Abstract
Glutamine is a major dietary amino acid that is both a fuel and nitrogen donor for healing tissues damaged by chemotherapy and radiation. Evidence supports the benefit of oral (enteral) glutamine to reduce symptoms and improve and/or maintain quality of life of cancer patients. Benefits include not only better nutrition, but also decreased mucosal damage (mucositis, stomatitis, pharyngitis, esophagitis, and enteritis). Glutamine supplementation in a high protein diet (10 grams/day) + disaccharides, such as sucrose and/or trehalose, is a combination that increases glutamine uptake by mucosal cells. This increased topical effect can reduce painful mucosal symptoms and ulceration associated with chemotherapy and radiation in the head and neck region, esophagus, stomach and small intestine. Topical and oral glutamine seem to be the preferred routes for this amino acid to promote mucosal healing during and after cancer treatment.
1. What Is Glutamine
Glutamine is an L-alpha-amino acid. It is the most abundant free amino acid in human blood. Glutamine is needed for several functions in the body including for the synthesis of proteins as well as an energy source. Glutamine can be synthesized by the body and can also be obtained from the diet if needed.
2. Importance of Glutamine
Glutamine is an important nitrogen donor in intracellular metabolism and in the maintenance of intestinal tract, immune cells, and muscle [,,,,,]. Weight loss in cancer patients is common, but sarcopenia (loss of muscle mass) is associated with increased complications and significantly worse survival [,,,]. Glutamine is a preferred fuel for both lymphocytes [,] and gastrointestinal (GI) tract [], thus it plays an important role in helping to defend against infections and to assist mucosa in being a barrier against infection (Figure 1). Glutamine has a central role in intracellular metabolism and acts as a nitrogen shuttle between muscle and other tissues; it is at a high and relatively stable concentration in plasma and red blood cells and at a much higher concentration in muscle compared to other amino acids [,,,,]. Since plasma glutamine concentrations are only minimally affected over time by either glutamine ingestion or infusion, muscle can be considered as a “bank” and the liver can be considered as the “banker” (Figure 2) [].
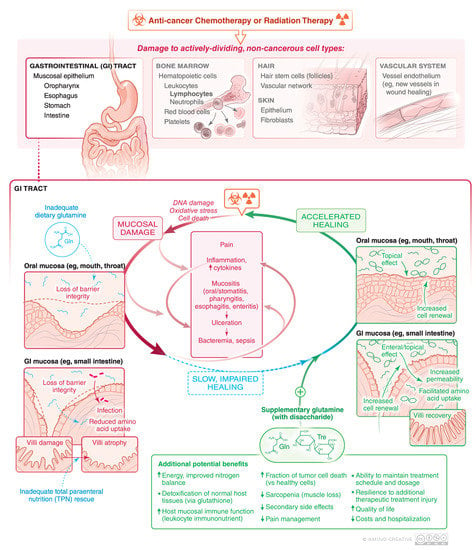
Figure 1.
Physical damage and amino acid malnutrition can both contribute to slow healing and worse mucosal injury from cancer chemotherapy and/or radiation. Dietary glutamine may ameliorate some of these side effects of cancer therapy.

Figure 2.
Pivotal role of glutamine stored in muscle (glutamine “bank”), normal high glutamine concentration in plasma, and liver amino acid metabolism (switch hitter) to facilitate steady state glutamine for mucosal health in (A) healthy anabolic state versus (B) during catabolic states including injury from cancer therapy, malnutrition, and tissue damage. In the catabolic state of mucositis from cancer therapy injury, topical glutamine + disaccharide can be helpful.
3. Oral Mucositis from Cancer Therapy: A Common Problem
Oral Mucositis (OM) refers to inflammation and ulceration of the oral mucosa as a side-effect of cancer therapy [,,,,,,,,]. OM and esophagitis can occur secondary to systemic chemotherapy for cancer [,,], high-dose chemotherapy as a hematopoietic transplant preparative regimen [,,,,,,,,,,,] or due to radiation therapy (RT) for head and neck (H&N) cancer [,,,,,] or if the oropharynx or esophagus is in field during and after radiation of bone metastases. OM is a common problem and occurs in about 20–40% of patients receiving conventional chemotherapy for solid tumors, about 80% of patients receiving high dose chemotherapy prior to a hematopoietic stem cell transplantation (HSCT) and almost all patients receiving RT for H&N cancer [,,]. Ulcerative OM and esophagitis are extremely painful, with many patients needing systemic opioids such as morphine or fentanyl for pain management [,,,,].
