | 8 | 1 | 4 | ||||
| Marital status | 0.43 | |||||||||
| Married or living as married | 27 | 52 | 15 | 60 | 12 | 44 | ||||
| Single (never married) | 13 | 25 | 6 | 24 | 7 | 26 | ||||
| Divorced or separated | 9 | 17 | 4 | 16 | 5 | 19 | ||||
| Widowed | 3 | 6 | 0 | 0 | 3 | 11 | ||||
| Disease Characteristics | ||||||||||
| Time since cancer diagnosis | 0.57 | |||||||||
| Mean | 4.9 | 5.4 | 4.5 | |||||||
| SD | 5.9 | 6.8 | 5.0 | |||||||
| Range | <1 to 27 | <1 to 27 | <1 to 21 | |||||||
| Primary cancer diagnosis | 0.18 | |||||||||
| Breast | 25 | 47 | 9 | 35 | 16 | 59 | ||||
| Metastatic breast | 6 | 11 | 5 | 19 | 1 | 4 | ||||
| Blood | 5 | 9 | 2 | 8 | 3 | 11 | ||||
| Female reproductive | 4 | 8 | 3 | 12 | 1 | 4 | ||||
| Multiple cancers specified | 6 | 11 | 2 | 8 | 4 | 15 | ||||
| Other | 7 | 13 | 5 | 19 | 2 | 7 | ||||
| Ever received chemotherapy | ||||||||||
| Yes | 29 | 56 | 14 | 56 | 15 | 56 | 0.97 | |||
| No | 23 | 44 | 11 | 44 | 12 | 44 | ||||
| Health Status | ||||||||||
| Perceived health | 0.87 | |||||||||
| Excellent | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Very good | 15 | 31 | 8 | 33 | 7 | 28 | ||||
| Good | 23 | 47 | 11 | 46 | 12 | 48 | ||||
| Fair | 10 | 20 | 4 | 17 | 6 | 24 | ||||
| Poor | 1 | 2 | 1 | 4 | 0 | 0 | ||||
| Body Mass Index, kg/m2 | 0.84 | |||||||||
| Underweight (<18.5) | 2 | 4 | 1 | 4 | 1 | 4 | ||||
| Normal (18.5–24.9) | 17 | 32 | 10 | 38 | 7 | 26 | ||||
| Overweight (25.0–29.9) | 13 | 25 | 6 | 23 | 7 | 26 | ||||
| Obese (>30) | 21 | 40 | 9 | 35 | 12 | 44 | ||||
| In the last month, ate enough plant-based foods | 0.73 | |||||||||
| None of the time | 1 | 2 | 1 | 4 | 0 | 0 | ||||
| A little of the time | 7 | 14 | 4 | 17 | 3 | 12 | ||||
