Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria for the Systematic Review
2.2. Search Strategy
2.3. Data Collection Process
2.4. Studies Included in the Meta-Analysis
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
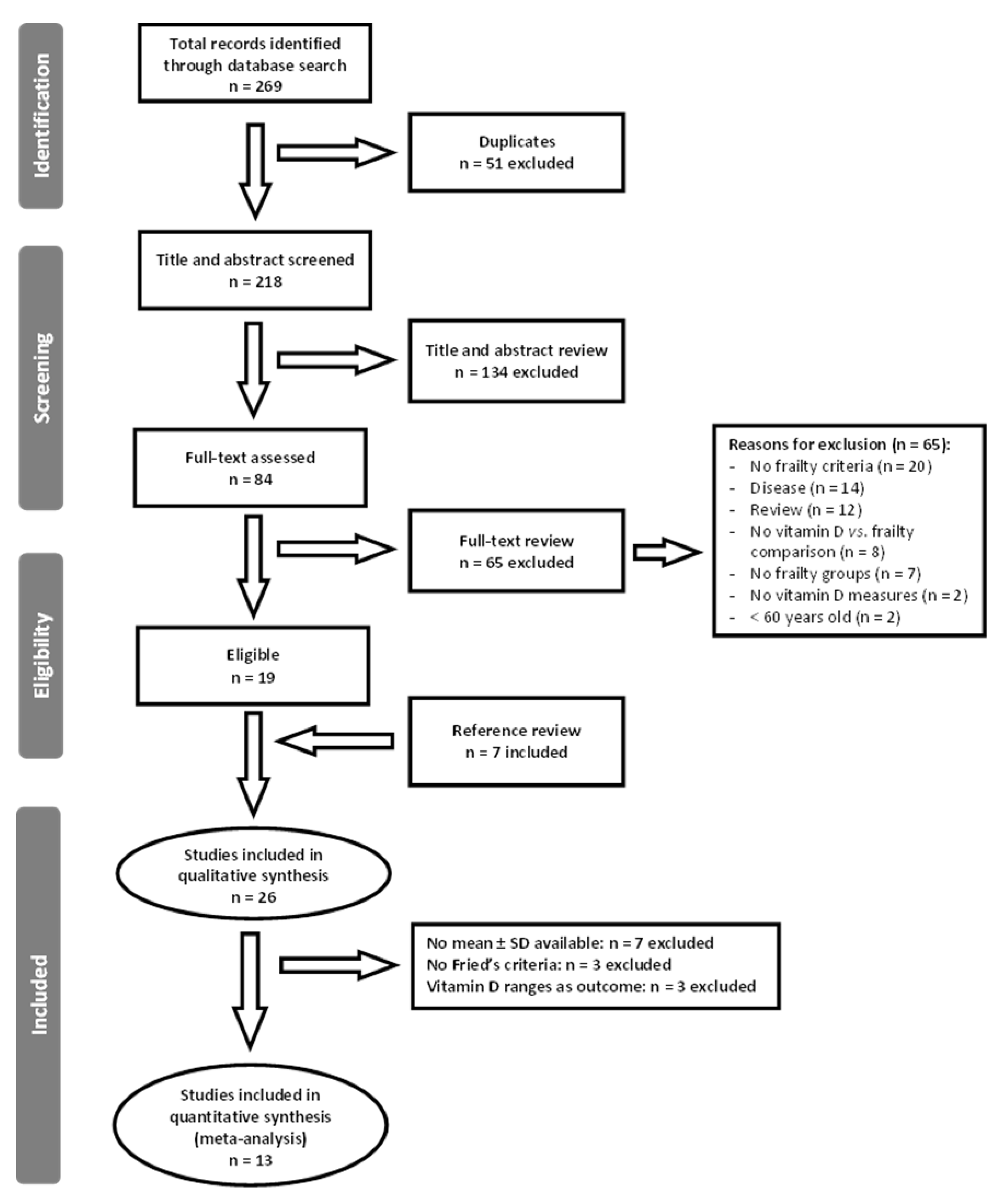
3.1. Literature Search Results
3.2. Characteristics of Included Studies
3.3. Meta-Analysis Frailty Group vs. Non-Frailty Group
3.4. Meta-Analysis Pre-Frailty Group vs. Non-Frailty Group
3.5. Meta-Analysis Frailty Group vs. Pre-Frailty Group
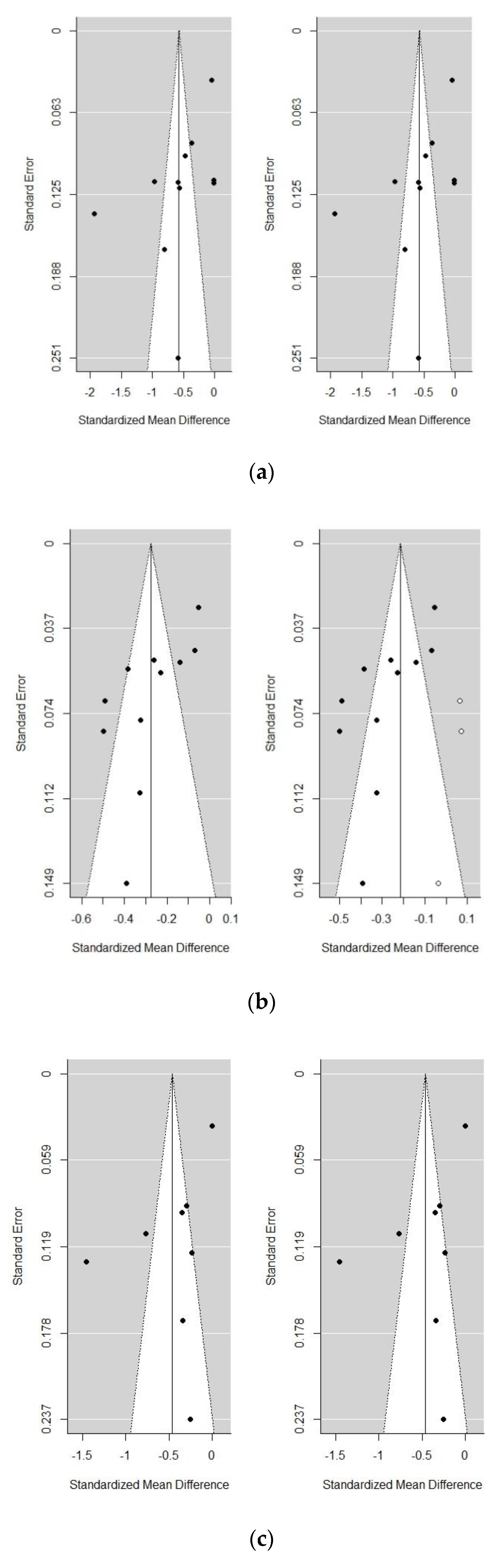
3.6. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardus, A.; Panizo, S.; Encinas, M.; Dolcet, X.; Gallego, C.; Aldea, M.; Fernandez, E.; Valdivielso, J.M. 1,25-Dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis 2009, 204, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Richart, T.; Li, Y.; Staessen, J.A. Renal Versus Extrarenal Activation of Vitamin D in Relation to Atherosclerosis, Arterial Stiffening, and Hypertension. Am. J. Hypertens. 2007, 20, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D: Extraskeletal Health. Rheum. Dis. Clin. N. Am. 2012, 38, 141–160. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What Dietetics Practitioners Need to Know. J. Am. Diet. Assoc. 2011, 111, 524–527. [Google Scholar] [CrossRef]
- Ganji, V.; Zhang, X.; Tangpricha, V. Serum 25-Hydroxyvitamin D Concentrations and Prevalence Estimates of Hypovitaminosis D in the U.S. Population Based on Assay-Adjusted Data. J. Nutr. 2012, 142, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Greene-Finestone, L.; Berger, C.; De Groh, M.; Hanley, D.; Hidiroglou, N.; Sarafin, K.; Poliquin, S.; Krieger, J.; Richards, J.; Goltzman, D.; et al. 25-Hydroxyvitamin D in Canadian adults: Biological, environmental, and behavioral correlates. Osteoporos. Int. 2011, 22, 1389–1399. [Google Scholar] [CrossRef]
- Lips, P. Vitamin, D. Deficiency and Secondary Hyperparathyroidism in the Elderly: Consequences for Bone Loss and Fractures and Therapeutic Implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- Souberbielle, J.C.; Cormier, C.; Kindermans, C.; Gao, P.; Cantor, T.; Forette, F.; Baulieu, E.E. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J. Clin. Endocrinol. Metab. 2001, 86, 3086–3090. [Google Scholar] [CrossRef]
- De Jongh, R.T.; Van Schoor, N.M.; Lips, P. Changes in vitamin D endocrinology during aging in adults. Mol. Cell. Endocrinol. 2017, 453, 144–150. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K. Frailty, Sarcopenia, and Hormones. Endocrinol. Metab. Clin. N. Am. 2013, 42, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Tangen, C.; Walston, J.; Newman, A.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.; Mogilner, A.; Rockwood, K. Accumulation of Deficits as a Proxy Measure of Aging. Sci. World 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collard, R.; Boter, H.; Schoevers, R.; Voshaar, R. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Schöttker, B.; Rothenbacher, D.; Perna, L.; Müller, H.; Brenner, H. Serum 25-hydroxyvitamin D levels and incident diabetes mellitus type 2: A competing risk analysis in a large population-based cohort of older adults. Eur. J. Epidemiol. 2013, 28, 267–275. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Ewing, S.K.; Fredman, L.; Hochberg, M.C.; Cauley, J.A.; Hillier, T.A.; Cummings, S.R.; Yaffe, K.; Cawthon, P.M. Circulating 25-Hydroxyvitamin D Levels and Frailty Status in Older Women. J. Clin. Endocrinol. Metab. 2010, 95, 5266–5273. [Google Scholar] [CrossRef] [Green Version]