The intense mouth and esophageal pain from mucosal injury as well as enteritis results in significant nutritional compromise, which can lead to weight loss, impaired healing, and decreased resistance to infection [,,]. Because of malnutrition, feeding through a gastrostomy tube or total parenteral nutrition may be needed [,]. Quality of life is markedly reduced [,]. Maintenance of oral hygiene becomes difficult. The oral ulcerations can get secondarily infected, which may lead to bacteremia in patients with immunosuppression due to high dose chemotherapy. Cost of care is increased due to costs associated with pain management, nutritional support, infection control, and additional hospitalization. Perhaps most importantly, severe OM can necessitate a reduction in chemotherapy dosage or a break in RT and may discourage patients from getting therapy in a timely manner or at all, potentially affecting the overall success of cancer therapy [,,,,,,,,].
The pathogenesis of OM is complex and multifactorial (Figure 1) [,,,,,,,,,,,]. The primary etiology is direct damage from chemotherapy or RT to the oral epithelium. Table 1 lists common chemotherapy agents and how these often cause cytopenias and GI side effects including nausea, malnutrition, and mucosal damage (mucositis, stomatitis, esophagitis, enteritis) independently. In particular, the basal cells of the oral epithelium become unable to replenish the mucosa by their normally rapid division []. The cancer therapy also causes a variety of additional effects in the oral, esophageal, and intestinal mucosa, including activation of various inflammatory pathways, leading to up-regulation of inflammatory cytokines and other tissue-damaging molecules [,,,,,]. Once ulceration develops, the lesions become colonized by the local flora which can further aggravate the injury and impair healing. The overall result is a sustained period of oral and/or alimentary canal damage, inflammation and sometimes ulceration, with healing occurring one to several weeks after radiation and/or chemotherapy. If healing is slow, if may be difficult to sustain the intended schedule of chemotherapy or radiation therapy as illustrated in Figure 1. Conversely, accelerated healing can not only improve quality of life (QOL) but also facilitate timely cancer treatment with fewer secondary therapy-related side effects. Development of a “therapeutic alliance” to get feedback and provide options, to make each cycle of chemotherapy better and avoid a catabolic state, is an important role of, not only the oncologist, but also caregivers, dieticians, and nurses [].

Table 1.
Chemotherapy drugs and radiation-associated^ side effects.
4. Suggested Use of Glutamine in Mucositis Management from MASCC/ISOO 2019 Guidelines
A number of clinical studies have evaluated the use of glutamine to prevent or treat OM in various cancer populations. The Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) recently published systematic reviews of the literature on various interventions for OM and evidence-based clinical practice guidelines [,,,,,]. These include newer guidelines related to glutamine []. In patients receiving concurrent chemotherapy and radiation for H&N cancer, a suggestion was made in favor of the use of oral (PO) and/or swish and spit glutamine for the prevention of OM. This suggestion was based on Level II evidence, which was derived from two randomized controlled trials (RCTs). In these studies, the use of oral glutamine resulted in a significant reduction of severity of OM [,]. In addition, it also reduced the duration of OM in one study and the associated pain in another study. In one of these studies of H&N cancer patients, the glutamine was delivered as a “swish and swallow” liquid formulation, which indicates the possibility of a topical effect as well [,,].
There are also multiple studies supporting use of oral (PO) glutamine or parenteral for OM in other populations including patients with solid cancers and patients receiving HSCT [,,,]. These studies are summarized by Yarom et al. []. However, due to inadequate or conflicting data in these groups, no MASCC/ISOO guideline was possible for oral glutamine in these other populations.
On the other hand, in patients undergoing HSCT, the guidelines include a recommendation against the use of parenteral (intravenous) glutamine for the prevention of OM. This recommendation was based on the results of six RCTs, of which two found parenteral glutamine to be effective and four did not demonstrate a beneficial effect []. Furthermore, in one study of HSCT patients, a correlation of parenteral glutamine treatment with relapse and mortality was documented []. Taking into account all the above factors, the panel recommended against the use of parenteral glutamine for prevention of OM in patients undergoing HSCT.
The above results suggest that oral glutamine can be beneficial for management of OM and that there could be a positive topical effect. This approach is supported by results of a pilot RCT in which glutamine used topically as a mouth rinse and not swallowed was found to be effective in reducing severity and duration of OM [].
5. Potential Mechanisms of Glutamine Activity in Mucositis Management
Neutrophils, macrophages, and lymphocytes are needed for mucosal barrier immune defenses. Since glutamine is fuel for leukocytes, topical/oral/enteral glutamine may contribute to mucosal healing by not only a direct effect on mucosal epithelial cells, but also by improvement in host mucosal immune function and ability to resist microbial invasion [,,,,,,].
