Daily Protein and Energy Intake Are Not Associated with Muscle Mass and Physical Function in Healthy Older Individuals—A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Dietary Records
2.3. Physical Activity Level
2.4. Identification of Under- and Overreporters
2.5. Coefficient of Variation for Protein Distribution
2.6. Appendicular Lean Mass
2.7. Muscle Strength and Functional Capability
2.8. Food Questionnaire
2.9. Groups Division
2.10. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Total Daily Energy and Protein Intake and Distribution per Main Meal
3.3. Daily Protein Intakes and the ASMI, Grip Strength, MVC, 400 m Gait Time, 30 s Chair Stand
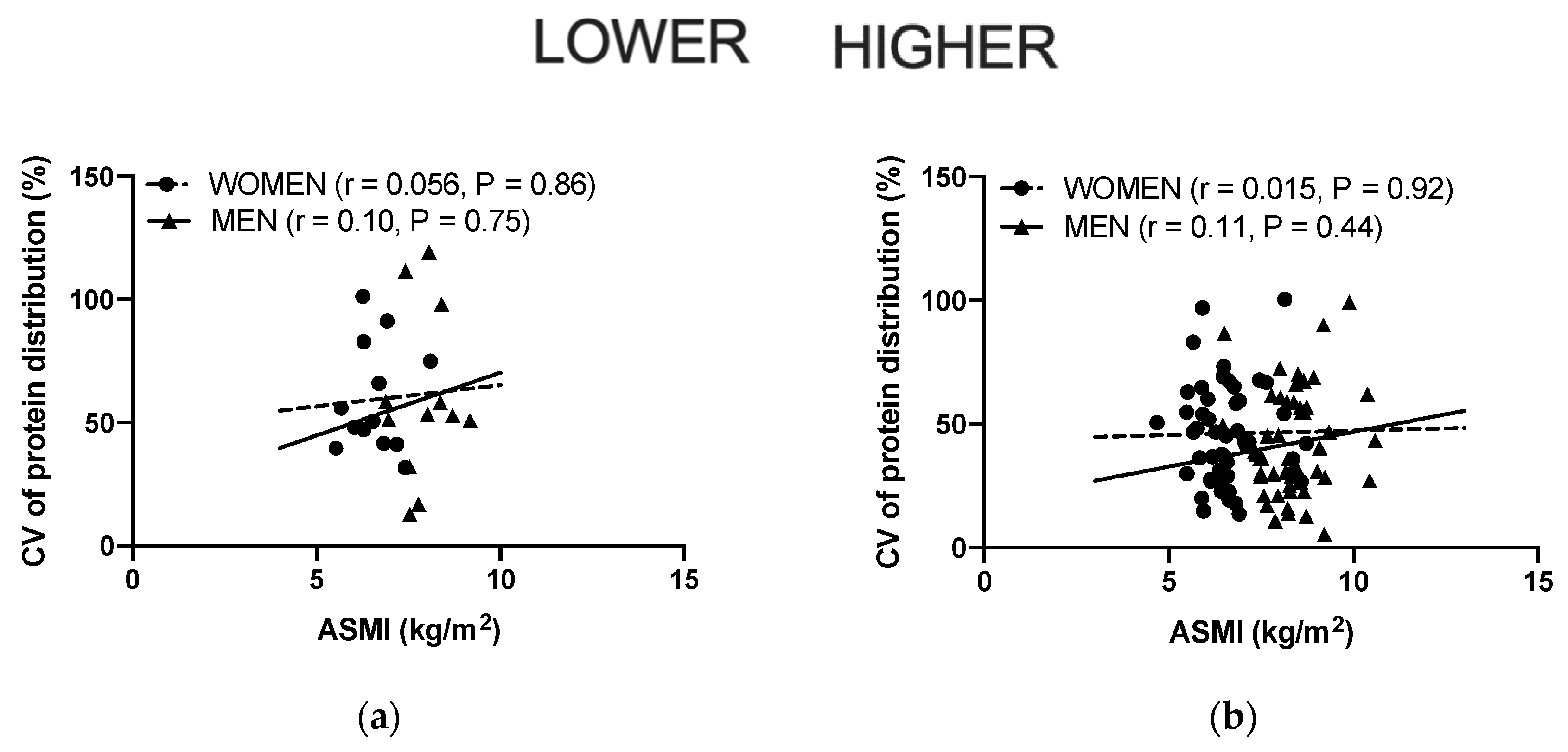
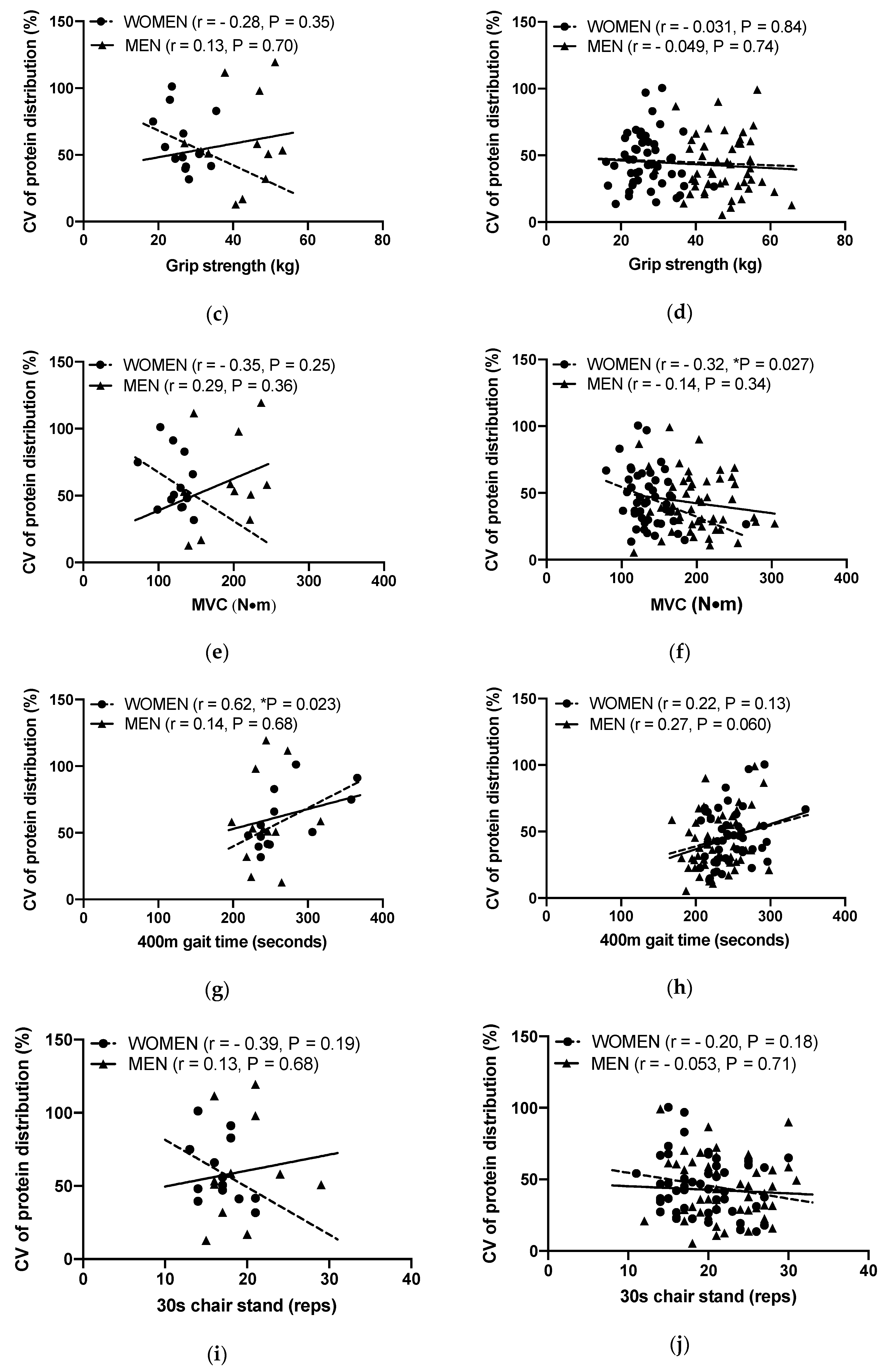
3.4. Associations between the Protein Distribution and ASMI, Grip Strength, MVC, 400 m Gait Time and 30 s Chair Stand
3.5. Associations between Protein (g/kg aBW) and Energy (kJ/kg aBW) Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paddon-Jones, D.; Campbell, W.W.; Jacques, P.F.; Kritchevsky, S.B.; Moore, L.L.; Rodriguez, N.R.; van Loon, L.J. Protein and healthy aging. Am. J. Clin. Nutr. 2015, 101, 1339S–1345S. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R.; Cifelli, A.M.; Kostas, G.; Kim, I.-Y. Optimizing protein intake in adults: Interpretation and application of the recommended dietary allowance compared with the acceptable macronutrient distribution range. Adv. Nutr. Int. Rev. J. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Traylor, D.A.; Gorissen, S.H.; Phillips, S.M. Perspective: Protein requirements and optimal intakes in aging: Are we ready to recommend more than the recommended daily allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. Ser. A Biol. Sci. Med Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney-Martin, G.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein Requirements during Aging. Nutrients 2016, 8, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergie. Scientific opinion on dietary reference values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition (2002: Geneva, S., Food and Agriculture Organization of the United Nations, World Health Organization, United Nations University. In Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007; Volume 935.
