The Effect of Leucine-Enriched Essential Amino Acid Supplementation on Anabolic and Catabolic Signaling in Human Skeletal Muscle after Acute Resistance Exercise: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessment of One-Repetition Maximum
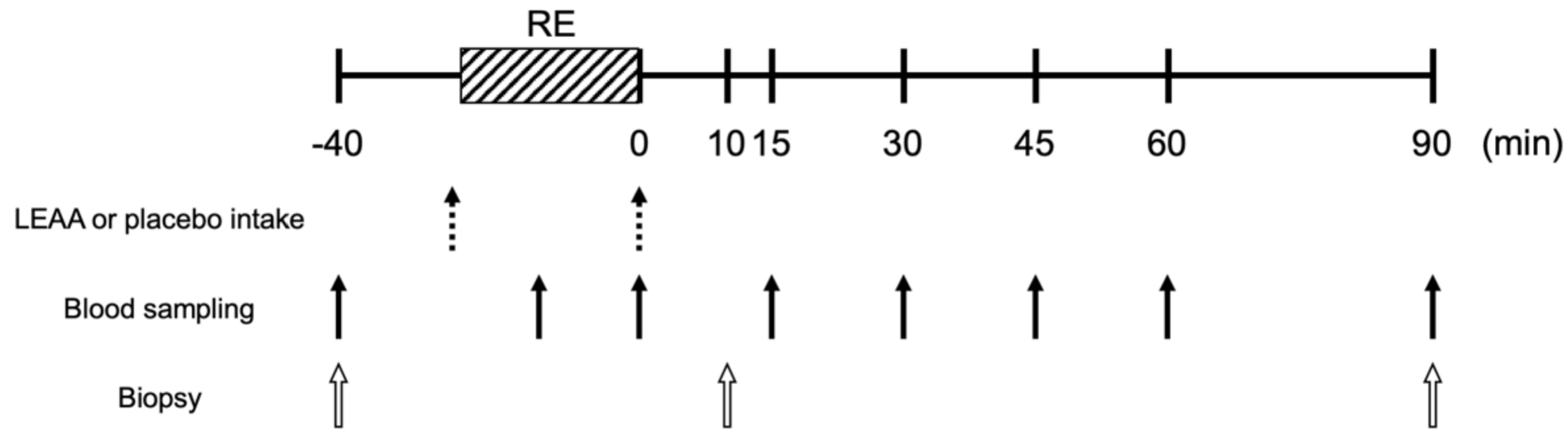
2.3. Study Protocol
2.4. Blood Insulin, Glucose, Lactate and Amino Acid Concentrations
2.5. Western Blotting
2.6. RNA Extraction and Real-Time qPCR
2.7. Statistical Analysis
3. Results
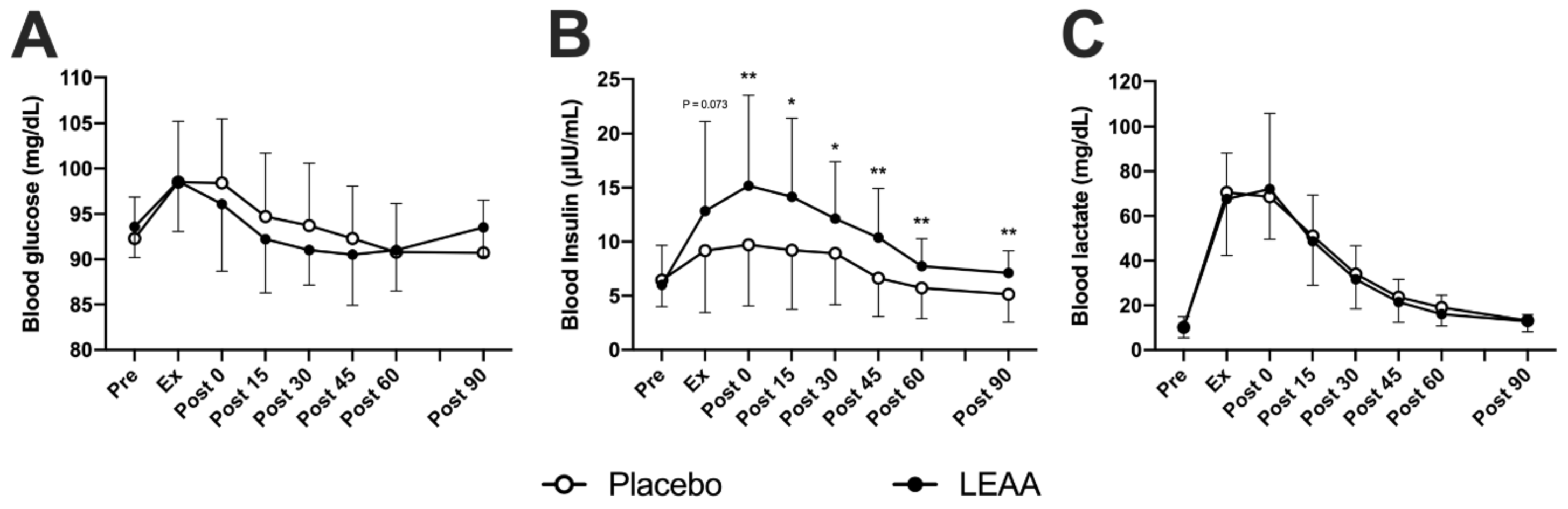
3.1. Blood Insulin, Lactate and Glucose Concentrations
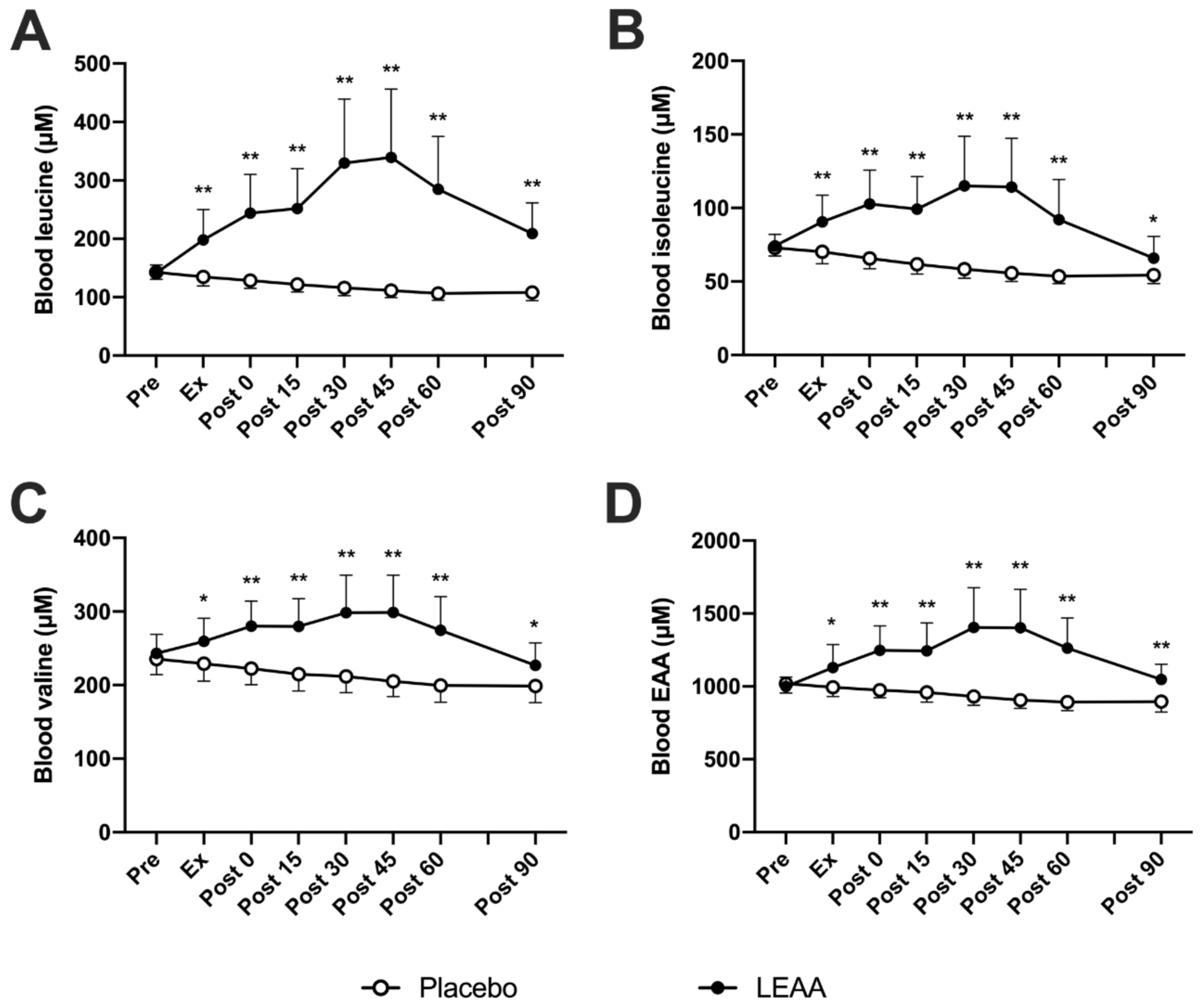
3.2. Plasma BCAAs and Total EAA Concentrations
3.3. mTORC1 Signaling
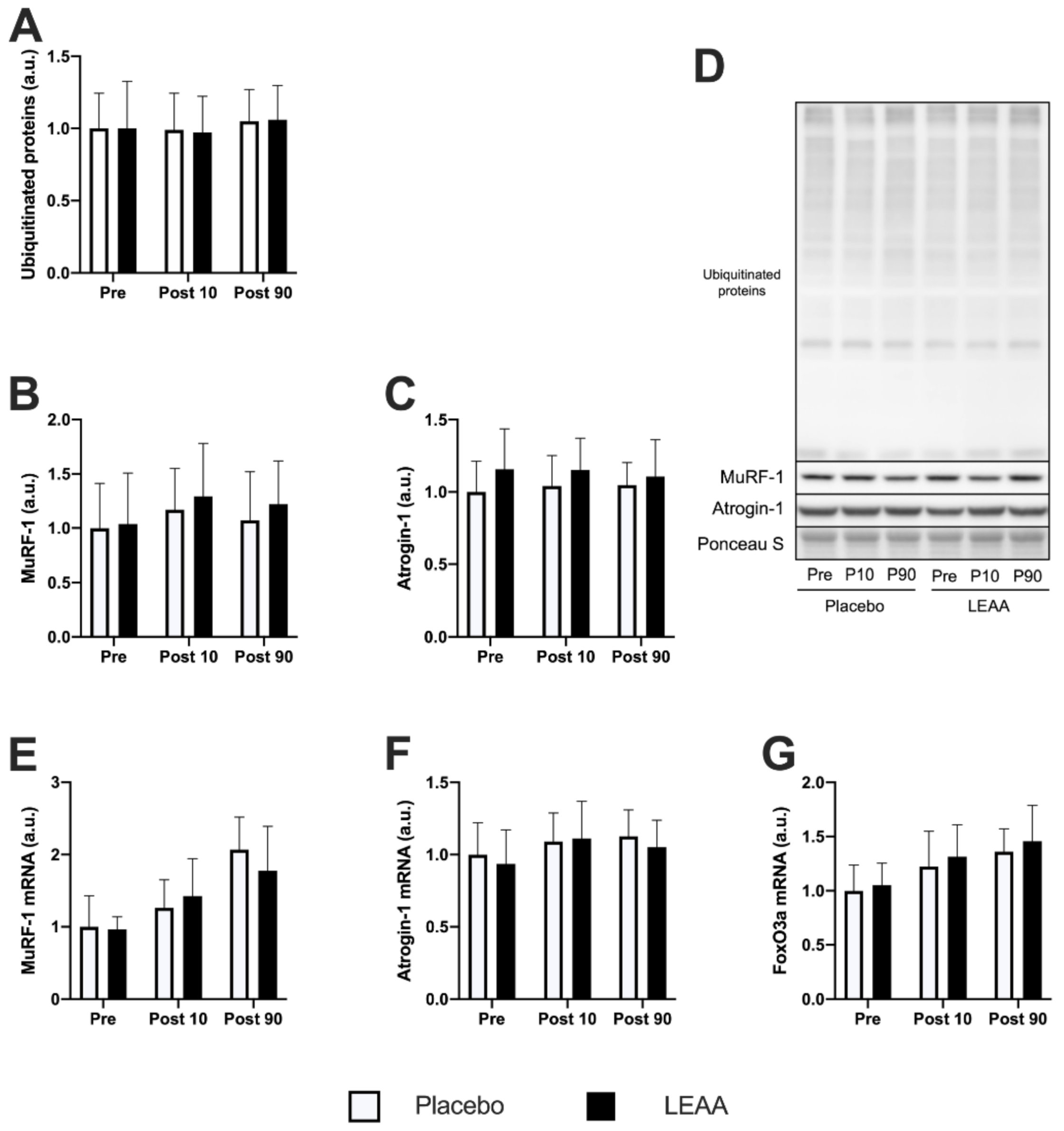
3.4. Ubiquitin–Proteasome System-Related Factors
3.5. Inflammatory Cytokines
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colonetti, T.; Grande, A.J.; Milton, K.; Foster, C.; Alexandre, M.C.; Uggioni, M.L.; Rosa, M.I. Effects of whey protein supplement in the elderly submitted to resistance training: Systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2017, 68, 257–264. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Li, M.; Liu, F. Effect of whey protein supplementation during resistance training sessions on body mass and muscular strength: A meta-analysis. Food Funct. 2019, 10, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.; McGlory, C.; Phillips, S. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Fry, C.S.; Drummond, M.J.; Gundermann, D.M.; Walker, D.K.; Glynn, E.L.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J. Nutr. 2011, 141, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Baptista, I.L.; Leal, M.L.; Artioli, G.G.; Aoki, M.S.; Fiamoncini, J.; Turri, A.O.; Curi, R.; Miyabara, E.H.; Moriscot, A.S. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 2010, 41, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-García, A.D.; Columbus, D.A.; Manjarín, R.; Nguyen, H.V.; Suryawan, A.; Orellana, R.A.; Davis, T.A. Leucine supplementation stimulates protein synthesis and reduces degradation signal activation in muscle of newborn pigs during acute endotoxemia. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E791–E801. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Damas, F.; Phillips, S.; Vechin, F.C.; Ugrinowitsch, C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. 2015, 45, 801–807. [Google Scholar] [CrossRef]
- Ogasawara, R.; Fujita, S.; Hornberger, T.A.; Kitaoka, Y.; Makanae, Y.; Nakazato, K.; Naokata, I. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci. Rep. 2016, 6, 31142. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.; Baehr, L.M.; Marcotte, G.R.; Chason, C.M.; Tolento, L.; Gomes, A.V.; Bodine, S.C.; Baar, K. Acute resistance exercise activates rapamycin-sensitive and-insensitive mechanisms that control translational activity and capacity in skeletal muscle. J. Physiol. 2016, 594, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, R.; Suginohara, T. Rapamycin-insensitive mechanistic target of rapamycin regulates basal and resistance exercise–induced muscle protein synthesis. FASEB J. 2018, 32, 5824–5834. [Google Scholar] [CrossRef] [PubMed]
- You, J.-S.; McNally, R.M.; Jacobs, B.L.; Privett, R.E.; Gundermann, D.M.; Lin, K.-H.; Steinert, N.D.; Goodman, C.A.; Hornberger, T.A. The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J. 2018, 33, 4021–4034. [Google Scholar] [CrossRef]
- Witard, O.C.; Tieland, M.; Beelen, M.; Tipton, K.D.; van Loon, L.J.; Koopman, R. Resistance exercise increases postprandial muscle protein synthesis in humans. Med. Sci. Sports Exerc. 2009, 41, 144–154. [Google Scholar] [CrossRef]
- Dreyer, H.C.; Fujita, S.; Cadenas, J.G.; Chinkes, D.L.; Volpi, E.; Rasmussen, B.B. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J. Physiol. 2006, 576, 613–624. [Google Scholar] [CrossRef]
- Mascher, H.; Tannerstedt, J.; Brink-Elfegoun, T.; Ekblom, B.; Gustafsson, T.; Blomstrand, E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E43–E51. [Google Scholar] [CrossRef]