- Sergi, G.; Veronese, N.; Fontana, L.; De Rui, M.; Bolzetta, F.; Zambon, S.; Corti, M.C.; Baggio, G.; Toffanello, E.D.; Crepaldi, G.; et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: The Pro.V.A. Study. J. Am. Coll. Cardiol. 2015, 65, 976–983. [Google Scholar] [CrossRef]
- Smit, E.; Crespo, C.J.; Michael, Y.; Ramirez-Marrero, F.A.; Brodowicz, G.R.; Bartlett, S.; Andersen, R.E. The effect of vitamin D and frailty on mortality among non-institutionalized US older adults. Eur. J. Clin. Nutr. 2012, 66, 1024–1028. [Google Scholar] [CrossRef]
- Krams, T.; Cesari, M.; Guyonnet, S.; Abellan van Kan, G.; Cantet, C.; Vellas, B.; Rolland, Y. Is the 25-hydroxy-vitamin D serum concentration a good marker of frailty? J. Nutr. Health Aging 2016, 20, 1034–1039. [Google Scholar] [CrossRef]
- Schoufour, J.D.; Echteld, M.A.; Boonstra, A.; Groothuismink, Z.M.A.A.; Evenhuis, H.M. Biochemical measures and frailty in people with intellectual disabilities. Age Ageing 2016, 45, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.Y.; Lee, J.Y.; Kim, D.H. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: A systematic review and dose-response meta-analysis. BMC Geriatr. 2018, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, P.; Liu, P.; Hao, Q.; Chen, S.; Dong, B.; Wang, J. Association of vitamin D deficiency and frailty: A systematic review and meta-analysis. Maturitas 2016, 94, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Dersimonian, R.; Laird, N. Meta-Analysis in Clinical Trials *. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Ríos, A.I.; Guerrero, J.M.; García-garcía, F.J.; Rodríguez-mañas, L.; Medrano-campillo, P.; De, M.A.; Lanza, T.; Alvarez-sánchez, N.; Carrillo-vico, A. Associations between frailty and serum N-terminal propeptide of type I procollagen and 25-hydroxyvitamin D in older Spanish women: The Toledo Study for Healthy Aging. EXG 2015, 69, 79–84. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Guerrero, J.M.; García-García, F.J.; Rodríguez-Mañas, L.; Cruz-Chamorro, I.; Lardone, P.J.; Carrillo-Vico, A. Homocysteine levels are associated with bone resorption in pre-frail and frail Spanish women: The Toledo Study for Healthy Aging. Exp. Gerontol. 2018, 108, 201–208. [Google Scholar] [CrossRef]
- Chang, C.-I.; Chan, D.-C.D.; Kuo, K.-N.; Hsiung, C.A.; Chen, C.-Y. Vitamin D insufficiency and frailty syndrome in older adults living in a Northern Taiwan community. Arch. Gerontol. Geriatr. 2010, 50 (Suppl. 1), S17–S21. [Google Scholar] [CrossRef]
- Ensrud, K.; Blackwell, T.; Cauley, J.; Cummings, S.; Barrett-Connor, J.; Dam, T.; Hoffman, A.; Shikany, J.; Lane, N.; Stefanick, M.; et al. Circulating 25-hydroxyvitamin D Levels and Frailty Status in Older Men: The Osteoporotic Fractures in Men Study. J. Am. Geriatr. Soc. 2012, 59, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.; Xue, Q.; Cappola, A.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.; Leng, S.; Semba, R.; Walston, J.; et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J. Gerontol. A Biol. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Robledo, L.M.; Ávila-funes, J.A.; Amieva, H.; Meillon, C.; Acosta, J.L.; Navarrete-reyes, A.P.; Muñoz-valle, J.F.; Torres-carrillo, N.M.; Miguel, L.; Ávila-funes, J.A.; et al. Association of low serum 25-hydroxyvitamin D levels with the frailty syndrome in Mexican community-dwelling elderly. Aging Male 2016, 19, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V.; Naganathan, V.; Cumming, R.G.