Association of Dietary Micronutrient Intake with Pulmonary Tuberculosis Treatment Failure Rate: ACohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
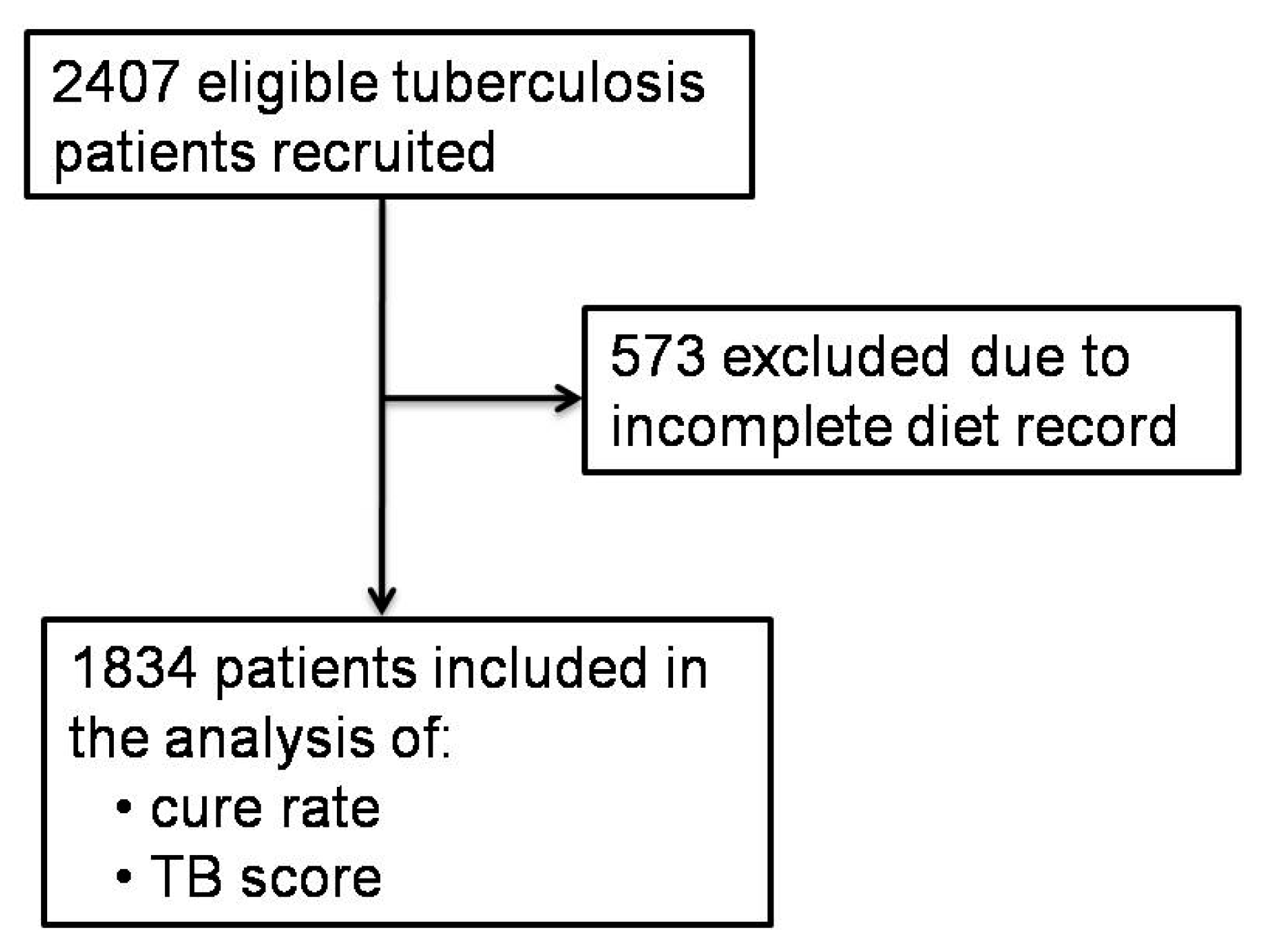
2.2. Study Design and Population
2.3. Procedure
2.4. Variables
2.5. Data Quality Control
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Dheda, K.; Barry, C.E., III; Maartens, G. Tuberculosis. Lancet 2016, 387, 1211–1226. [Google Scholar] [CrossRef]
- Hoyt, K.J.; Sarkar, S.; White, L.; Joseph, N.M.; Salgame, P.; Lakshminarayanan, S.; Muthaiah, M.; Vinod Kumar, S.; Ellner, J.J.; Roy, G.; et al. Effect of malnutrition on radiographic findings and mycobacterial burden in pulmonary tuberculosis. PLoS ONE 2019, 14, e0214011. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Saravanan, N.; Bethunaickan, R.; Tripathy, S. Malnutrition: Modulator of Immune Responses in Tuberculosis. Front. Immunol. 2017, 8, 1316. [Google Scholar] [CrossRef] [Green Version]
- Estrella, J.L.; Kan-Sutton, C.; Gong, X.; Rajagopalan, M.; Lewis, D.E.; Hunter, R.L.; Eissa, N.T.; Jagannath, C. A Novel In Vitro Human Macrophage Model to Study the Persistence of Mycobacterium tuberculosis Using Vitamin D(3) and Retinoic Acid Activated THP-1 Macrophages. Front. Microbiol. 2011, 2, 67. [Google Scholar] [CrossRef] [Green Version]