- Clarke, B.A.; Drujan, D.; Willis, M.S.; Murphy, L.O.; Corpina, R.A.; Burova, E.; Rakhilin, S.V.; Stitt, T.N.; Patterson, C.; Latres, E.; et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007, 6, 376–385. [Google Scholar] [CrossRef]
- Cohen, S.; Brault, J.J.; Gygi, S.P.; Glass, D.J.; Valenzuela, D.M.; Gartner, C.; Latres, E.; Goldberg, A.L. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell Biol. 2009, 185, 1083–1095. [Google Scholar] [CrossRef]
- Polge, C.; Heng, A.E.; Jarzaguet, M.; Ventadour, S.; Claustre, A.; Combaret, L.; Bechet, D.; Matondo, M.; Uttenweiler-Joseph, S.; Monsarrat, B.; et al. Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J. 2011, 25, 3790–3802. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Mitchell, C.J.; West, D.W.; Philp, A.; Marcotte, G.R.; Baker, S.K.; Baar, K.; Phillips, S.M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012, 590, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Phillips, B.E.; Williams, J.P.; Rankin, D.; Lund, J.N.; Wilkinson, D.J.; Smith, K.; Atherton, P.J. The impact of delivery profile of essential amino acids upon skeletal muscle protein synthesis in older men: Clinical efficacy of pulse vs. bolus supply. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E450–E457. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef]
- Moberg, M.; Apró, W.; Ekblom, B.; Hall, G.V.; Holmberg, H.-C.; Blomstrand, E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am. J. Physiol. Cell Physiol. 2016, 310, C874–C884. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Bukhari, S.S.; Phillips, B.E.; Limb, M.C.; Cegielski, J.; Brook, M.S.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Williams, J.P. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018, 37, 2011–2021. [Google Scholar] [CrossRef]
- Bukhari, S.S.I.; Phillips, B.E.; Wilkinson, D.J.; Limb, M.C.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1056–E1065. [Google Scholar]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehab. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Peake, J.; Nosaka, K.K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar]
