A Greater Flavonoid Intake Is Associated with Lower Total and Cause-Specific Mortality: A Meta-Analysis of Cohort Studies
Abstract
:1. Introduction
2. Methods
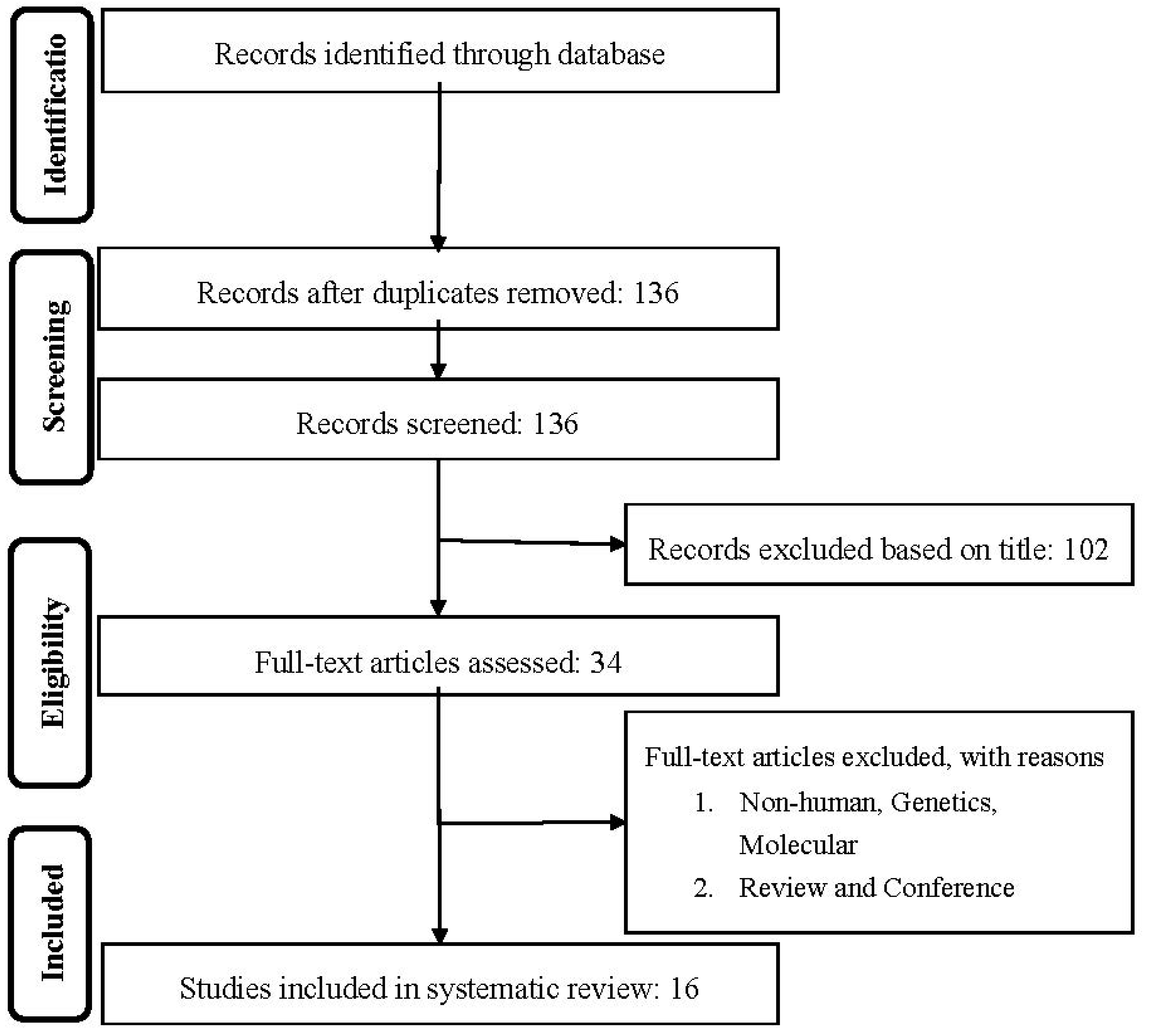
2.1. Literature Search and Study Selection
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis and Statistical Analyses
2.5. Publication Bias
3. Results
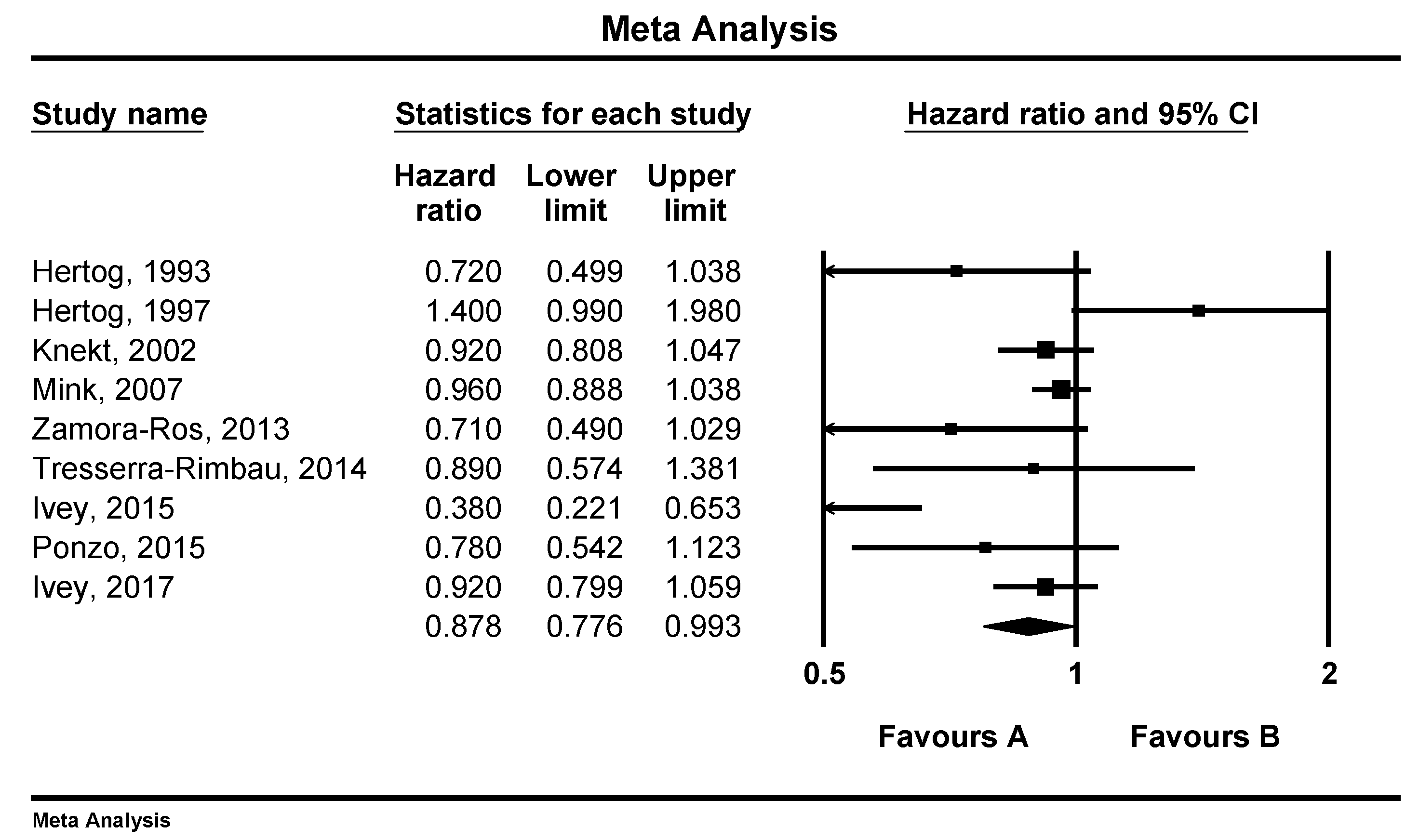
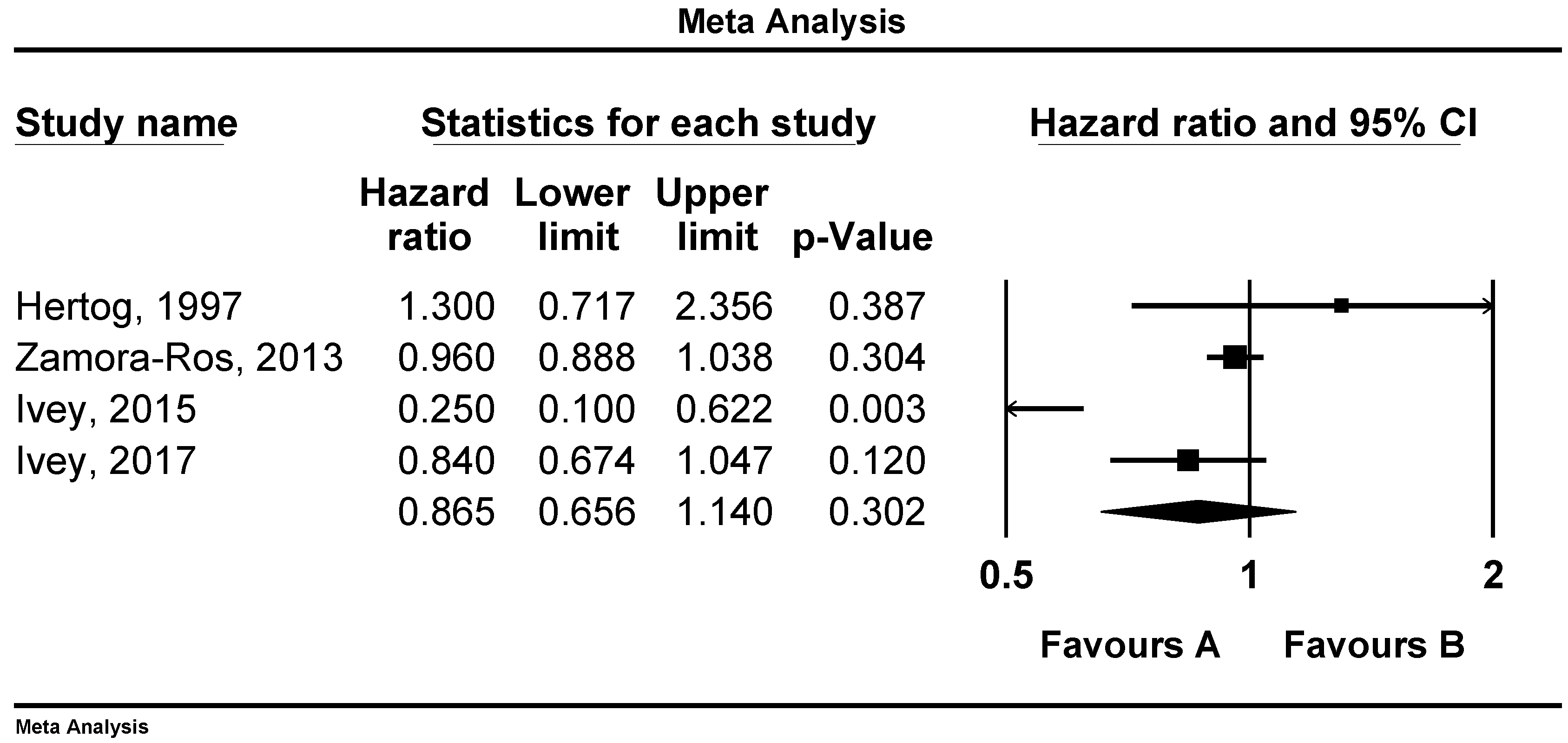
3.1. Associations of Flavonoid Intake with All Cause, CVD and Stroke Mortality
3.2. Sensitivity Analysis
3.3. Publication Bias
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.O.; Abate, K.H.; Abd-Allah, F.; Ahmed, M.; Alam, K.; Alam, T.; Alvis-Guzman, N.; Ansari, H.; Arnlov, J.; et al. The Burden of Cardiovascular Diseases Among US States, 1990–2016. JAMA Cardiol. 2018, 3, 375–389. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Clin. Nutr. ESPEN 2017, 20, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann. Intern. Med. 1996, 125, 384–389. [Google Scholar] [CrossRef]
- Hertog, M.G.; Sweetnam, P.M.; Fehily, A.M.; Elwood, P.C.; Kromhout, D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: The Caerphilly Study. Am. J. Clin. Nutr. 1997, 65, 1489–1494. [Google Scholar] [CrossRef]
- Hirvonen, T.; Pietinen, P.; Virtanen, M.; Ovaskainen, M.L.; Hakkinen, S.; Albanes, D.; Virtamo, J. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology 2001, 12, 62–67. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Geleijnse, J.M.; Launer, L.J.; Van der Kuip, D.A.; Hofman, A.; Witteman, J.C. Inverse association of tea and flavonoid intakes with incident myocardial infarction: The Rotterdam Study. Am. J. Clin. Nutr. 2002, 75, 880–886. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Rexrode, K.M.; Hu, F.; Albert, C.M.; Chae, C.U.; Rimm, E.B.; Stampfer, M.J.; Manson, J.E. Dietary intakes of flavonols and flavones and coronary heart disease in US women. Am. J. Epidemiol. 2007, 165, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, Y.; Iso, H.; Ishihara, J.; Okada, K.; Inoue, M.; Tsugane, S. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: The Japan Public Health Center-based (JPHC) study cohort I. Circulation 2007, 116, 2553–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Tuomainen, T.P.; Kurl, S.; Salonen, J.T. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2008, 100, 890–895. [Google Scholar] [CrossRef] [Green Version]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Jimenez, C.; Cleries, R.; Agudo, A.; Sanchez, M.J.; Sanchez-Cantalejo, E.; Molina-Montes, E.; Navarro, C.; Chirlaque, M.D.; Maria Huerta, J.; et al. Dietary flavonoid and lignan intake and mortality in a Spanish cohort. Epidemiology 2013, 24, 726–733. [Google Scholar] [CrossRef]
- Ivey, K.L.; Hodgson, J.M.; Croft, K.D.; Lewis, J.R.; Prince, R.L. Flavonoid intake and all-cause mortality. Am. J. Clin. Nutr. 2015, 101, 1012–1020. [Google Scholar] [CrossRef] [Green Version]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; Lopez-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Ponzo, V.; Goitre, I.; Fadda, M.; Gambino, R.; De Francesco, A.; Soldati, L.; Gentile, L.; Magistroni, P.; Cassader, M.; Bo, S. Dietary flavonoid intake and cardiovascular risk: A population-based cohort study. J. Transl. Med. 2015, 13, 218. [Google Scholar] [CrossRef] [Green Version]
- Ivey, K.L.; Jensen, M.K.; Hodgson, J.M.; Eliassen, A.H.; Cassidy, A.; Rimm, E.B. Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br. J. Nutr. 2017, 117, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.M.; Liu, Y.J.; Huang, Y.; Yu, H.J.; Yuan, S.; Tang, B.W.; Wang, P.G.; He, Q.Q. Dietary total flavonoids intake and risk of mortality from all causes and cardiovascular disease in the general population: A systematic review and meta-analysis of cohort studies. Mol. Nutr. Food Res. 2017, 61, 1601003. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, G.; Bacchetti, T.; Sahebkar, A. Effect of statin therapy on paraoxonase-1 status: A systematic review and meta-analysis of 25 clinical trials. Prog. Lipid Res. 2015, 60, 50–73. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother. Res. PTR 2014, 28, 633–642. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.C.; Mikhailidis, D.P.; Toth, P.P.; Muntner, P.; Ursoniu, S.; Mosterou, S.; Glasser, S.; Martin, S.S.; Jones, S.R.; et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: A systematic review and meta-analysis. Pharmacol. Res. 2016, 103, 236–252. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Metaanalysis (Vers. 2); Biostat. Inc.: Englewood Cliffs, NJ, USA, 2005. [Google Scholar]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and smoking-related cancer risk: A meta-analysis. PLoS ONE 2013, 8, e75604. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ Clin. Res. Ed. 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrime, M.G.; Bauer, S.R.; McDonald, A.C.; Chowdhury, N.H.; Coltart, C.E.; Ding, E.L. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J. Nutr. 2011, 141, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Naidoo, N.; Landberg, R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: Review of recent findings. Curr. Opin. Lipidol. 2013, 24, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: A meta-analysis. Eur. J. Epidemiol. 2011, 26, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Takatsuka, N.; Shimizu, H. Soy and fish oil intake and mortality in a Japanese community. Am. J. Epidemiol. 2002, 156, 824–831. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kayaba, K.; Ishikawa, S. Soy and Soy Products Intake, All-Cause Mortality, and Cause-Specific Mortality in Japan: The Jichi Medical School Cohort Study. Asia-Pac. J. Public Health 2015, 27, 531–541. [Google Scholar] [CrossRef]
- Kim, A.; Chiu, A.; Barone, M.K.; Avino, D.; Wang, F.; Coleman, C.I.; Phung, O.J. Green tea catechins decrease total and low-density lipoprotein cholesterol: A systematic review and meta-analysis. J. Am. Diet. Assoc. 2011, 111, 1720–1729. [Google Scholar] [CrossRef]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Ruel, G.; Couillard, C. Evidences of the cardioprotective potential of fruits: The case of cranberries. Mol. Nutr. Food Res. 2007, 51, 692–701. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Gluba, A.; Mikhailidis, D.P.; Nowak, P.; Bielecka-Dabrowa, A.; Rysz, J.; Banach, M. The role of polyphenols in cardiovascular disease. Med. Sci. Monit. 2010, 16, Ra110–Ra119. [Google Scholar] [PubMed]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292s–297s. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A. Polyphenols and cardiovascular disease: A critical summary of the evidence. Mini Rev. Med. Chem. 2011, 11, 1186–1190. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Kengne, A.P.; Mikhailidis, D.P.; Toth, P.P.; Ray, K.K.; Banach, M. Dietary food patterns and glucose/insulin homeostasis: A cross-sectional study involving 24,182 adult Americans. Lipids Health Dis. 2017, 16, 192. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Gao, H.K.; Kengne, A.P. Inflammatory Markers Are Positively Associated with Serum trans-Fatty Acids in an Adult American Population. J. Nutr. Metab. 