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Associations between frailty and serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations in older australian men: The concord health and ageing in men project. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1112–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, G.; Tanabe, M. Frailty is Highly Prevalent and Associated with Vitamin D Deficiency in Male Nursing Home Residents. J. Am. Geriatr. Soc. 2016, 64, e33–e35. [Google Scholar] [CrossRef] [PubMed]
- Michelon, E.; Blaum, C.; Semba, R.D.; Xue, Q.-L.; Ricks, M.O.; Fried, L.P. Vitamin and carotenoid status in older women: Associations with the frailty syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martínez, R.; Fernández-garrido, J.; Buigues, C.; Martinez-martinez, M.; Cantero-díaz, L.; Santamaría-carrillo, Y.; Serra-catalá, N.; Peris, C.; Cauli, O. Serum vitamin D and functional impairment in octogenarian women. Appl. Nurs. Res. 2016, 30, e10–e14. [Google Scholar] [CrossRef]
- Pabst, G.; Zimmermann, A.K.; Huth, C.; Koenig, W.; Ludwig, T.; Zierer, A.; Peters, A.; Thorand, B. Association of low 25-hydroxyvitamin D levels with the frailty syndrome in an aged population: Results from the KORA-Age Augsburg study. J. Nutr. Health Aging 2015, 19, 258–264. [Google Scholar] [CrossRef]
- Puts, M.T.E.; Visser, M.; Twisk, J.W.R.; Deeg, D.J.H.; Lips, P. Endocrine and inflammatory markers as predictors of frailty. Clin. Endocrinol. 2005, 63, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, J.; Núñez, E.; Ruiz, V.; Bonanad, C.; Fernández, J.; Cauli, O.; García-blas, S.; Mainar, L.; Valero, E.; Rodríguez-borja, E.; et al. Usefulness of Clinical Data and Biomarkers for the Identi fi cation of Frailty After Acute Coronary Syndromes. Can. J. Cardiol. 2015, 31, 1–7. [Google Scholar] [CrossRef]
- Semba, R.D.; Bartali, B.; Zhou, J.; Blaum, C.; Ko, C.-W.; Fried, L.P. Low Serum Micronutrient Concentrations Predict Frailty Among Older Women Living in the Community. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 594–599. [Google Scholar] [CrossRef]
- Shardell, M.; Hicks, G.E.; Miller, R.R.; Kritchevsky, S.; Andersen, D.; Bandinelli, S.; Cherubini, A.; Ferrucci, L. Association of low vitamin D levels with the frailty syndrome in men and women. J. Gerontol. A. Biol. Sci. Med. Sci. 2009, 64, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Shardell, M.; D’Adamo, C.; Alley, D.E.; Miller, R.R.; Hicks, G.E.; Milaneschi, Y.; Semba, R.D.; Cherubini, A.; Bandinelli, S.; Ferrucci, L. Serum 25-hydroxyvitamin D, transitions between frailty states, and mortality in older adults: The invecchiare in Chianti study. J. Am. Geriatr. Soc. 2012, 60, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Santos, A.R.; Afonso, C.; Santos, A.; Borges, N.; Moreira, P.; Padrão, P.; Fonseca, I.; Amaral, T.F. The association between 25(OH)D levels, frailty status and obesity indices in older adults. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tajar, A.; Lee, D.M.; Pye, S.R.; O’Connell, M.D.L.; Ravindrarajah, R.; Gielen, E.; Boonen, S.; Vanderschueren, D.; Pendleton, N.; Finn, J.D.; et al. The association of frailty with serum 25-hydroxyvitamin d and parathyroid hormone levels in older european men. Age Ageing 2013, 42, 352–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, S.; Decke, S.; Gala, T.; De Las, H.; Linkohr, B.; Koenig, W.; Ladwig, K.H.; Peters, A.; Thorand, B. Prospective association of vitamin D with frailty status and all-cause mortality in older adults: Results from the KORA-Age Study. Prev. Med. 2015, 73, 40–46. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhan, J.; Tang, Z.; Huang, W.; Tan, P.; Gao, S.; Ma, C.; Jian, Z.; Liu, Y. Vitamin D Binding Protein Affects the Correlation of 25 (OH) D and Frailty in the Older Men. Int. J. Endocrinol. 2014, 2014, 543783. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm-Leen, E.R.; Hall, Y.N.; Deboer, I.H.; Chertow, G.M. Vitamin D deficiency and frailty in older Americans. J. Intern. Med. 2010, 268, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, Y.Y.E.; McCaul, K.A.; Yeap, B.B.; Hankey, G.J.; Flicker, L. Low vitamin D status is an independent predictor of increased frailty and all-cause mortality in older men: The health in men study. J. Clin. Endocrinol. Metab. 2013, 98, 3821–3828. [Google Scholar] [CrossRef] [Green Version]
- Mitri, J.; Pittas, A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef] [Green Version]
- Pittas, A.; Nelson, J.; Hillmann, W.; Garganta, C.; Nathan, D.; Hu, F.; Dawson-Hughes, B.; Diabetes Prevention Program Research Group. Plasma 25-Hydroxyvitamin D and Progression to Diabetes in Patients at Risk for Diabetes. An ancillary analysis in the Diabetes Prevention Program. Diabetes Care 2012, 35, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Ozfirat, Z.; Chowdhury, T.A. Vitamin D deficiency and type 2 diabetes. Postgrad. Med. J. 2010, 86, 18–25. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Hollis, B.; Rimm, E. A Prospective Study of 25-Hydroxy-Vitamin D and Risk of Myocardial Infarction in Men. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judd, S.E.; Tangpricha, V. Vitamin D Deficiency and Risk for Cardiovascular Disease. Am. J. Med. Sci. 2009, 338, 40–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shui, I.M.; Mucci, L.A.; Kraft, P.; Tamimi, R.M.; Lindstrom, S.; Penney, K.L.; Nimptsch, K.; Hollis, B.W.; Dupre, N.; Platz, E.A.; et al. Vitamin D—Related Genetic Variation, Plasma Vitamin D, and Risk of Lethal Prostate Cancer: A Prospective Nested Case—Control Study. J. Natl. Cancer Inst. 2012, 104, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabetta, J.R.; Depetrillo, P.; Cipriani, R.J.; Smardin, J.; Burns, L.A.; Marie, L. Serum 25-Hydroxyvitamin D and the Incidence of Acute Viral Respiratory Tract Infections in Healthy Adults. PLoS ONE 2010, 5, e11088. [Google Scholar] [CrossRef] [PubMed]
- Agmon-levin, N.; Kivity, S.; Tzioufas, A.G.; López, M.; Rozman, B.; Efes, I.; Shapira, Y.; Shamis, A.; Amital, H.; Youinou, P.; et al. Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjögren’s syndrome. J. Autoimmun. 2012, 39, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; März, W.; Drechsler, C.; Ritz, E.; Zittermann, A.; Cavalier, E.; Pieber, T.R.; Lappe, J.M.; Grant, W.B.; et al. Vitamin D, cardiovascular disease and mortality. Clin. Endocrinol. 2011, 75, 575–584. [Google Scholar] [CrossRef]
- Snijder, M.B.; Schoor, N.M.; Van Pluijm, S.M.F.; Dam, R.M.; Van Visser, M. Vitamin D Status in Relation to One-Year Risk of Recurrent Falling in Older Men and Women. J. Clin. Endocrinol. Metab. 2006, 91, 2980–2985. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D Receptor Expression in Human Muscle Tissue Decreases with Age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Marcinkowska, E. A run for a membrane vitamin d receptor. NeuroSignals 2001, 10, 341–349. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Vitamin D and Muscle Function. Osteoporos. Int. 2002, 13, 187–194. [Google Scholar] [CrossRef]