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.H.; Gomez, A.; Bergan, J.G.; Lee, T.C.; Monckeberg, F.; Chichester, C.O. Comparative nitrogen balance study between young and aged adults using three levels of protein intake from a combination wheat-soy-milk mixture. Am. J. Clin. Nutr. 1978, 31, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, W.W.; Johnson, C.A.; McCabe, G.P.; Carnell, N.S. Dietary protein requirements of younger and older adults. Am. J. Clin. Nutr. 2008, 88, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kröger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkilä, A.T. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br. J. Nutr. 2016, 115, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- McLean, R.R.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; Sahni, S. Dietary protein intake is protective against loss of grip strength among older adults in the framingham offspring cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahni, S.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; McLean, R.R. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef] [Green Version]
- ten Haaf, D.S.; van Dongen, E.J.; Nuijten, M.A.; Eijsvogels, T.M.; de Groot, L.C.; Hopman, M.T. Protein intake and distribution in relation to physical functioning and quality of life in community-dwelling elderly people: Acknowledging the role of physical activity. Nutrients 2018, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016. [Google Scholar] [CrossRef]
- Bohe, J.; Low, J.F.; Wolfe, R.R.; Rennie, M.J. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J. Physiol. 2001, 532, 575–579. [Google Scholar] [CrossRef]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007, 582, 813–823. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2013, 99, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.H.; Oikawa, S.Y.; Phillips, S.M. Dietary protein to maintain muscle mass in aging: A case for per-meal protein recommendations. J. Frailty Aging 2016, 5, 49–58. [Google Scholar] [CrossRef]
- Phillips, S.M.; Martinson, W. Nutrient-rich, high-quality, protein-containing dairy foods in combination with exercise in aging persons to mitigate sarcopenia. Nutr. Rev. 2019, 77, 216–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millward, D.J. Macronutrient intakes as determinants of dietary protein and amino acid adequacy. J. Nutr. 2004, 134, 1588S–1596S. [Google Scholar] [CrossRef]