2017, 2017, 3848201. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Karimi, E.; Rezaie, P.; Ferns, G.A. Effects of conjugated linoleic acid supplementation on serum C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Cardiovasc. Ther. 2017, 35. [Google Scholar] [CrossRef]
- Mazidi, M.; Gao, H.K.; Vatanparast, H.; Kengne, A.P. Impact of the dietary fatty acid intake on C-reactive protein levels in US adults. Medicine 2017, 96, e5736. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.P.; Mikhailidis, D.P.; Cicero, A.F.; Banach, M. Effects of selected dietary constituents on high-sensitivity C-reactive protein levels in U.S. adults. Ann. Med. 2017, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- van Die, M.D.; Bone, K.M.; Williams, S.G.; Pirotta, M.V. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014, 113, E119–E130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.H.; Liu, Z. Soy food consumption and lung cancer risk: A meta-analysis using a common measure across studies. Nutr. Cancer 2013, 65, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef]
- Yang, P.M.; Tseng, H.H.; Peng, C.W.; Chen, W.S.; Chiu, S.J. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int. J. Oncol. 2012, 40, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Thomas, C.M.; Wood, R.C., 3rd; Wyatt, J.E.; Pendleton, M.H.; Torrenegra, R.D.; Rodriguez, O.E.; Harirforoosh, S.; Ballester, M.; Lightner, J.; Krishnan, K.; et al. Anti-neoplastic activity of two flavone isomers derived from Gnaphalium elegans and Achyrocline bogotensis. PLoS ONE 2012, 7, e39806. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.K.; Singh, A.P.; Singh, R.K.; Demartino, A.; Brard, L.; Vorsa, N.; Lange, T.S.; Moore, R.G. Anti-angiogenic activity of cranberry proanthocyanidins and cytotoxic properties in ovarian cancer cells. Int. J. Oncol. 2012, 40, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Toledo, A.C.; Sakoda, C.P.; Perini, A.; Pinheiro, N.M.; Magalhaes, R.M.; Grecco, S.; Tiberio, I.F.; Camara, N.O.; Martins, M.A.; Lago, J.H.; et al. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br. J. Pharmacol. 2013, 168, 1736–1749. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989s–1009s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, J.N., 3rd; Colantonio, L.D.; Howard, G.; Safford, M.M.; Banach, M.; Reynolds, K.; Cushman, M.; Muntner, P. Healthy lifestyle factors and incident heart disease and mortality in candidates for primary prevention with statin therapy. Int. J. Cardiol. 2016, 207, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Touvier, M.; Druesne-Pecollo, N.; Kesse-Guyot, E.; Andreeva, V.A.; Fezeu, L.; Galan, P.; Hercberg, S.; Latino-Martel, P. Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: A prospective cohort study. Breast Cancer Res. Treat. 2013, 137, 225–236. [Google Scholar] [CrossRef]
- Luo, J.; Gao, Y.T.; Chow, W.H.; Shu, X.O.; Li, H.; Yang, G.; Cai, Q.; Li, G.; Rothman, N.; Cai, H.; et al. Urinary polyphenols, glutathione S-transferases copy number variation, and breast cancer risk: Results from the Shanghai women’s health study. Mol. Carcinog. 2012, 51, 379–388. [Google Scholar] [CrossRef] [Green Version]

| Author, Year and Reference | Country, Region/Cohort | Men (%) | Mean Age (Years) | Follow-Up Time (Years) | No. of Cases (Outcomes) | No. of Subjects | Outcomes (Mortality) | Main Confounders |
|---|---|---|---|---|---|---|---|---|
| Hertog (1993) [4] | Netherlands, Zutphen Elderly Study | 100 | 65–84 | 5 | 43, 185 | 805 | CHD, All-cause | Age, BMI, smoking, serum total and HDL-C, systolic blood pressure, intake of total energy, saturated fatty acids, cholesterol, alcohol, coffee, vitamin C, vitamin E, beta-carotene, dietary fiber, history of MI |
| Rimm (1996) [6] | USA, Health Professionals follow-up Study | 100 | 40–75 | 6 | 140 | 34,789 | CHD | Age, BMI, smoking, diabetes, profession, hypertension, high cholesterol levels, family history of CHD, intake of vitamin E, alcohol, dietary fiber, carotene and saturated fat |
| Hertog (1997) [7] | UK, Caerphilly study | 100 | 45–59 | 14 | 131, 334 | 1900 | IHD, All-cause | Age, BMI, smoking, systolic blood pressure, serum total cholesterol, history of IHD at baseline, social class, intakes of total energy, alcohol, fat, vitamin C, vitamin E, and beta-carotene |
| Hirvonen (2001) [8] | Finland, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study | 100 | 50–69 | 6.1 | 815 | 25,372 | CHD | Age, BMI, smoking, systolic and diastolic blood pressure, serum total cholesterol, HDL-C, diabetes, CHD history, marital status, educational level and physical activity |