- Yuk, J.-M.; Shin, D.-M.; Lee, H.-M.; Yang, C.-S.; Jin, H.S.; Kim, K.-K.; Lee, Z.-W.; Lee, S.-H.; Kim, J.-M.; Jo, E.-K. Vitamin D3 Induces Autophagy in Human Monocytes/Macrophages via Cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soh, A.Z.; Chee, C.B.E.; Wang, Y.T.; Yuan, J.M.; Koh, W.P. Dietary Intake of Antioxidant Vitamins and Carotenoids and Risk of Developing Active Tuberculosis in a Prospective Population-Based Cohort Study. Am. J. Epidemiol. 2017, 186, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Fox, G.J.; Lee, R.S.; Lucas, M.; Khan, F.A.; Proulx, J.-F.; Hornby, K.; Jung, S.; Benedetti, A.; Behr, M.A.; Menzies, D. Inadequate Diet Is Associated with Acquiring Mycobacterium tuberculosis Infection in an Inuit Community. A Case–Control Study. Ann. Am. Thorac. Soc. 2015, 12, 1153–1162. [Google Scholar] [CrossRef]
- Hemilä, H.; Kaprio, J. Vitamin E supplementation may transiently increase tuberculosis risk in males who smoke heavily and have high dietary vitamin C intake. Br. J. Nutr. 2008, 100, 896–902. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. First Trimester Microelements and their Relationships with Pregnancy Outcomes and Complications. Nutrients 2020, 12, 1108. [Google Scholar] [CrossRef]
- Tam, E.; Keats, E.C.; Rind, F.; Das, J.K.; Bhutta, A.Z.A. Micronutrient Supplementation and Fortification Interventions on Health and Development Outcomes among Children Under-Five in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Disease Control and Prevention, Ministry of Health of China. The Chinese National Tuberculosis Prevention and Control Guideline; Xiao, D., Ed.; China Union Medical University Press: Beijing, China, 2008.