- Kim, I.Y.; Shin, Y.A.; Schutzler, S.E.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Quality of meal protein determines anabolic response in older adults. Clin. Nutr. 2018, 37, 2076–2083. [Google Scholar] [CrossRef]
- Yoshii, N.; Sato, K.; Ogasawara, R.; Nishimura, Y.; Shinohara, Y.; Fujita, S. Effect of mixed meal and leucine intake on plasma amino acid concentrations in young men. Nutrients 2018, 10, 1543. [Google Scholar] [CrossRef] [Green Version]
- Reitelseder, S.; Tranberg, B.; Agergaard, J.; Dideriksen, K.; Højfeldt, G.; Merry, M.E.; Storm, A.C.; Poulsen, K.R.; Hansen, E.T.; van Hall, G.; et al. Phenylalanine stable isotope tracer labeling of cow milk and meat and human experimental applications to study dietary protein-derived amino acid availability. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Kim, I.Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.; Wolfe, R.R.; Ferrando, A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef]
- Steffee, W.P.; Goldsmith, R.S.; Pencharz, P.B.; Scrimshaw, N.S.; Young, V.R. Dietary protein intake and dynamic aspects of whole body nitrogen metabolism in adult humans. Metab. Clin. Exp. 1976, 25, 281–297. [Google Scholar] [CrossRef]
- Forslund, A.H.; Hambraeus, L.; Olsson, R.M.; El-Khoury, A.E.; Yu, Y.M.; Young, V.R. The 24-h whole body leucine and urea kinetics at normal and high protein intakes with exercise in healthy adults. Am. J. Physiol. 1998, 275, E310–E320. [Google Scholar] [CrossRef]
- Young, V.R.; El-Khoury, A.E.; Raguso, C.A.; Forslund, A.H.; Hambraeus, L. Rates of urea production and hydrolysis and leucine oxidation change linearly over widely varying protein intakes in healthy adults. J. Nutr. 2000, 130, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Højfeldt, G.; Bülow, J.; Agergaard, J.; Asmar, A.; Schjerling, P.; Simonsen, L.; Bülow, J.; van Hall, G.; Holm, L. Impact of habituated dietary protein intake on fasting and postprandial whole-body protein turnover and splanchnic amino acid metabolism in elderly men: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cardon-Thomas, D.K.; Riviere, T.; Tieges, Z.; Greig, C.A. Dietary protein in older adults: Adequate daily intake but potential for improved distribution. Nutrients 