| Some of the time | 19 | 39 | 8 | 33 | 11 | 44 | ||||
| Most of the time | 16 | 33 | 9 | 38 | 7 | 28 | ||||
| All of the time | 6 | 12 | 2 | 8 | 4 | 16 |
| Coping with Cancer in the Kitchen Intervention | Printed Materials Control | Adjusted Difference (95% CI) between Intervention and Control Groups b | ||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Baseline (n = 24) | Post-Intervention (n = 23) | 15-Week Follow-Up (n = 15) | Baseline (n = 25) | Post-Intervention (n = 24) | 15-Week Follow-Up (n = 15) | Post-Intervention | 15-Week Follow-Up |
| Primary Outcomes | ||||||||
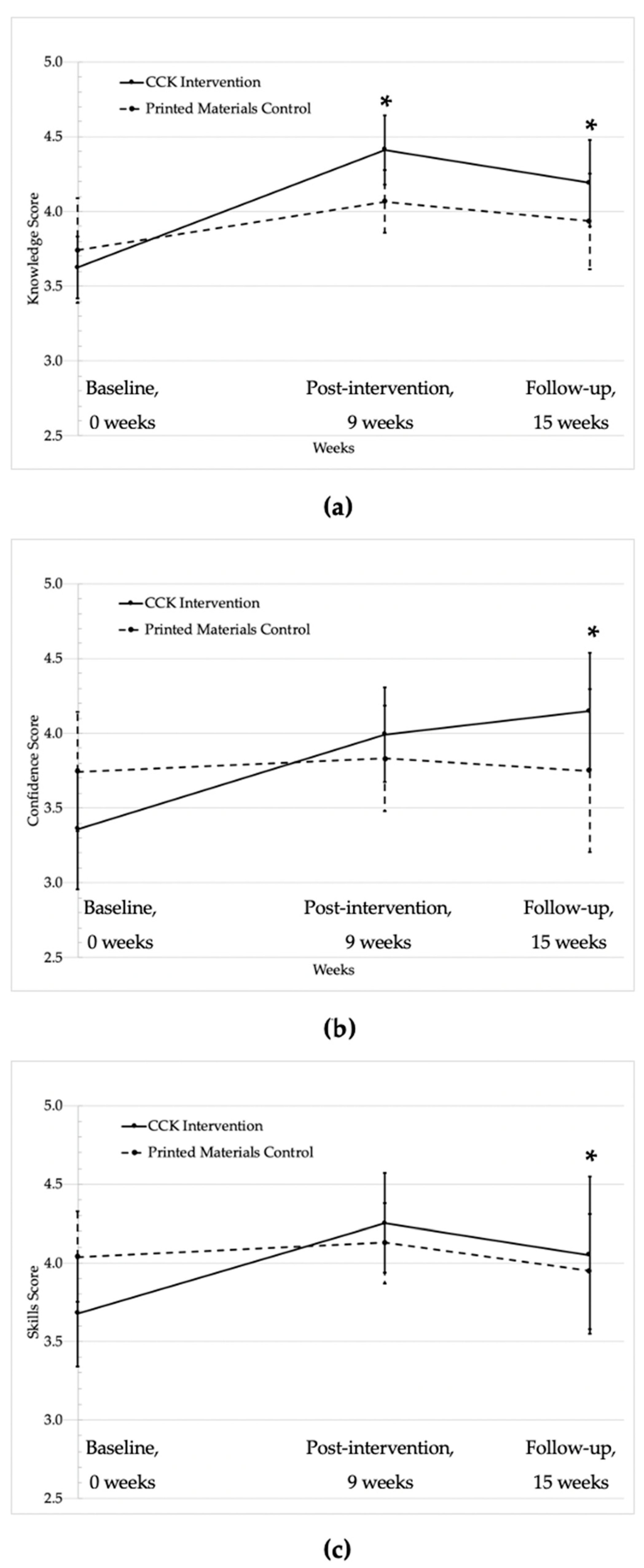
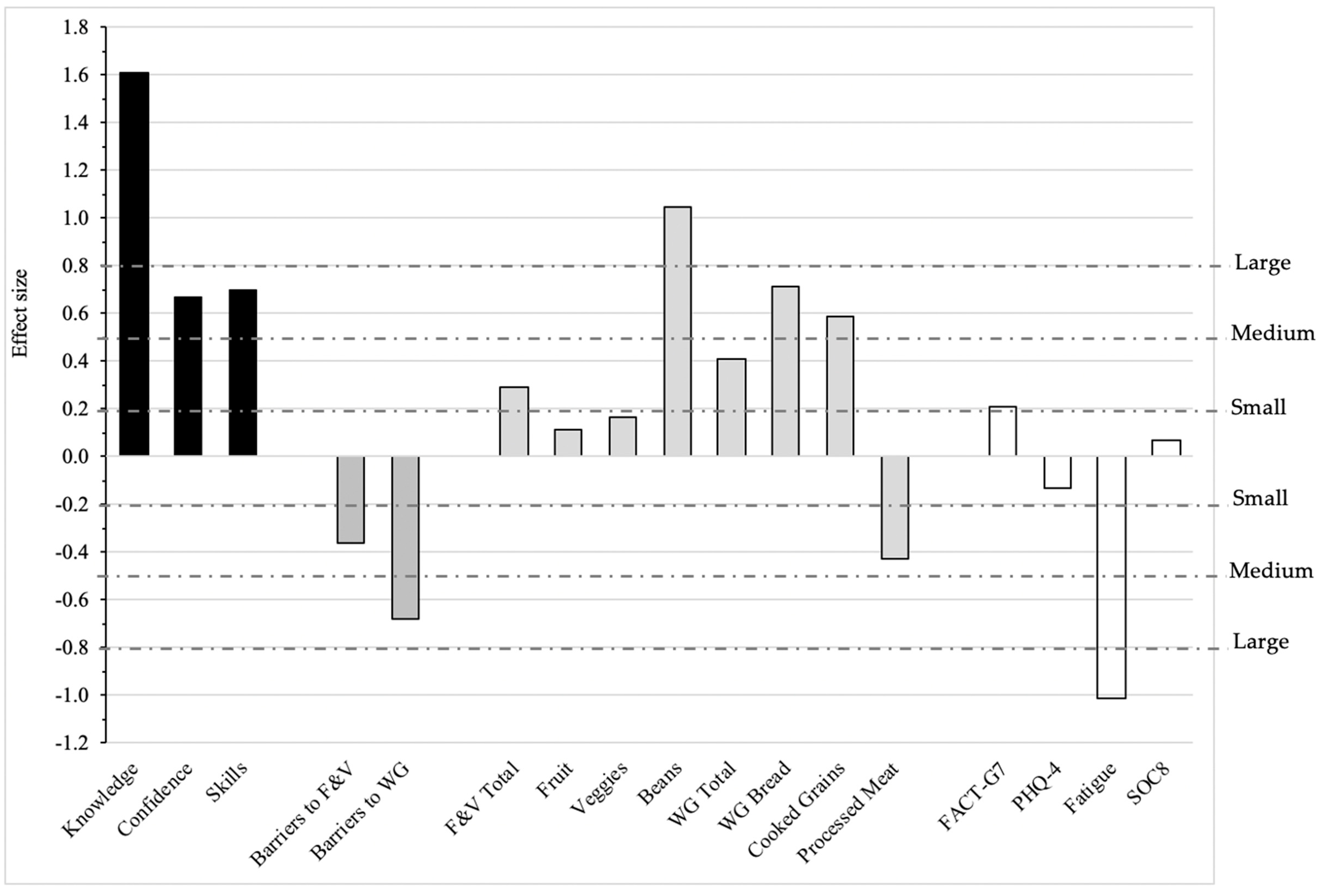
| Knowledge about a Plant-Based Diet | 0.36 * (0.06, 0.67) | 0.54 * (0.11, 0.98) | ||||||
| Mean | 3.6 | 4.4 | 4.2 | 3.7 | 4.1 | 3.9 | ||
| SD | 0.5 | 0.5 | 0.5 | 0.8 | 0.5 | 0.6 | ||
| Within-arm mean difference | +0.8 c,* | +0.6 d,* | +0.4 e | +0.2 f | ||||
| Confidence Preparing a Variety of Plant Foods | 0.36 (−0.02, 0.74) | 0.83 * (0.23, 1.42) | ||||||
| Mean | 3.4 | 4.0 | 4.1 | 3.7 | 3.8 | 3.7 | ||
| SD | 0.9 | 0.8 | 1.0 | 1.0 | 0.8 | 0.7 | ||
| Within-arm mean difference | +0.6 * | +0.7 * | +0.1 | 0 | ||||
| Skills to Practice a Plant-Based Diet | 0.28 (−0.09, 0.64) | 0.65 * (0.16, 1.14) | ||||||
| Mean | 3.7 | 4.3 | 4.1 | 4.0 | 4.1 | 3.9 | ||
| SD | 0.8 | 0.7 | 0.9 | 0.7 | 0. | 0.7 | ||
| Within-arm mean difference | +0.6 * | +0.4 * | +0.1 | −0.1 | ||||
| Perceived Barriers | ||||||||
| Perceived Barriers to Eating More Fruits and Vegetables | −0.37 * (−0.64, −0.10) | NA | ||||||
| Mean | 2.6 | 2.4 | -- | 2.4 | 2.5 | -- | ||
| SD | 0.6 | 0.7 | -- | 0.8 | 0.8 | -- | ||
| Within-arm mean difference | −0.2 * | -- | +0.1 | -- | ||||