- Lin, S.; Gao, T.; Sun, C.; Jia, M.; Liu, C.; Ma, X.; Ma, A. Association of dietary patterns and endoscopic gastric mucosal atrophy in an adult Chinese population. Sci. Rep. 2019, 9, 16567. [Google Scholar] [CrossRef] [Green Version]
- Institute of Nutrition and Health, Chinese Center for Disease Control and Prevention. China Food Composition Tables, 6th ed.; Yang, Y., Ed.; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Wejse, C.; Gustafson, P.; Nielsen, J.; Gomes, V.F.; Aaby, P.; Andersen, P.L.; Sodemann, M. TBscore: Signs and symptoms from tuberculosis patients in a low-resource setting have predictive value and may be used to assess clinical course. Scand. J. Infect. Dis. 2008, 40, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Karyadi, E.; West, C.E.; Schultink, W.; Nelwan, R.H.; Gross, R.; Amin, Z.; Dolmans, W.M.; Schlebusch, H.; van der Meer, J.W. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: Effects on clinical response and nutritional status. Am. J. Clin. Nutr. 2002, 75, 720–727. [Google Scholar] [CrossRef]
- Jaganath, D.; Mupere, E. Childhood tuberculosis and malnutrition. J. Infect. Dis. 2012, 206, 1809–1815. [Google Scholar] [CrossRef] [Green Version]
- Box, G.E.P.; Tidwell, P.W. Transformation of the Independent Variables. Technometrics 1962, 4, 531–550. [Google Scholar] [CrossRef]
- Chinese Nutrition Society (Ed.) Chinese Dietary Reference Intakes; Science Press: Beijing, China, 2013. [Google Scholar]
- Vilcheze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R., Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013, 4, 1881. [Google Scholar] [CrossRef] [Green Version]
- Vilchèze, C.; Kim, J.; Jacobs, W.R., Jr. Vitamin C Potentiates the Killing of Mycobacterium tuberculosis by the First-Line Tuberculosis Drugs Isoniazid and Rifampin in Mice. Antimicrob. Agents Chemother. 2018, 62, e02165-17. [Google Scholar] [CrossRef] [Green Version]
- Pakasi, T.A.; Karyadi, E.; Suratih, N.M.D.; Salean, M.; Darmawidjaja, N.; Bor, H.; van der Velden, K.; Dolmans, W.M.V.; van der Meer, J.W.M. Zinc and vitamin A supplementation fails to reduce sputum conversion time in severely malnourished pulmonary tuberculosis patients in Indonesia. Nutr. J. 2010, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, A.A.; Peters, T.J.; Little, P. Design, analysis and presentation of factorial randomised controlled trials. BMC Med. Res. Methodol. 2003, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedrezazadeh, E.; Ostadrahimi, A.; Mahboob, S.; Assadi, Y.; Ansarin, K.; Shakoori, P.; Pourmoghaddam, M. Vitamin E-Selenium Supplement and Clinical Responses of Active Pulmonary Tuberculosis. Tanaffos 2006, 5, 49–55. [Google Scholar]