- Buford, T.W.; Cooke, M.B.; Willoughby, D.S. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Euro. J. Appl. Physiol. 2009, 107, 463–471. [Google Scholar] [CrossRef]
- Vella, L.; Caldow, M.K.; Larsen, A.E.; Tassoni, D.; Della Gatta, P.A.; Gran, P.; Russell, A.P.; Cameron-Smith, D. Resistance exercise increases NF-κB activity in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 302, R667–R673. [Google Scholar] [CrossRef]
- Louis, E.; Raue, U.; Yang, Y.; Jemiolo, B.; Trappe, S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J. Appl. Physiol. 2007, 103, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Frantz, J.D.; Tawa, N.E.; Melendez, P.A.; Oh, B.-C.; Lidov, H.G.; Hasselgren, P.-O.; Frontera, W.R.; Lee, J.; Glass, D.J. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.; Zhang, G.; Fattah, E.A.A.; Eissa, N.T.; Li, Y.-P. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011, 25, 99–110. [Google Scholar] [CrossRef]
- Shimomura, Y.; Inaguma, A.; Watanabe, S.; Yamamoto, Y.; Muramatsu, Y.; Bajotto, G.; Sato, J.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 236–244. [Google Scholar] [CrossRef]
- Earle, R.W.; Baechle, T.R. Principles of test selection. In Essentials of Strength Training and Conditioning; Haff, G.G., Triplett, N.T., Eds.; Human Kinetics: Champaign, IL, USA, 2008; pp. 249–258. [Google Scholar]
- Shimbo, K.; Oonuki, T.; Yahashi, A.; Hirayama, K.; Miyano, H. Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Sp. 2009, 23, 1483–1492. [Google Scholar] [CrossRef]
- Yoshida, H.; Kondo, K.; Yamamoto, H.; Kageyama, N.; Ozawa, S.-I.; Shimbo, K.; Muramatsu, T.; Imaizumi, A.; Mizukoshi, T.; Masuda, J. Validation of an analytical method for human plasma free amino acids by high-performance liquid chromatography ionization mass spectrometry using automated precolumn derivatization. J. Chromatogr. B 2015, 998, 88–96. [Google Scholar] [CrossRef]
- Takegaki, J.; Sase, K.; Fujita, S. Repeated bouts of resistance exercise attenuate mitogen-activated protein-kinase signal responses in rat skeletal muscle. Biochem. Biophys. Res. Commun. 2019, 520, 73–78. [Google Scholar] [CrossRef]