| Perceived Barriers to Eating More Whole Grains | −0.24 (−0.56, 0.07) | NA | ||||||
| Mean | 2.6 | 2.3 | -- | 2.6 | 2.5 | -- | ||
| SD | 0.5 | 0.7 | -- | 0.6 | 0.7 | -- | ||
| Within-arm mean difference | −0.3 * | -- | −0.1 | -- | ||||
| Dietary Intake | ||||||||
| Total Fruit and Vegetable, cup equivalents per day g | 0.17 (−0.13, 0.47) | −0.19 (−0.55, 0.17) | ||||||
| Mean | 2.78 | 2.99 | 2.86 | 2.64 | 2.70 | 2.76 | ||
| SD | 0.73 | 0.85 | 0.84 | 0.76 | 0.76 | 0.88 | ||
| Within-arm mean difference | +0.21 | +0.08 | +0.06 | +0.12 | ||||
| Whole Grains Total, ounce equivalents per day h | 0.06 (−0.18, 0.30) | 0.01 (−0.24, 0.27) | ||||||
| Mean | 0.71 | 0.83 | 0.82 | 0.78 | 0.82 | 0.89 | ||
| SD | 0.30 | 0.46 | 0.36 | 0.49 | 0.39 | 0.43 | ||
| Within-arm mean difference | +0.12 | +0.11 | +0.04 | +0.11 | ||||
| Processed Meat, times per day | −0.08 * (−0.15, −0.02) | −0.09 * (−0.18, −0.01) | ||||||
| Mean | 0.14 | 0.04 | 0.04 | 0.08 | 0.10 | 0.11 | ||
| SD | 0.23 | 0.05 | 0.05 | 0.15 | 0.18 | 0.17 | ||
| Range | −0.10 * | −0.10 * | +0.02 | +0.03 | ||||
| Quality of Life | ||||||||
| General Quality of Life, FACT-G7 Score | 0.63 (−1.45, 2.71) | −0.09 (−3.54, 3.36) | ||||||
| Mean | 17.1 | 18.1 | 17.1 | 17.8 | 18.0 | 18.4 | ||
| SD | 5.1 | 5.0 | 6.1 | 5.9 | 5.7 | 4.2 | ||
| Within-arm mean difference | +1.0 | 0 | +0.2 | +0.6 | ||||
| Psychological Distress, PHQ-4 | -0.83 (−2.13, 0.46) | −0.66 (−2.92, 1.60) | ||||||
| Mean | 3.25 | 2.87 | 3.53 | 2.67 | 3.17 | 2.73 | ||
| SD | 2.83 | 2.82 | 3.40 | 2.41 | 3.51 | 3.06 | ||
| Within-arm mean difference | −0.38 | +0.28 | +0.50 | +0.06 | ||||
| Fatigue, range 0–10 | −0.76 (−2.09, 0.57) | −0.22 (−1.59, 1.14) | ||||||
| Mean | 5.9 | 3.8 | 4.1 | 5.1 | 4.3 | 3.7 | ||