- Neyrolles, O.; Wolschendorf, F.; Mitra, A.; Niederweis, M. Mycobacteria, metals, and the macrophage. Immunol. Rev. 2015, 264, 249–263. [Google Scholar] [CrossRef]
- Grobler, L.; Nagpal, S.; Sudarsanam, T.D.; Sinclair, D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst. Rev. 2016, 2016, CD006086. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xiong, K.; Wang, Q.; Zhao, S.; Liu, Y.; Ma, A. Adjunctive vitamin A and D during pulmonary tuberculosis treatment: A randomized controlled trial with a 2 × 2 factorial design. Food Funct. 2020, 11, 4672–4681. [Google Scholar] [CrossRef]
- Saraff, V.; Shaw, N. Sunshine and vitamin D. Arch. Dis. Child 2016, 101, 190–192. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
| Successful (n = 1710) 1 | Failed (n = 124) 1 | P 2 | Number of Patients with Insufficient Micronutrient Intake (Percentage) 3 | |
|---|---|---|---|---|
| Age | 55.0 (28.0) | 59.5 (29.0) | 0.002 | - |
| Gender (male) | 1357 (79.4%) | 91 (73.4%) | 0.12 | - |
| BMI (kg/m2) 4 | 20.7 (2.7) | 21.1 (2.7) | 0.13 | - |
| Smoking | 467 (27.3%) | 34 (27.4%) | 0.98 | - |
| Excessive drinking | 601 (35.1%) | 42 (33.9%) | 0.77 | - |
| Education | 0.1 | - | ||
| Primary school and below | 971 (56.8%) | 81 (65.3%) | ||
| Middle and high school | 701 (41.0%) | 39 (31.5%) | ||
| High education | 38 (2.2%) | 4 (3.2%) | ||
| Occupation (farmers) | 1602 (93.7%) | 122 (98.4%) | 0.03 | - |
| Presence of lung cavity | 230 (13.5%) | 16 (12.9%) | 0.86 | |
| Newly diagnosed (newly) | 1571 (92.4%) | 100 (82.6%) | <0.001 | - |
| Anemia | 13 (0.8%) | 2 (1.6%) | 0.58 | - |
| Hypertension | 54 (3.2%) | 5 (4.1%) | 0.59 | - |
| Diabetes | 37 (2.2%) | 5 (4.0%) | 0.18 | - |
| Exercise (adequate) | 970 (56.8%) | 64 (51.6%) | 0.26 | - |
| Exposure to sunshine (>40 min) | 1463 (85.6%) | 96 (77.4%) | 0.01 | - |
| Total energy intake (kcal/d) | 1572.1 (766.4) | 1615.4 (775.3) | 0.51 | - |
| Vitamin A (ug RAE/d) 5 | 229.5 (317.0) | 245.7 (352.6) | 0.57 | 1690 (92.1%) |
| Thiamine (mg/d) | 1.0 (0.7) | 1.1 (0.7) | 0.3 | 1276 (69.6%) |