2017, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Rousset, S.; Patureau Mirand, P.; Brandolini, M.; Martin, J.F.; Boirie, Y. Daily protein intakes and eating patterns in young and elderly French. Br. J. Nutr. 2003, 90, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Berner, L.A.; Becker, G.; Wise, M.; Doi, J. Characterization of dietary protein among older adults in the United States: Amount, animal sources, and meal patterns. J. Acad. Nutr. Diet. 2013, 113, 809–815. [Google Scholar] [CrossRef]
- Calloway, D.H.; Spector, H. Nitrogen balance as related to caloric and protein intake in active young men. Am. J. Clin. Nutr. 1954, 2, 405–412. [Google Scholar] [CrossRef]
- Munro, H.N. Energy and protein intakes as determinants of nitrogen balance. Kidney Int. 1978, 14, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Carbone, J.W.; McClung, J.P.; Pasiakos, S.M. Recent advances in the characterization of skeletal muscle and whole-body protein responses to dietary protein and exercise during negative energy balance. Adv. Nutr. 2018. [Google Scholar] [CrossRef]
- Millward, D.J.; Jackson, A.A. Protein/energy ratios of current diets in developed and developing countries compared with a safe protein/energy ratio: Implications for recommended protein and amino acid intakes. Public Health Nutr. 2004, 7, 387–405. [Google Scholar] [CrossRef] [Green Version]
- Bechshoft, R.L.; Reitelseder, S.; Hojfeldt, G.; Castro-Mejia, J.L.; Khakimov, B.; Ahmad, H.F.; Kjaer, M.; Engelsen, S.B.; Johansen, S.M.; Rasmussen, M.A.; et al. Counteracting age-related loss of skeletal muscle mass: A clinical and ethnological trial on the role of protein supplementation and training load (CALM intervention study): Study protocol for a randomized controlled trial. Trials 2016, 17, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacht, R.S.; Lind, V.M.; Bechshoft, R.L.; Hojfeldt, G.; Reitelseder, S.; Jensen, T.; Jespersen, P.A.; Sandris Nielsen, D.; Holm, L.; Tetens, I. Investigating risk of suboptimal macro and micronutrient intake and their determinants in older danish adults with specific focus on protein intake—A cross-sectional study. Nutrients 2019, 11, 795. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Cunningham, J.J. Body composition as a determinant of energy expenditure: A synthetic review and a proposed general prediction equation. Am. J. Clin. Nutr. 1991, 54, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E. The sensitivity and specificity of the Goldberg cut-off for EI:BMR for identifying diet reports of poor validity. Eur. J. Clin. Nutr. 2000, 54, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but not amount of protein intake is associated with frailty: A cross-sectional investigation in the region of Nurnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Mertz, K.H.; Reitelseder, S.; Jensen, M.; Lindberg, J.; Hjulmand, M.; Schucany, A.; Binder Andersen, S.; Bechshoeft, R.L.; Jakobsen, M.D.; Bieler, T.; et al. Influence of between-limb asymmetry in muscle mass, strength, and power on functional capacity in healthy older adults. Scand. J. Med. Sci. Sports 2019, 29, 1901–1908. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Newman, A.B.; Simonsick, E.M.; Naydeck, B.L.; Boudreau, R.M.; Kritchevsky, S.B.; Nevitt, M.C.; Pahor, M.; Satterfield, S.; Brach, J.S.; Studenski, S.A.; et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006, 295, 2018–2026. [Google Scholar] [CrossRef] [Green Version]
- Hengeveld, L.M.; Pelgröm, A.D.; Visser, M.; Boer, J.M.; Haveman-Nies, A.; Wijnhoven, H.A. Comparison of protein intake per eating occasion, food sources of protein and general characteristics between community-dwelling older adults with a low and high protein intake. Clin. Nutr. ESPEN 2019, 29, 165–174. [Google Scholar] [CrossRef] [PubMed]
- de Hollander, E.; Van Zutphen, M.; Bogers, R.P.; Bemelmans, W.J.; De Groot, L. The impact of body mass index in old age on cause-specific mortality. J. Nutr. Health Aging 2012, 16, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Pitkäniemi, J.; Gao, W.; Heine, R.; Pyörälä, K.; Söderberg, S.; Stehouwer, C.D.; Zethelius, B.; Tuomilehto, J.; Laatikainen, T. Relationship between body mass index and mortality among Europeans. Eur. J. Clin. Nutr. 2012, 66, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and all-cause mortality in older adults: A meta-analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K. Nutritional recommendations for the management of sarcopenia. J. Am. Med Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Farsijani, S.; Payette, H.; Morais, J.A.; Shatenstein, B.; Gaudreau, P.; Chevalier, S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am. J. Clin. Nutr. 2017, 106, 113–124. [Google Scholar] [CrossRef]