| Knekt (2002) [9] | Finland, Finnish mobile clinic health examination survey | -- | 54.0 ± 10.6 | 28 | 681, 2085 | 9131 | IHD, All-cause | Age, sex, geographic area, occupation, blood pressure, smoking, serum cholesterol, BMI, diabetes, intakes of energy, cholesterol, saturated fatty acids, fiber, vitamin E, vitamin C and beta-carotene |
| Geleijnse (2002) [10] | Netherlands, Rotterdam Study | 38.3 | >55 | 5.6 | 30 | 4807 | MI | Age, sex, BMI, smoking, education level, daily intakes of alcohol, coffee, polyunsaturated fat, saturated fat, fiber, vitamin E, and total energy |
| Mink (2007) [11] | USA, Iowa Women’s Health Study | 0 | 55–69 | 16 | 2316, 7091 | 34,489 | CVD, All-cause | Age, BMI, waist-to-hip ratio, smoking, energy intake, marital status, education, blood pressure, diabetes, physical activity and estrogen use |
| Lin (2007) [12] | USA, Nurses’ Health Study | 0 | 30–55 | 12 | 324 | 66,360 | CHD | Age, BMI, current smoking, parental history of MI at an age <60 years, history of hypertension, hypercholesterolemia and diabetes, menopausal status, hormone replacement therapy, use of aspirin, multivitamin and vitamin E supplements, physical activity, alcohol consumption and total energy intake |
| Kokubo (2007) [13] | Japan, Japan Public Health Center-Based Study | 25.8 | 40–59 | 12.5 | 1538 | 40,462 | CVD | Age, sex, BMI, smoking, alcohol use, history of hypertension or diabetes, hypolipidemic drugs, education level, sports, dietary intake of fruits, vegetables, fish, salt, and energy |
| Mursu (2008) [14] | Finland, Kuopio Ischemic Heart Disease Risk Factor Study | 100 | 42–60 | 15.2 | 153 | 1950 | CVD | Age, examination years, BMI, systolic blood pressure, hypertension medication, serum HDL-C and LDL-C, serum TAG, maximal oxygen uptake, smoking, CVD in family, diabetes, alcohol intake, energy-adjusted intake of folate and vitamin E, total fat (percentage of energy) and saturated fat intake (percentage of energy) |
| McCullough (2012) [15] | USA, Cancer Prevention Study II Nutrition Cohort | 38.8 | 69.5 | 7 | 2771 | 98,469 | CVD | Age, sex, BMI, smoking, beer and liquor intake, history of hypertension and dyslipidemia, family history of MI, physical activity, energy intake, aspirin use, hormone replacement therapy (in women only) |