| Nicotinic acid(mg NE/d) 5 | 11.4 (6.7) | 11.2 (6.5) | 0.47 | 1278 (69.7%) |
| Riboflavin (mg/d) | 0.6 (0.4) | 0.6 (0.3) | 0.15 | 1775 (96.8) |
| Vitamin C (mg/d) | 50.0 (60.5) | 40.0 (63.0) | 0.15 | 1492 (81.4%) |
| Vitamin E (mg α-TE/d) 5 | 11.2 (10.4) | 13.7 (13.3) | 0.07 | 1147 (62.5%) |
| K (mg/d) | 1464.5 (789.6) | 1539.4 (814.6) | 0.33 | 1471 (80.2%) |
| Na (mg/d) 6 | 422.8 (866.1) | 407.6 (812.5) | 0.26 | 1517 (82.7%) |
| Ca (mg/d) | 269.7 (199.5) | 303.5 (223.9) | 0.09 | 1824 (99.5%) |
| Mg (mg/d) | 290.6 (169.4) | 315.5 (194.0) | 0.05 | 1112 (60.6%) |
| P (mg/d) | 951.3 (481.0) | 978.0 (580.3) | 0.15 | 431 (23.5%) |
| Fe (mg/d) | 17.5 (9.4) | 18.6 (9.7) | 0.12 | 434 (23.7%) |
| Zn (mg/d) | 8.6 (4.6) | 9.2 (5.0) | 0.14 | 1369 (74.6%) |
| Cu (mg/d) | 1.7 (1.0) | 1.9 (1.1) | 0.02 | 155 (8.5%) |
| Se (μg/d) | 37.4 (22.3) | 39.6 (22.9) | 0.88 | 1631 (88.9%) |
| Mn (mg/d) | 5.7 (3.6) | 6.3 (3.8) | 0.06 | - |
| Group | Univariate | Multivariate 2 | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | Ptrend | OR (95% CI) | P | Ptrend | ||
| Age | - | 1.02 (1.01, 1.03) | 0.003 | - | 1.01 (1.00, 1.03) | 0.04 | - |
| Gender 1 | - | 0.72 (0.47, 1.09) | 0.12 | - | 0.67 (0.42, 1.07) | 0.09 | - |
| BMI | - | 1.05 (0.99, 1.12) | 0.13 | - | 1.06 (0.99, 1.13) | 0.10 | - |
| Smoking 1 | - | 1.01 (0.67, 1.51) | 0.98 | - | 1.09 (0.69, 1.72) | 0.73 | - |
| Excessive drinking 1 | - | 1.06 (0.72, 1.56) | 0.77 | - | 0.85 (0.55, 1.31) | 0.46 | - |
| Occupation 1 | - | 0.24 (0.06, 1.00) | 0.05 | - | 0.28 (0.07, 1.21) | 0.09 | - |
| Newly diagnosed 1 | - | 2.56 (1.55, 4.23) | <0.001 | - | 2.18 (1.27, 3.74) | 0.005 | - |
| Exposure to sunshine 1 | - | 1.73 (1.11, 2.69) | 0.02 | - | 1.88 (1.17, 3.02) | 0.009 | - |
| Vitamin A (ug RAE/d) 3 | <147.5 | 0.77 (0.50,1.18) | 0.23 | 0.14 | 0.71 (0.44, 1.14) | 0.15 | 0.12 |
| 147.5–346.0 | 0.68 (0.44, 1.07) | 0.09 | 0.72 (0.44, 1.16) | 0.17 | |||
| ≥346.0 | - | - | - | ||||
| Thiamine (mg/d) | <0.8 | 1.15 (0.73, 1.81) | 0.56 | 0.9 | 1.22 (0.60, 2.47) | 0.59 | 0.58 |
| 0.8–1.3 | 1.10 (0.71, 1.71) | 0.66 | 1.03 (0.57, 1.86) | 0.92 | |||
| ≥1.3 | - | - | - | ||||
| Nicotinic acid (mg NE/d) 3 | <9.5 | 1.19 (0.76, 1.85) | 0.45 | 0.37 | 0.72 (0.36, 1.43) | 0.35 | 0.32 |
| 9.5–13.6 | 0.98 (0.62, 1.56) | 0.94 | 0.82 (0.48, 1.41) | 0.47 | |||
| ≥13.6 | - | - | - | ||||
| Riboflavin (mg/d) | <0.5 | 0.68 (0.34, 1.36) | 0.27 | 0.14 | 0.69 (0.34, 1.39) | 0.30 | 0.28 |