Interestingly, resilience of lymphocyte recovery, as measured by absolute lymphocyte count (ALC) after the very first cycle of chemotherapy, has been associated with a better prognosis in a variety of malignancies including acute lymphoblastic leukemia as well as tumors such as osteosarcoma, Ewing sarcoma, and rhabdomyosarcoma [,,,,,,]. It is possible that better nutrition, with amino acids including glutamine as fuel for lymphocytes, could contribute to ALC recovery and/or resilience. Animal models and human studies have shown glutamine supplementation improves the ability to resist the toxic effects of radiation to the GI tract [,,,,,].
Detoxification and resilience to free radical damage by chemotherapy (e.g., doxorubicin or cyclophosphamide) and/or radiation of normal tissues and tumors can involve the antioxidant glutathione. Since glutamine is a substrate for glutathione synthesis, adequate mucosal cell glutamine may contribute towards improved healing after chemotherapy and radiation damage [,] as well as, interestingly, the simultaneous inhibition of glutathione levels in tumors, too [,,,]. Furthermore, decreased inflammatory cytokines in normal cells and increased pro-apoptosis proteins in cancer cells were observed with glutamine + disaccharide supplementation []. Thus, glutamine can contribute to selective improvement in host cell resilience, less inflammation, and decreased ability of tumors to detoxify chemotherapy or resist radiation, i.e. an improved therapeutic index of the anti-cancer therapy.
Finally, fewer complications and improved survival have been seen in cancer patients that have less muscle loss, also known as sarcopenia [,,]. This underscores the importance of enteral nutrition and activity to maintain muscle mass rather than having muscle become a back-up system to provide amino acids including glutamine in catabolic oncology patients. Figure 2 illustrates this dynamic physiology and the importance of glutamine in enteral nutrition in cancer patients.
6. Bioavailability of Oral Glutamine Locally vs. Systemically in Mucositis Management
Although glutamine is easily the most abundant plasma amino acid (~20%; [,]), it accounts for an even greater proportion of the intracellular fluid (ICF) amino acid pool (~60%) and an extremely high proportion of the amino acid pool of muscle [,]. Figure 2 illustrates the central role of glutamine in nitrogen homeostasis and how the catabolic state can decrease intracellular (muscle) glutamine >50% and plasma glutamine levels 20–30%. Thus, in the catabolic state caused by cancer, nausea, cellular injury, or poor enteral glutamine intake, muscle glutamine stores may be called upon to provide glutamine to help to withstand and repair damage. This is because tissues are either glutamine consumers (high glutaminase activity in most organs and leucocytes and fibroblasts) or glutamine exporters (high glutamine synthetase activity in muscle and brain).
The liver is a “switch-hitter” that is endowed with periportal hepatocytes that have high glutaminase activity to process glutamine from intestinal absorption and perivenous hepatocytes with high glutamine synthetase activity to provide glutamine to the plasma to nourish the GI-tract when enteral glutamine is lacking. This allows the GI tract to get a constant supply of glutamine to use as a respiratory fuel by balancing glutamine luminal absorption when enteral glutamine is plentiful with plasma extraction when it is not. Since skeletal muscle accounts for 38% of body mass in men and 30% in women [], the contribution of muscle to glutamine homeostasis via the liver is a very significant one. Ammonia produced by intestinal glutaminase is cleared by the liver. Thus, both gut and liver contribute to glutamine homeostasis in the plasma and for mucosal surface metabolism (Figure 2).
Glutamine solubility is low (25 g/L); thus, suspensions are needed for topical oral and enteral supplementation; disaccharides can facilitate mucosal uptake []. Valencia et al. showed that in normal volunteers after ingestion of repeated daily high dose glutamine 0.3 g/kg/day, plasma glutamine remained at ~500 uM with only a minimal change (~20% increase) in plasma glutamine after this large daily dose after 10 days. This illustrates that the normally high concentration of glutamine is difficult to increase and probably of little relevance to tumors since glutamine is “always high” [].
Glutamine supplementation without disaccharide has been also shown to have some benefit in other studies against OM associated with BMT [,,]. Glutamine suspension with disaccharide to supply intraluminal glutamine for oral mucosa, esophagus, and intestines resulted serendipitously in not only a good-tasting formulation, but also a more effective treatment against mucositis than a glutamine-aspartame formulation. The glutamine-disaccharide combination facilitated >100-fold increased glutamine absorption by mucosal cells [,]. The glutamine-disaccharide formulation was effective against OM in a pilot study [] as well as two randomized, placebo double-blind trials in cancer patients getting anthracycline based chemotherapy and autologous bone marrow transplant chemotherapy [,,,,].