- Stewart, J.W.; Lee Alekel, D.; Ritland, L.M.; Van Loan, M.; Gertz, E.; Genschel, U. Serum 25-hydroxyvitamin D is related to indicators of overall physical fitness in healthy postmenopausal women. Menopause 2009, 16, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Wicherts, I.S.; Van Schoor, N.M.; Boeke, A.J.P.; Visser, M.; Deeg, D.J.H.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Rejnmark, L. Effects of vitamin D on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Dewansingh, P.; Melse-Boonstra, A.; Krijnen, W.P.; Van der Schans, C.P.; Jager-Wittenaar, H.; Van den Heuvel, E.G.H.M. Supplemental protein from dairy products increases body weight and vitamin D improves physical performance in older adults: A systematic review and meta-analysis. Nutr. Res. 2018, 49, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halfon, M.; Phan, O.; Teta, D. Vitamin D: A Review on Its Effects on Muscle Strength, the Risk of Fall, and Frailty. Biomed. Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avenell, A.; Mak, J.C.; O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014, 1465–1858. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: A trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014, 2, 307–320. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Etten, E.; Van Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Pérez, D.; Sánchez-Flores, M.; Maseda, A.; Lorenzo-López, L.; Millán-Calenti, J.; Gostner, J.; Fuchs, D.; Pásaro, E.; Laffon, B.; Valdiglesias, V. Frailty in Older Adults Is Associated With Plasma Concentrations of Inflammatory Mediators but Not With Lymphocyte Subpopulations. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agbalalah, T.; Hughes, S.F.; Freeborn, E.J.; Mushtaq, S. Impact of vitamin D supplementation on endothelial and inflammatory markers in adults: A systematic review. J. Steroid Biochem. Mol. Biol. 2017, 173, 292–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Tuohimaa, P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.K.; Somerville, M.J.; Yoong, L.K.K.; Bergeron, C.; Haussler, M.R.; Crapper McLachlan, D.R. Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: Correlation with calbindin-28k mRNA levels. Mol. Brain Res. 1992, 13, 239–250. [Google Scholar] [CrossRef]
- Annweiler, C.; Allali, G.; Allain, P.; Bridenbaugh, S.; Schott, A.M.; Kressig, R.W.; Beauchet, O. Vitamin D and cognitive performance in adults: A systematic review. Eur. J. Neurol. 2009, 16, 1083–1089. [Google Scholar] [CrossRef]
- Przybelski, R.; Agrawal, S.; Krueger, D.; Engelke, J.A.; Walbrun, F.; Binkley, N. Rapid correction of low vitamin D status in nursing home residents. Osteoporos. Int. 2008, 19, 1621–1628. [Google Scholar] [CrossRef]
- Rondanelli, M.; Trotti, R.; Opizzi, A.; Solerte, S.B. Relationship among nutritional status, pro/antioxidant balance and cognitive performance in a group of free-living healthy elderly. Minerva Med. 2007, 98, 639–645. [Google Scholar]