- Ogasawara, R.; Akimoto, T.; Umeno, T.; Sawada, S.; Hamaoka, T.; Fujita, S. MicroRNA expression profiling in skeletal muscle reveals different regulatory patterns in high and low responders to resistance training. Physiol. Genom. 2016, 48, 320–324. [Google Scholar] [CrossRef]
- Rennie, M.J.; Bohe, J.; Smith, K.; Wackerhage, H.; Greenhaff, P. Branched-chain amino acids as fuels and anabolic signals in human muscle. J. Nutr. 2006, 136, 264S–268S. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Volpi, E.; Rasmussen, B.B. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Borgenvik, M.; Apró, W.; Blomstrand, E. Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am. J. Physiol. Endocrin. Metab. 2012, 302, E510–E521. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Koba, T.; Hamada, K.; Sakurai, M.; Higuchi, T.; Miyata, H. Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J. Sports Med. Phys. Fit. 2009, 49, 424–431. [Google Scholar]
- Kato, H.; Miura, K.; Nakano, S.; Suzuki, K.; Bannai, M.; Inoue, Y. Leucine-enriched essential amino acids attenuate inflammation in rat muscle and enhance muscle repair after eccentric contraction. Amino Acids 2016, 48, 2145–2155. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Nelson, A.R.; Raymond, F.; Metairon, S.; Mansourian, R.; Clarke, J.; Stellingwerff, T.; Phillips, S.M. Protein-leucine ingestion activates a regenerative inflammo-myogenic transcriptome in skeletal muscle following intense endurance exercise. Physiol. Genom. 2016, 48, 21–32. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Gundermann, D.M.; Walker, D.K.; Reidy, P.T.; Borack, M.S.; Drummond, M.J.; Arora, M.; Volpi, E.; Rasmussen, B.B. Leucine-Enriched Amino Acid Ingestion after Resistance Exercise Prolongs Myofibrillar Protein Synthesis and Amino Acid Transporter Expression in Older Men. J. Nutr. 2014, 144, 1694–1702. [Google Scholar] [CrossRef]
- Drummond, M.J.; Fry, C.S.; Glynn, E.L.; Dreyer, H.C.; Dhanani, S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J. Physiol. 2009, 587, 1535–1546. [Google Scholar] [CrossRef]