| 0.5–0.7 | 0.85 (0.49, 1.48) | 0.56 | 0.85 (0.49, 1.49) | 0.57 | |||
| ≥0.7 | - | - | - | ||||
| Vitamin C (mg/d) | <32.0 | 0.87 (0.54, 1.39) | 0.56 | 0.19 | 1.80 (1.07, 3.04) | 0.03 | 0.02 |
| 32.0–70.0 | 1.18 (0.76, 1.82) | 0.46 | 1.39 (0.85, 2.29) | 0.19 | |||
| ≥70.0 | - | - | - | ||||
| Vitamin E (mg α-TE/d) 3 | <8.6 | 1.55 (0.99, 2.42) | 0.05 | 0.05 | 1.23 (0.59, 2.54) | 0.58 | 0.69 |
| 8.6–15.2 | 1.06 (0.66, 1.71) | 0.80 | 0.99 (0.58, 1.70) | 0.98 | |||
| ≥15.2 | - | - | - | ||||
| K (mg/d) | <1229.4 | 1.20 (0.77, 1.86) | 0.43 | 0.41 | 1.29 (0.55, 3.02) | 0.56 | 0.55 |
| 1229.4–1731.4 | 1.00 (0.63, 1.59) | 0.99 | 1.20 (0.67, 2.17) | 0.54 | |||
| ≥1731.4 | - | - | - | ||||
| Na (mg/d) 4 | <249.9 | 1.09 (0.68, 1.74) | 0.72 | 0.45 | 1.48 (0.90, 2.43) | 0.12 | 0.19 |
| 249.9–716.0 | 1.39 (0.89, 2.18) | 0.15 | 1.05 (0.64, 1.71) | 0.86 | |||
| ≥716.0 | - | - | - | ||||
| Ca (mg/d) | <211.0 | 1.29 (0.83, 2.00) | 0.26 | 0.23 | 1.02 (0.54, 1.91) | 0.96 | 0.95 |
| 211.0–340.5 | 1.00 (0.63, 1.59) | 0.99 | 0.91 (0.54, 1.53) | 0.72 | |||
| ≥340.5 | - | - | - | ||||
| Mg (mg/d) | <240.0 | 1.54 (1.00, 2.39) | 0.05 | 0.02 | 0.33 (0.14, 0.79) | 0.01 | 0.01 |
| 240.0–354.0 | 0.93 (0.58, 1.51) | 0.78 | 0.50 (0.28, 0.90) | 0.02 | |||
| ≥354.0 | - | - | - | ||||
| P (mg/d) | <801.0 | 1.58 (1.01, 2.47) | 0.05 | 0.1 | 0.75 (0.28, 2.02) | 0.56 | 0.43 |
| 801.0–1116.0 | 1.13 (0.70, 1.82) | 0.62 | 0.59 (0.30, 1.15) | 0.12 | |||
| ≥1116.0 | - | - | - | ||||
| Fe (mg/d) | <14.6 | 1.10 (0.72, 1.69) | 0.66 | 0.13 | 0.90 (0.33, 2.44) | 0.84 | 0.84 |
| 14.6–20.4 | 0.77 (0.48, 1.22) | 0.26 | 1.35 (0.70, 2.60) | 0.37 | |||
| ≥20.4 | - | - | - | ||||
| Zn (mg/d) | <7.2 | 1.17 (0.74, 1.85) | 0.49 | 0.9 | 2.52 (1.25, 5.08) | 0.01 | 0.02 |
| 7.2–10.2 | 1.15 (0.73, 1.80) | 0.55 | 1.29 (0.76, 2.19) | 0.34 | |||
| ≥10.2 | - | - | - | ||||
| Cu (mg/d) | <1.4 | 1.41 (0.91, 2.19) | 0.12 | 0.01 | 0.48 (0.22, 1.02) | 0.06 | 0.10 |
| 1.4–2.0 | 0.81 (0.49, 1.34) | 0.41 | 0.77 (0.45, 1.32) | 0.34 | |||
| ≥2.0 | - | - | - | ||||
| Se (μg/d) | <30.6 | 1.46 (0.92, 2.31) | 0.11 | 0.67 | 1.05 (0.61, 1.83) | 0.86 | 0.88 |
| 30.6–44.5 | 1.36 (0.85, 2.17) | 0.20 | 0.77 (0.46, 1.29) | 0.32 | |||
| ≥44.5 | - | - | - | ||||
| Mn (mg/d) | <4.6 | 1.19 (0.77, 1.82) | 0.43 | 0.05 | 0.71 (0.33, 1.51) | 0.37 | 0.44 |
| 4.6–6.8 | 0.75 (0.46, 1.21) | 0.23 | 0.86 (0.50, 1.50) | 0.61 | |||