- Kelaiditi, E.; Canevelli, M.; Andrieu, S.; Del Campo, N.; Soto, M.E.; Vellas, B.; Cesari, M. Frailty Index and Cognitive Decline in Alzheimer’s Disease: Data from the Impact of Cholinergic Treatment USe Study. J. Am. Geriatr. Soc. 2016, 64, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Cavanelli, M.; Abellan van Kan, G.; Ousset, P.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A/I.A.G.G) international consensus group. J. Nutr. Health Aging 2013, 17, 262. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Cognitive frailty: A new geriatric syndrome? Eur. Geriatr. Med. 2015, 6, 408–411. [Google Scholar] [CrossRef]

| Study | First Author Country | Study Design | Population (Mean Age ± SD) (years) | Case Population (Mean Age ± SD) (years) | Control Population (Mean Age ± SD) (years) | Frailty Criteria | Outcome and Assay Method | Results |
|---|---|---|---|---|---|---|---|---|
| Alvarez-Ríos et al., 2015 [26] | Spain | Cross-sectional | n = 592 female | n = 61 frail (median 78, IQR 75–83) n = 232 pre-frail (median 74, IQR 71–78) | n = 299 non-frail (median 72, IQR 69–76) | Fried’s phenotype | 25(OH)D electro-chemiluminescence | ↓ 25(OH)D in pre-frail and frail vs. non-frail subjects |
| Alvarez-Sánchez et al., 2018 [27] | Spain | Cross-sectional | n = 631 female (median 74, IQR 70–77) | n = 61 frail n = 245 pre-frail | n = 325 non-frail | Fried’s phenotype | 25(OH)D chemiluminescence immunoassay | ↓ 25(OH)D with frailty |
| Chang et al., 2010 [28] | Taiwan | Cross-sectional | n = 215 | n = 21 frail (72.1 ± 4.4) male/female: 6/15 n = 119 pre-frail (71.4 ± 3.8) male/female: 49/70 | n = 75 non-frail (70.3 ± 3.7) male/female: 32/43 | Fried’s phenotype | 25(OH)D RIA | ↓ 25(OH)D with frailty using Fried’s phenotype |
| n = 26 frail (71.9 ± 3.7) male/female: 7/19 n = 52 pre-frail (71.5 ± 3.6) male/female: 14/38 | n = 137 non-frail (70.8 ± 4.0) male/female: 66/71 | Edmonton Frail Scale | The association between 25(OH)D and frailty is attenuated using the Edmonton Frail Scale | |||||
| Ensrud et al., 2010 [16] | USA | Cross-sectional Longitudinal (4.5-year follow-up) | n = 6307 female > 69 years old n = 4551 female | n = 1065 frail (n.p.) n = 3047 pre-frail (n.p.) | n = 2195 non-frail (n.p.) n = 4551 non-frail (non-frail + pre-frail) (n.p.) | Fried’s phenotype | 25(OH)D LC-MS/MS | Lower (<20 ng/mL) and higher (≥30 ng/mL) levels of 25(OH)D were moderately associated with higher odds of frailty at baseline Lower levels of 25(OH)D (<20 ng/mL) were modestly associated with an increased risk of incident frailty or death at follow-up |
| Ensrud et al., 2012 [29] | USA | Cross-sectional | n = 1606 male >65 years old | n = 130 frail n = 731 pre-frail | n = 745 non-frail | Fried’s phenotype | 25(OH)D LC-MS/MS | ↑ % frail male with ↓ 25(OH)D |
| Fried et al., 2009 [30] | USA | Cross-sectional | n = 704 female 70–79 years old | n = 90 frail n = 330 pre-frail | n = 284 non-frail | Fried’s phenotype | 25(OH)D Radioreceptor assay | ↓ 25(OH)D with frailty (nearly significant, p = 0.08) |
| Gutierrez-Robledo et al., 2015 [31] | Mexico | Cross-sectional | n = 331 | n = 209 frail (78.9 ± 6.4) male/female: 86/123 | n = 122 non-frail (79.9 ± 4.7) male/female: 66/56 | Fried’s phenotype (modified) | 25(OH)D ELISA | ↓ 25(OH)D in frail vs. non-frail subjects |
| Hirani et al., 2013 [32] | Australia | Cross-sectional | n = 1511 male 70–79 years old | n.p | n.p | Fried’s phenotype (modified) | 25(OH)D 1,25D RIA | ↓ 25(OH)D and 1,25D with frailty |
| Kojima & Tanabe, 2016 [33] | UK | Cross-sectional | n = 152 male (70.3 ± 12.8) | n = 124 frail (71.9 ± 13.1) | n = 28 non-frail (62.8 ± 8.6) | FI (34 items) | 25(OH)D n.p | ↓25(OH) with frailty |