| SD | 2.1 | 2.4 | 2.3 | 2.3 | 2.6 | 2.0 | ||
| Within-arm mean difference | −2.1 * | −1.8 * | −0.8 | −1.4 | ||||
| Emotional Support, SOC8 T-score | 0.17 (−3.23, 3.57) | 1.67 (−3.80, 7.13) | ||||||
| Mean | 44.1 | 44.8 | 47.7 | 45.6 | 46.1 | 46.9 | ||
| SD | 9.9 | 8.4 | 9.8 | 11.2 | 11.2 | 12.6 | ||
| Within-arm mean difference | +0.7 | +3.6 | +0.5 | +1.3 | ||||
| Perceived Control Over Course of Cancer, range 0–4 | −0.18 (−0.63, 0.27) | 0.34 (−0.20, 0.88) | ||||||
| Mean | 1.4 | 1.5 | 1.9 | 1.1 | 1.4 | 1.0 | ||
| SD | 1.1 | 0.9 | 0.8 | 1.2 | 1.0 | 1.1 | ||
| Within-arm mean difference | +0.1 | +0.5 | +0.3 * | −0.1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, M.F.; Li, Z.; Habedank, M. A Randomized Controlled Trial Testing the Effectiveness of Coping with Cancer in the Kitchen, a Nutrition Education Program for Cancer Survivors. Nutrients 2020, 12, 3144. https://doi.org/10.3390/nu12103144
Miller MF, Li Z, Habedank M. A Randomized Controlled Trial Testing the Effectiveness of Coping with Cancer in the Kitchen, a Nutrition Education Program for Cancer Survivors. Nutrients. 2020; 12(10):3144. https://doi.org/10.3390/nu12103144
Chicago/Turabian StyleMiller, Melissa Farmer, Zhongyu Li, and Melissa Habedank. 2020. "A Randomized Controlled Trial Testing the Effectiveness of Coping with Cancer in the Kitchen, a Nutrition Education Program for Cancer Survivors" Nutrients 12, no. 10: 3144. https://doi.org/10.3390/nu12103144
APA StyleMiller, M. F., Li, Z., & Habedank, M. (2020). A Randomized Controlled Trial Testing the Effectiveness of Coping with Cancer in the Kitchen, a Nutrition Education Program for Cancer Survivors. Nutrients, 12(10), 3144. https://doi.org/10.3390/nu12103144