| Placebo | Leucine-Enriched Essential Amino Acid (LEAA) | p-Value (t-Test) | |
|---|---|---|---|
| Age (years) | 21.4 ± 1.3 | 21.8 ± 1.5 | 0.535 |
| Height (cm) | 172.5 ± 6.5 | 171.8 ± 5.8 | 0.782 |
| Weight (kg) | 64.4 ± 9.3 | 61.4 ± 5.0 | 0.377 |
| BMI (kg/m2) | 21.5 ± 1.9 | 20.9 ± 1.9 | 0.443 |
| One-repetition maximum (1-RM) | |||
| Leg Extension (kg) | 118.8 ± 27.5 | 126.1 ± 15.7 | 0.475 |
| Leg Curl (kg) | 84.7 ± 21.2 | 85.4 ± 10.1 | 0.926 |
| LEAA (g) | Placebo (g) | |
|---|---|---|
| l-Leucine | 1.00 | |
| l-Isoleucine | 0.27 | |
| l-Valine | 0.28 | |
| l-Threonine | 0.23 | |
| l-Methionine | 0.08 | |
| l-Histidine hydrochloride | 0.04 | |
| l-Lysine hydrochloride | 0.42 | |
| l-Tryptophan | 0.02 | |
| l-Phenylalanine | 0.17 | |
| Maltitol | 0.08 | 2.69 |
| Excipient and Perfume | 0.35 | 0.25 |
| Total | 2.94 | 2.94 |
| Item | Placebo | LEAA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ex | P 0 | P 15 | P 30 | P 45 | P 60 | P 90 | Ex | P 0 | P 15 | P 30 | P 45 | P 60 | P 90 | |
| Glucose | ↑ *** | ↑ *** | → | → | → | → | → | ↑ ** | → | → | → | → | → | → |
| Insulin | ↑ * | ↑ ** | ↑ * | ↑ * | → | → | → | ↑ *** | ↑ *** | ↑ *** | ↑ ** | ↑* | → | → |
| Lactate | ↑ *** | ↑ *** | ↑ *** | ↑ *** | ↑ ** | → | → | ↑ *** | ↑ *** | ↑ *** | ↑ ** | → | → | → |
| Leucine | ↓ ** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | → | ↑ ** | ↑ *** | ↑ *** | ↑ *** | ↑ *** | → |
| Isoleucine | → | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | → | ↑ ** | ↑ * | ↑ *** | ↑ *** | → | → |
| Valine | ↓ * | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | → | ↑ * | ↑ * | ↑ *** | ↑ *** | → | → |
| Total EAA | → | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | ↓ *** | → | ↑ *** | ↑ ** | ↑ *** | ↑ *** | ↑ *** | → |
| Placebo | LEAA | |||
|---|---|---|---|---|
| Post 10 | Post 90 | Post 10 | Post 90 | |
| Protein Content/Phosphorylation | ||||
| P/T Akt | ↑ *** | → | ↑ *** | ↑ ** |
| P/T mTOR | → | → | ↑ * | ↑ *** |
| P/T p70S6K | → | ↑ † | → | ↑ *** |