| Krams et al., 2016 [19] | France | Cross-sectional | n = 321 (82.94 ± 5.89) male/female: 128/193 | n = 146 frail (84.10 ± 5.77) male /female: 54/92 | n = 148 non-frail (81.61 ± 5.59) male/female: 60/88 | Fried’s phenotype (modified) | 25(OH)D Chemiluminescence immunoassay | 25(OH)D levels were not significantly correlated with frailty |
| Michelon et al., 2006 [34] | USA | Cross-sectional | n = 754 female | n = 86 frail (mean 75.8, 95% CI 75.1–76.5) n = 337 pre-frail (mean 74.4, 95% CI 74.1–74.7) | n = 331 non-frail (mean 73.1, 95% CI 73.7–74.4) | Fried’s phenotype | 25(OH)D Radioreceptor assay | ↓ 25(OH)D with frailty |
| Navarro-Martínez et al., 2016 [35] | Spain | Cross-sectional | n = 104 female (mean = 84) | n = 60 Frail n = 22 Pre-frail | n = 22 non-frail | Fried’s phenotype | 25(OH)D GC/MS | ↓ 25(OH)D in pre-frail and frail vs. non-frail subjects |
| Pabst et al., 2015 [36] | Germany | Cross-sectional | n = 940 (75.6 ± 6.5) male/female: 478/762 | n = 38 Frail n = 351 Pre-frail | n = 551 non-frail | Fried’s phenotype | 25(OH)D ECLIA System | ↓ 25(OH)D with frailty |
| Puts et al., 2005 [37] | The Netherlands | Cross-sectional | n = 1271 | n = 242 frail (79.2 ± 6.2) male/female: 91/151 | n = 1029 non-frail (74.5 ± 6.3) male/female: 531/498 | Nine frailty indicators | 25(OH)D Competitive binding protein assay | ↓ 25(OH)D with frailty |
| Longitudinal (3-year follow-up) | n = 885 | n = 125 frail (78.2 ± 6.2) male/female: 56/69 | n = 760 non-frail (73.4 ± 5.9) male/female: 382/378 | ↓ 25(OH)D with frailty | ||||
| Sanchis et al., 2015 [38] | Spain | Cross-sectional | n = 342 male/female: 194/138 | n = 116 frail (81 ± 7) male/female: 47/69 | n = 226 non-frail (77 ± 7) male/female: 77/149 | Fried’s phenotype | 25(OH)D n.p. | ↓ vitamin D in frail vs. non-frail subjects 25(OH)D < 9 ng/mL was found as an independent predictor of frailty |
| Semba et al., 2006 [39] | USA | Longitudinal (3-year follow-up) | n = 766 female | n = 250 frail (80.4 ± 7.9) | n = 516 non-frail (76.1 ± 7.4) | Fried’s phenotype | 25(OH)D Radioreceptor assay | ↓ 25(OH)D with frailty |
| Sergi et al., 2015 [17] | Italy | Cross-sectional | n = 1567 | n = 491 pre-frail (1 positive criterion) (75.18 ± 6.86) male/female: 166/325 n = 209 pre-frail (2 positive criteria) (77.77 ± 7.48) male/female: 47/162 | n = 867 non-frail (71.68 ± 5.55) male/female: 404/463 | Fried’s phenotype (modified) | 25(OH)D RIA | ↓ 25(OH)D with increasing pre-frailty severity |
| Shardell et al., 2009 [40] | USA | Cross-sectional | n = 1005 | Male: n = 39 frail (80.8 ± 7.8) n = 151 pre-frail (76.2 ± 7.1) Female: n = 64 frail (82.3 ± 7.4) n = 237 pre-frail (76.1 ± 7.5) | Male: n = 242 non-frail (71.7 ± 5.5) Female: n = 243 non-frail (72.9 ± 6.1) | Fried’s phenotype | 25(OH)D RIA | Strong associations of ↓ 25(OH)D with frailty in men and weak association in women |
| Shardell et al., 2012 [41] | USA | Cross-sectional | n = 1005 | n = 100 frail n = 354 pre-frail | n = 471 non-frail | Fried’s phenotype | 25(OH)D RIA | Participants with ≥20 ng/mL of 25(OH)D had lower prevalence of frailty |
| Smit et al., 2012 [18] | USA | Cross-sectional | n = 4731 | n = 453 frail (73.6 ± 0.6) male/female: 140/313 n = 1915 pre-frail (72.0 ± 0.4) male/female: 705/1210 | n = 2363 non-frail (69.4 ± 0.3) male/female: 1099/1264 | Fried’s phenotype (modified) | 25(OH)D RIA | ↓ 25(OH)D with frailty |
| Longitudinal (12-year follow-up) | ↑ Risk of death with frailty and low serum 25(OH)D | |||||||