| ≥6.8 | - | - | - | ||||
| Group | Univariate | Multivariate 2 | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | Ptrend | OR (95% CI) | P | Ptrend | ||
| Age | - | 1.02 (1.01, 1.02) | <0.001 | - | 1.01 (1.00, 1.02) | 0.01 | - |
| Gender 3 | - | 0.76 (0.61, 0.95) | 0.02 | - | 0.80 (0.62, 1.04) | 0.09 | - |
| BMI | - | 0.90 (0.87, 0.93) | <0.001 | - | 0.91 (0.87, 0.94) | <0.001 | - |
| Smoking 3 | - | 1.11 (0.91, 1.37) | 0.31 | - | 0.90 (0.70, 1.14) | 0.38 | - |
| Excessive drinking 3 | - | 3.78 (3.07, 4.65) | <0.001 | - | 3.10 (2.46, 3.89) | <0.001 | - |
| Occupation 3 | 0.54 (0.36, 0.82) | 0.003 | - | 0.44 (0.28, 0.70) | 0.001 | - | |
| Newly diagnosed 3 | 1.79 (1.27, 2.52) | 0.001 | - | 1.60 (1.10, 2.33) | 0.02 | - | |
| Exposure to sunshine 3 | 2.90 (2.20, 3.81) | <0.001 | - | 2.40 (1.78, 3.24) | <0.001 | - | |
| Vitamin A (ug RAE/d) 4 | <147.5 | 0.82 (0.65, 1.02) | 0.08 | 0.11 | 0.64 (0.49, 0.83) | 0.001 | 0.002 |
| 147.5–346.0 | 0.96 (0.77,1.20) | 0.71 | 0.90 (0.70, 1.16) | 0.42 | |||
| ≥346.0 | - | - | - | ||||
| Thiamine (mg/d) | <0.8 | 0.78 (0.62, 0.99) | 0.04 | 0.08 | 0.84 (0.57, 1.24) | 0.38 | 0.48 |
| 0.8–1.3 | 0.55 (0.44, 0.69) | <0.001 | 0.72 (0.53, 0.98) | 0.04 | |||
| ≥1.3 | - | - | - | ||||
| Nicotinic acid (mg NE/d) 4 | <9.5 | 0.88 (0.70, 1.10) | 0.27 | 0.17 | 0.59 (0.41, 0.86) | 0.01 | 0.01 |
| 9.5–13.6 | 0.75 (0.60, 0.94) | 0.01 | 0.87 (0.65, 1.16) | 0.34 | |||
| ≥13.6 | - | - | - | ||||
| Riboflavin (mg/d) | <0.5 | 0.67 (0.54, 0.85) | 0.001 | 0.001 | 0.61 (0.42, 0.88) | 0.01 | 0.002 |
| 0.5–0.7 | 0.57 (0.45, 0.74) | <0.001 | 0.67 (0.50, 0.91) | 0.01 | |||
| ≥0.7 | - | - | - | ||||
| Vitamin C (mg/d) | <32.0 | 1.90 (1.52, 2.39) | <0.001 | <0.001 | 1.49 (1.14, 1.96) | 0.004 | 0.002 |
| 32.0–70.0 | 1.56 (1.24, 1.96) | <0.001 | 1.32 (1.02, 1.71) | 0.04 | |||
| ≥70.0 | - | - | - | ||||
| Vitamin E (mg α-TE/d) 4 | <8.6 | 1.12 (0.89, 1.40) | 0.33 | 0.59 | 0.86 (0.59, 1.26) | 0.45 | 0.27 |
| 8.6–15.2 | 0.85 (0.68, 1.07) | 0.17 | 0.83 (0.62, 1.11) | 0.21 | |||
| ≥15.2 | - | - | - | ||||
| K (mg/d) | <1229.4 | 1.10 (0.88, 1.37) | 0.42 | 0.48 | 0.61 (0.39, 0.95) | 0.03 | 0.03 |
| 1229.4–1731.4 | 0.89 (0.71, 1.11) | 0.29 | 0.85 (0.61, 1.18) | 0.32 | |||
| ≥1731.4 | - | - | - | ||||
| Na (mg/d) 5 | <249.9 | 1.19 (0.95, 1.50) | 0.12 | 0.09 | 0.91 (0.70, 1.19) | 0.50 | 0.56 |
| 249.9–716.0 | 1.18 (0.94, 1.48) | 0.15 | 0.92 (0.72, 1.19) | 0.54 | |||