| P/T rpS6 | → | ↑ * | → | ↑ *** |
| P/T 4EBP1 | ↓ † | → | → | → |
| P/T eEF2 | → | → | → | → |
| Ubiquitinated Proteins | → | → | → | → |
| MuRF-1 | → | → | → | → |
| Atrogin-1 | → | → | → | → |
| mRNA Expression | ||||
| murf-1 | → | ↑ *** | ↑ ** | ↑ *** |
| atrogin-1 | → | ↑ * | ↑ * | ↑ † |
| foxo3a | ↑ ** | ↑ *** | ↑ ** | ↑ *** |
| il-1beta | → | ↑ *** | → | ↑ ** |
| il-6 | → | ↑ ** | → | ↑ ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takegaki, J.; Sase, K.; Yasuda, J.; Shindo, D.; Kato, H.; Toyoda, S.; Yamada, T.; Shinohara, Y.; Fujita, S. The Effect of Leucine-Enriched Essential Amino Acid Supplementation on Anabolic and Catabolic Signaling in Human Skeletal Muscle after Acute Resistance Exercise: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients 2020, 12, 2421. https://doi.org/10.3390/nu12082421
Takegaki J, Sase K, Yasuda J, Shindo D, Kato H, Toyoda S, Yamada T, Shinohara Y, Fujita S. The Effect of Leucine-Enriched Essential Amino Acid Supplementation on Anabolic and Catabolic Signaling in Human Skeletal Muscle after Acute Resistance Exercise: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients. 2020; 12(8):2421. https://doi.org/10.3390/nu12082421
Chicago/Turabian StyleTakegaki, Junya, Kohei Sase, Jun Yasuda, Daichi Shindo, Hiroyuki Kato, Sakiko Toyoda, Toshiyuki Yamada, Yasushi Shinohara, and Satoshi Fujita. 2020. "The Effect of Leucine-Enriched Essential Amino Acid Supplementation on Anabolic and Catabolic Signaling in Human Skeletal Muscle after Acute Resistance Exercise: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial" Nutrients 12, no. 8: 2421. https://doi.org/10.3390/nu12082421
APA StyleTakegaki, J., Sase, K., Yasuda, J., Shindo, D., Kato, H., Toyoda, S., Yamada, T., Shinohara, Y., & Fujita, S. (2020). The Effect of Leucine-Enriched Essential Amino Acid Supplementation on Anabolic and Catabolic Signaling in Human Skeletal Muscle after Acute Resistance Exercise: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients, 12(8), 2421. https://doi.org/10.3390/nu12082421