- Kim, I.Y.; Schutzler, S.; Schrader, A.M.; Spencer, H.J.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: A randomized-controlled trial. Clin. Nutr. 2018, 37, 488–493. [Google Scholar] [CrossRef]
- Hudson, J.L.; Campbell, W.W. Protein distribution and muscle-related outcomes: Does the evidence support the concept? Nutrients 2020, 12, 1441. [Google Scholar] [CrossRef]
- Gingrich, A.; Spiegel, A.; Kob, R.; Schoene, D.; Skurk, T.; Hauner, H.; Sieber, C.C.; Volkert, D.; Kiesswetter, E. Amount, distribution, and quality of protein intake are not associated with muscle mass, strength, and power in healthy older adults without functional limitations-an enable study. Nutrients 2017, 9, 1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; Van Loon, L.J.; de Groot, L.C. Dietary protein intake in dutch elderly people: A focus on protein sources. Nutrients 2015, 7, 9697–9706. [Google Scholar] [CrossRef]
- Smeuninx, B.; Greig, C.A.; Breen, L. Amount, source and pattern of dietary protein intake across the adult lifespan: A cross-sectional study. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almoosawi, S.; Winter, J.; Prynne, C.J.; Hardy, R.; Stephen, A.M. Daily profiles of energy and nutrient intakes: Are eating profiles changing over time? Eur. J. Clin. Nutr. 2012, 66, 678–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
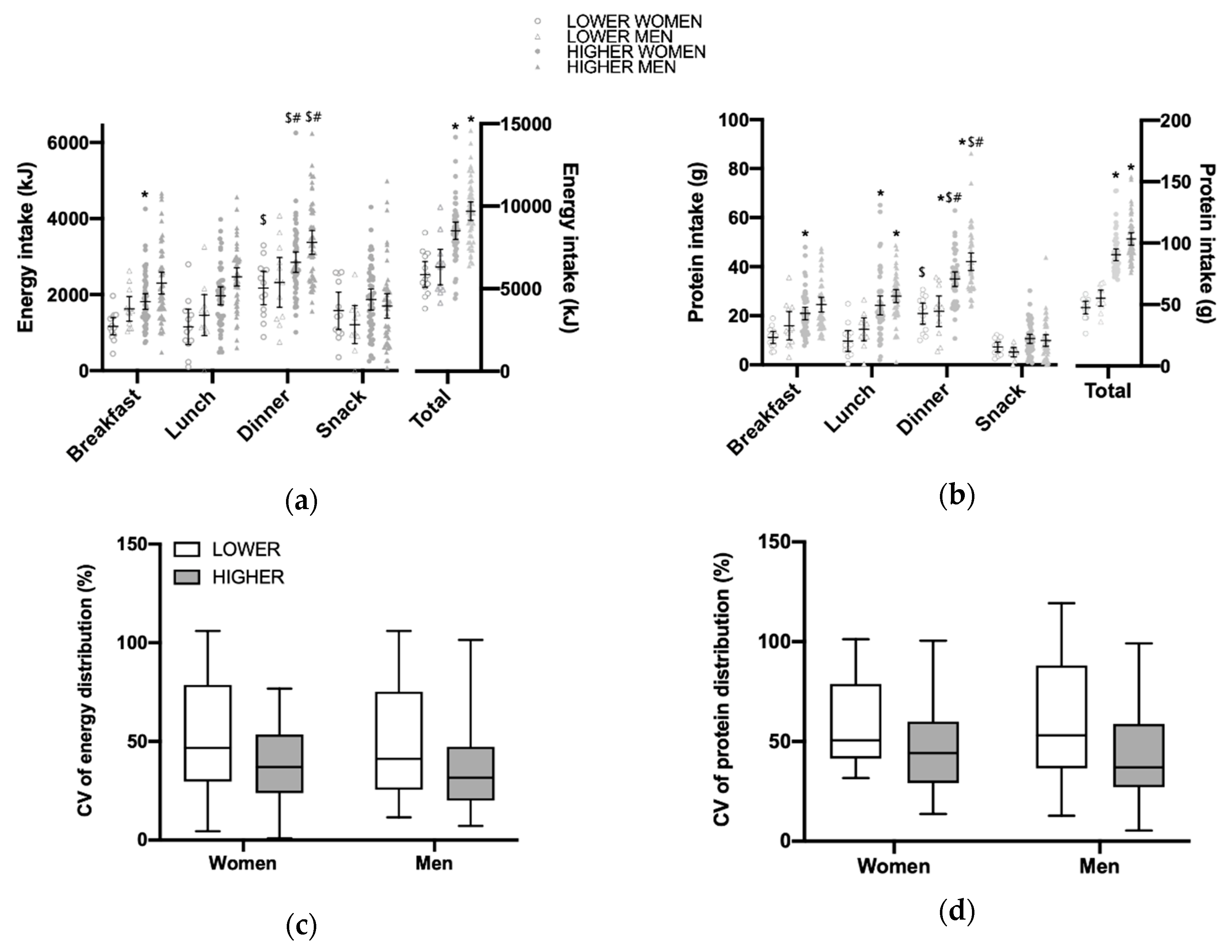
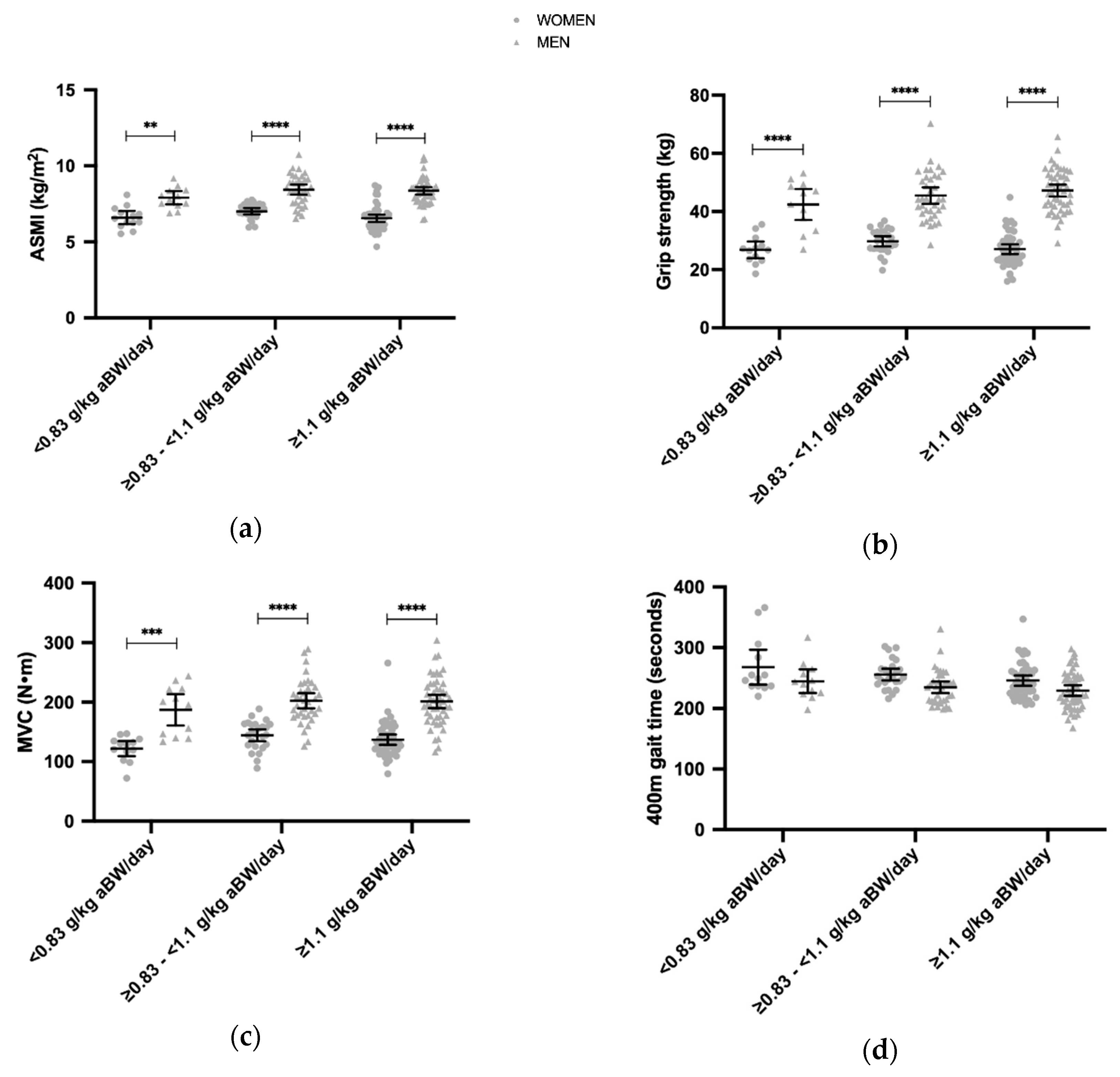
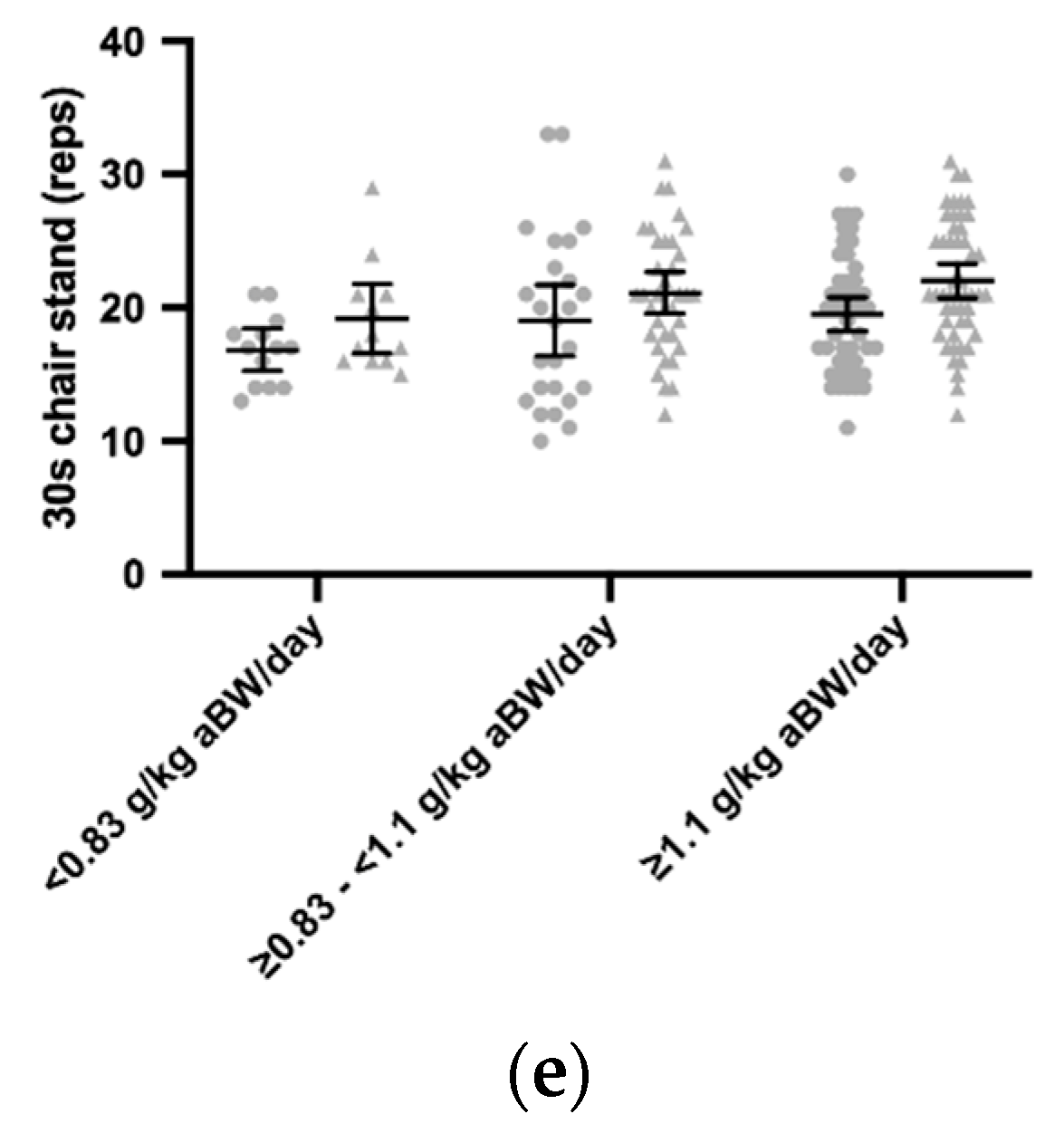
| All (n = 184) | Women Lower (n = 13) | Women Higher (n = 48) | Men Lower (n = 12) | Men Higher (n = 50) | p Women/Men | |
|---|---|---|---|---|---|---|
| Age (years) | 70.2 ± 3.9 | 71.7 ± 4.1 | 71.0 ± 4.0 | 71.8 ± 5.7 | 68.9 ± 3.5 | 0.52/0.04 |
| Age range (years) | 65–82 | 65–80 | 65–81 | 66–82 | 65–78 | |
| Height (m) | 1.72 ± 0.10 | 1.65 ± 0.07 | 1.66 ± 0.06 | 1.79 ± 0.06 | 1.76 ± 0.06 | 0.93/0.30 |
| Body Weight (kg) | 74.9 ± 12.1 | 69.6 ± 7.7 | 65.4 ± 11.4 | 78.7 ± 7.2 | 79.0 ± 11.8 | 0.19/1.00 |
| BMI (kg/m2) | 25.4 ± 3.7 | 25.6 ± 4.0 | 23.8 ± 3.9 | 24.6 ± 2.2 | 25.4 ± 3.5 | 0.13/0.61 |
| aBW (kg) | 73 ± 8.7 | 67.5 ± 3.1 | 65.2 ± 7.0 | 78.4 ± 6.5 | 76.6 ± 7.6 | 0.23/0.44 |
| WB LBM (kg) | 48.5 ± 8.6 | 39.9 ± 2.6 | 40.2 ± 4.2 | 54.5 ± 4.3 | 55.0 ± 5.3 | 0.76/0.81 |
| App. LBM (kg) | 22.4 ± 4.6 | 18.2 ± 3.0 | 18.3 ± 2.0 | 25.4 ± 2.1 | 26.1 ± 3.3 | 0.84/0.43 |