| ≥716.0 | - | - | - | ||||
| Ca (mg/d) | <211.0 | 1.48 (1.18, 1.86) | 0.001 | 0.001 | 1.34 (0.96, 1.88) | 0.08 | 0.10 |
| 211.0–340.5 | 1.14 (0.91, 1.43) | 0.26 | 1.17 (0.88, 1.55) | 0.28 | |||
| ≥340.5 | - | - | - | ||||
| Mg (mg/d) | <240.0 | 1.41 (1.13, 1.77) | 0.003 | 0.004 | 2.07 (1.32, 3.23) | 0.001 | 0.001 |
| 240.0–354.0 | 1.04 (0.83, 1.30) | 0.73 | 1.50 (1.09, 2.07) | 0.01 | |||
| ≥354.0 | - | - | - | ||||
| P (mg/d) | <801.0 | 1.10 (0.88, 1.37) | 0.42 | 0.47 | 1.37 (0.81, 2.30) | 0.24 | 0.17 |
| 801.0–1116.0 | 0.90 (0.72, 1.13) | 0.38 | 1.26 (0.87, 1.83) | 0.22 | |||
| ≥1116.0 | - | - | - | ||||
| Fe (mg/d) | <14.6 | 1.16 (0.92, 1.45) | 0.21 | 0.26 | 1.48 (0.88, 2.49) | 0.14 | 0.13 |
| 14.6–20.4 | 0.83 (0.66, 1.04) | 0.11 | 1.12 (0.77, 1.62) | 0.57 | |||
| ≥20.4 | - | - | - | ||||
| Zn (mg/d) | <7.2 | 0.85 (0.68, 1.06) | 0.16 | 0.12 | 0.50 (0.35, 0.73) | <0.001 | <0.001 |
| 7.2–10.2 | 0.62 (0.50, 0.78) | <0.001 | 0.57 (0.43, 0.76) | <0.001 | |||
| ≥10.2 | - | - | - | ||||
| Cu (mg/d) | <1.4 | 0.95 (0.77, 1.19) | 0.67 | 0.51 | 0.80 (0.54, 1.18) | 0.26 | 0.39 |
| 1.4–2.0 | 0.73 (0.58, 0.92) | 0.006 | 0.82 (0.60, 1.11) | 0.19 | |||
| ≥2.0 | - | - | - | ||||
| Se (μg/d) | <30.6 | 1.00 (0.80, 1.25) | 0.98 | 0.95 | 1.02 (0.75, 1.39) | 0.90 | 0.98 |
| 30.6–44.5 | 0.95 (0.76, 1.18) | 0.63 | 1.04 (0.80, 1.36) | 0.77 | |||
| ≥44.5 | - | - | - | ||||
| Mn (mg/d) | <4.6 | 1.50 (1.20, 1.88) | <0.001 | <0.001 | 3.03 (2.01, 4.55) | <0.001 | <0.001 |
| 4.6–6.8 | 1.29 (1.03, 1.62) | 0.03 | 2.17 (1.57, 2.99) | <0.001 | |||
| ≥6.8 | - | - | - | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, K.; Wang, J.; Zhang, J.; Hao, H.; Wang, Q.; Cai, J.; Ma, A. Association of Dietary Micronutrient Intake with Pulmonary Tuberculosis Treatment Failure Rate: ACohort Study. Nutrients 2020, 12, 2491. https://doi.org/10.3390/nu12092491
Xiong K, Wang J, Zhang J, Hao H, Wang Q, Cai J, Ma A. Association of Dietary Micronutrient Intake with Pulmonary Tuberculosis Treatment Failure Rate: ACohort Study. Nutrients. 2020; 12(9):2491. https://doi.org/10.3390/nu12092491
Chicago/Turabian StyleXiong, Ke, Jinyu Wang, Jianwen Zhang, Haibo Hao, Qiuzhen Wang, Jing Cai, and Aiguo Ma. 2020. "Association of Dietary Micronutrient Intake with Pulmonary Tuberculosis Treatment Failure Rate: ACohort Study" Nutrients 12, no. 9: 2491. https://doi.org/10.3390/nu12092491