| Zamora-Ros (2013) [16] | Spain, EPIC-Spain cohort | 38 | 29–70 | 13.6 | 1915 | 40,622 | All-cause | Age, sex, BMI, education level, physical activity, smoking, lifetime alcohol consumption, total energy, vitamin C and fiber intake |
| Tresserra-Rimbau (2014) [18] | Spain, PREDIMED study | 45.3 | 55–80 | 4.8 | 327 | 7172 | All-cause | Age, smoking, BMI, diabetes, alcohol, total energy intake, physical activity, family history of CVD or cancer, aspirin use, antihypertensive drug use, use of oral hypoglycemic agents, insulin, other medication, intake of protein, saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids and cholesterol |
| Ivey (2015) [17] | Australia, Calcium Intake Fracture Outcome Age Related Extension Study | 0 | >75 | 5 | 78, 129 | 1063 | CVD, All-cause | Age, prevalent CVD and cancer, overweight or obesity, low fruit and vegetable intake, physical inactivity, current cigarette smoking, alcohol consumption |
| Ponzo (2015) [19] | Italy, Local Health Units of the province of Asti | - | 45–64 | 12 | 84, 220 | 1658 | CVD, All-cause | Age, sex, BMI, education, living in a rural area, METs, fiber and saturated fatty acid intakes, alcohol intake, smoking, systolic and diastolic blood pressure, total and HDL-c, fasting glucose, CRP, statin and aspirin use |
| Ivey (2017) [20] | USA, Nurses’ Health Study II. | 0 | 36.1 | 18 | 189, 1894 | 93,145 | CVD, All-cause | Age, BMI, smoking, menopausal status, family history of diabetes, cancer and MI, multivitamin supplement use, aspirin use, race, diabetes, hypercholesterolemia, hypertension, physical activity, energy intake, alcohol consumption and the Alternative Health Eating Index (minus alcohol) score |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazidi, M.; Katsiki, N.; Banach, M. A Greater Flavonoid Intake Is Associated with Lower Total and Cause-Specific Mortality: A Meta-Analysis of Cohort Studies. Nutrients 2020, 12, 2350. https://doi.org/10.3390/nu12082350
Mazidi M, Katsiki N, Banach M. A Greater Flavonoid Intake Is Associated with Lower Total and Cause-Specific Mortality: A Meta-Analysis of Cohort Studies. Nutrients. 2020; 12(8):2350. https://doi.org/10.3390/nu12082350
Chicago/Turabian StyleMazidi, Mohsen, Niki Katsiki, and Maciej Banach. 2020. "A Greater Flavonoid Intake Is Associated with Lower Total and Cause-Specific Mortality: A Meta-Analysis of Cohort Studies" Nutrients 12, no. 8: 2350. https://doi.org/10.3390/nu12082350