| EI (MJ/day) | 8.2 ± 2.1 | 6.0 ± 1.3 | 8.5 ± 1.8 | 6.3 ± 1.6 | 9.7 ± 2.0 | <0.001/<0.001 |
| Protein (Energy %) | 17.6 ± 4.0 | 14.3 ± 2.6 | 18.7 ± 4.8 | 15.9 ± 5.4 | 18.6 ± 3.1 | <0.01/0.03 |
| Protein (g/day) | 82.8 ± 22.2 | 49.0 ± 8.5 | 90.6 ± 16.7 | 55.2 ± 10.2 | 104.3 ± 17.9 | <0.001/<0.001 |
| Protein (g/kg BW/day) | 1.13 ± 0.34 | 0.70 ± 0.11 | 1.41 ± 0.30 | 0.70 ± 0.11 | 1.34 ± 0.25 | <0.001/<0.001 |
| Protein (g/kg aBW/day) | 1.15 ± 0.31 | 0.73 ± 0.12 | 1.39 ± 0.25 | 0.70 ± 0.11 | 1.37 ± 0.23 | |
| Goldberg Score | ||||||
| EI/BMR | 0.96 ± 0.24 | 0.77 ± 0.14 | 1.10 ± 0.24 | 0.65 ± 0.17 | 1.00 ± 0.19 | <0.001/<0.001 |
| Underreporters, n | 41,(22%) | 8,(53%) | 4,(8%) | 11,(85%) | 5,(11%) | |
| Overreporters, n | 18,(10%) | 0,(0%) | 9,(19%) | 0,(0%) | 6,(13%) | |
| Physical activity | ||||||
| Step counts (Steps/day) | 9740 ± 4358 | 9598 ± 3600 | 10,723 ± 4232 | 10,059 ± 5771 | 9297 ± 3392 | 0.036/0.30 |
| Total | Breakfast | Lunch | Dinner | |||||
|---|---|---|---|---|---|---|---|---|
| r | R2 | r | R2 | r | R2 | r | R2 | |
| Women | 0.69 | 0.48 | 0.72 | 0.52 | 0.82 | 0.68 | 0.56 | 0.32 |
| Men | 0.70 | 0.49 | 0.89 | 0.79 | 0.74 | 0.55 | 0.61 | 0.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Højfeldt, G.; Nishimura, Y.; Mertz, K.; Schacht, S.R.; Lindberg, J.; Jensen, M.; Hjulmand, M.; Lind, M.V.; Jensen, T.; Jespersen, A.P.; et al. Daily Protein and Energy Intake Are Not Associated with Muscle Mass and Physical Function in Healthy Older Individuals—A Cross-Sectional Study. Nutrients 2020, 12, 2794. https://doi.org/10.3390/nu12092794
Højfeldt G, Nishimura Y, Mertz K, Schacht SR, Lindberg J, Jensen M, Hjulmand M, Lind MV, Jensen T, Jespersen AP, et al. Daily Protein and Energy Intake Are Not Associated with Muscle Mass and Physical Function in Healthy Older Individuals—A Cross-Sectional Study. Nutrients. 2020; 12(9):2794. https://doi.org/10.3390/nu12092794
Chicago/Turabian StyleHøjfeldt, Grith, Yusuke Nishimura, Kenneth Mertz, Simon R. Schacht, Jonas Lindberg, Mikkel Jensen, Morten Hjulmand, Mads Vendelbo Lind, Tenna Jensen, Astrid Pernille Jespersen, and et al. 2020. "Daily Protein and Energy Intake Are Not Associated with Muscle Mass and Physical Function in Healthy Older Individuals—A Cross-Sectional Study" Nutrients 12, no. 9: 2794. https://doi.org/10.3390/nu12092794