| Sousa-Santos et al., 2018 [42] | Portugal | Cross-sectional | n = 1447 (mean 74, range 65–100) male/female: 610/873 | n = 310 frail n = 785 pre-frail | n = 352 non-frail | Fried’s phenotype | 25(OH)D Electro-chemiluminescence immunoassay | ↓ 25(OH)D with pre-frailty and frailty |
| Tajar et al., 2013 [43] | UK | Cross-sectional | n = 1504 male | n = 76 frail (72.9 ± 4.7) n = 552 pre-frail (70.9 ± 5.6) | n = 876 non-frail (68.4 ± 5.5) | Fried’s phenotype (modified) FI (43 items) | 25(OH)D RIA | Comparable results using Fried’s phenotype and FI. ↓ 25(OH)D associated with being pre-frail and frail |
| Vogt et al., 2015 [44] | Germany | Longitudinal (2.9-year follow-up) | n = 727 | n = 27 frail (n.p.) n = 252 pre-frail (n.p.) | n = 448 non-frail (n.p.) | Fried’s phenotype (modified) | 25(OH)D Chemiluminescence immunoassay | ↓ 25(OH)D with incident pre-frailty and combined pre-frailty and frailty |
| Wang et al., 2014 [45] | China | Cross-sectional | n = 516 male | n = 174 frail (81.9 ± 4.4) n = 182 pre-frail (74.6 ± 5.2) | n = 160 non-frail (72.7 ± 4.1) | Fried’s phenotype | 25(OH)D RIA | ↓ 25(OH)D levels across frailty categories |
| Wilhelm-Leen et al., 2010 [46] | USA | Cross-sectional | n = 5048 male/female: 2469/2579 | n.p. | n.p. | Fried’s phenotype (modified) | 25(OH)D RIA | ↓ 25(OH)D with frailty |
| Wong et al., 2013 [47] | Australia | Cross-sectional | n = 4203 male (70–88) | n.p. | n.p. | FRAIL scale | 25(OH)D Chemiluminescence immunoassay | ↓ 25(OH)D with frailty |
| Longitudinal (9.2-year follow-up) | n = 1625 male | n = 1625 non-frail (FRAIL scale = 0) | ↓ 25(OH)D with incident frailty |
| Comparison | No. of Studies | Heterogeneity | Meta-Analysis | Publication Bias (Egger’s Test) | Trim-and-Fill | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 | p-Value | SMD | 95% CI | p-Value | Z | p-Value | SMD | 95% CI | p-Value | ||
| All studies | |||||||||||
| Frailty vs. non-frailty | 12 | 99.76 | <0.0001 | −1.31 | −2.47, −0.15 | 0.0271 | −0.4838 | 0.6285 | |||
| Pre-frailty vs. non-frailty | 12 | 99.83 | <0.0001 | −0.79 | −1.58, −0.003 | 0.0491 | −0.3639 | 0.7159 | |||
| Frailty vs. pre-frailty | 9 | 99.61 | <0.0001 | −0.82 | −1.77, 0.13 | 0.09 | 0.4327 | 0.6652 | |||
| Sensitivity analysis without Smit et al. (2012) [18] | |||||||||||
| Frailty vs. non-frailty | 11 | 96.05 | <0.0001 | −0.57 | −0.87, −0.28 | 0.0002 | −1.3846 | 0.1662 | |||
| Pre-frailty vs. non-frailty | 11 | 88.35 | <0.0001 | −0.27 | −0.38, −0.17 | <0.0001 | −2.5312 | 0.0114 | −0.21 | −0.31, −0.12 | <0.0001 |
| Frailty vs. pre-frailty | 8 | 95.55 | <0.0001 | −0.46 | −0.78, −0.14 | 0.0048 | −0.4693 | 0.6388 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Pérez, D.; Sánchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.P.; Fernández-Tajes, J.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2286. https://doi.org/10.3390/nu12082286
Marcos-Pérez D, Sánchez-Flores M, Proietti S, Bonassi S, Costa S, Teixeira JP, Fernández-Tajes J, Pásaro E, Valdiglesias V, Laffon B. Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(8):2286. https://doi.org/10.3390/nu12082286
Chicago/Turabian StyleMarcos-Pérez, Diego, María Sánchez-Flores, Stefania Proietti, Stefano Bonassi, Solange Costa, Joao Paulo Teixeira, Juan Fernández-Tajes, Eduardo Pásaro, Vanessa Valdiglesias, and Blanca Laffon. 2020. "Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis" Nutrients 12, no. 8: 2286. https://doi.org/10.3390/nu12082286