Trehalose, a disaccharide that not only facilitates glutamine cellular entry, but also has protein stabilizing properties and slower degradation to glucose than sucrose is contained in a commercially available glutamine-disaccharide product []. The cost of trehalose has become significantly less because it is now a commercially available sweetener (Treha, Cargill). Advantages of trehalose compared to sucrose for mucosal cell glutamine uptake include no oral degradation (less possibility of caries), stability in the acidic pH of the stomach, and once absorbed it is metabolized by the kidney so there is less of a glucose and insulin “bump” than the more rapid degradation and absorption for sucrose.
Enteric injury and diarrhea associated with abdominal radiation or chemotherapy can be ameliorated with glutamine supplementation. This should simultaneously increase glutathione in normal tissues such as the heart [,]. Glutamine supplementation may also decrease tumor glutaminase activity and tumor glutathione; this then may inhibit tumor utilization of glutamine for fuel and also make tumors more susceptible to damage from intracellular chemotherapy metabolites [,,,,,,,].
Irinotecan is a prodrug that is converted to the active metabolite SN-38 by intestinal bacteria. Its therapeutic effect is a blessing, but the severe, and sometimes delayed, prolonged enteritis from SN-38’s effects on the small bowel can adversely impact QOL. Delayed enteritis (e.g., days 5–15 after a 5–day cycle of oral irinotecan (50–90 mg/m2 daily for five days) is from chemotherapy-associated intestinal injury of the proximal intestine, which is exposed to the highest SN-38 concentrations. This delayed enteritis can be significantly reduced and sometimes completely eliminated by using the combination of glutamine with disaccharide during irinotecan and continuing for 7–10 days after oral irinotecan [].
7. Is Glutamine Safe During Cancer Treatment
Although normal and tumor bearing humans and animals have a high concentration of plasma glutamine (~500 uM) [,,,,], does the tumor’s utilization of glutamine outweigh nutritional benefit? Glutamine is a supplement Generally Recognized as Safe (GRAS) by the FDA. The amount of glutamine in a normal diet is about 10 g/day but may be higher in a high protein diet []. Under catabolic conditions such as during and after chemotherapy and radiation therapy, it would be expected that there would be an increased need for glutamine in the diet and increased utilization by mucosal tissue as well as leucocytes. Mayers et al. recognized the importance of whole organism physiology to address the contribution of glutamine to not only disease, but also health maintenance in cancer []. For example, not enough protein and/or glutamine during cancer treatment would be expected to lead to sarcopenia and lymphopenia, more complications, and worse survival [,,]. Adequate glutamine could be achieved with a high protein diet of 10–20 g/day, but 20–40 g may be needed if there is damage and stress []. If nausea is expected and/or there is impending or overt OM, esophagitis, or enteritis decreasing protein ingestion, ~4 g supplementary glutamine + disaccharide swish and swallow of the twice/day (BID) may be a safe and reasonable supplementary dose and schedule [].
Although both glucose and glutamine are fuels for cancer cells by providing alpha ketoglutarate to the Krebs cycle to generate ATP [,], the contribution of tumor glutaminase to the Krebs cycle is probably much less than glucose. Glutamine can improve enteral nutrition, immune function and has differential effects on glutathione synthesis in tissue and tumors. One of the major challenges is knowing whether the glutamine level is always high in plasma and whether dietary modifications have any effect on glutamine metabolism at the site of tumors [,]. Overall, it would seem the benefits probably outweigh the deleterious effects of dietary protein including glutamine (summarized in Table 2). Furthermore, studies by Suzanne Klimberg’s group showed oral glutamine supplementation reduced toxicity from chemotherapy or radiation and had a protective effect in tumor bearing animals and was associated with improved survival Table 2 [].

Table 2.
Physiologic balancing act by glutamine. Damage Control + Tissue Regeneration and Tumor Suppression.
Furthermore, glutamine has also shown to decrease tumor incidence in mice genetically predisposed to develop squamous cell carcinoma []. The dynamic interplay between: mucosal /topical supplementation with a glutamine and disaccharide []; the enteral nutrition with protein adequate to avoid a catabolic state; the tendency to maintain a steady state plasma glutamine; and the intramuscular glutamine “reservoir” and liver glycogen stores is very complex and likely influenced by the intensity of cytotoxic therapy and radiation and the ongoing need for repair. Of all the amino acids that seem to ameliorate and/or prevent mucosal injury from chemotherapy or radiation, glutamine has been the most well studied. Because of its local effect, the glutamine swish and swallow approach is a simple but possibly significant step in ameliorating some of the toxic effects of cancer therapy and helping cancer patients keep nutritional goals on track. Table 3 details ways to supplement diets with glutamine.

Table 3.
Available supplements with glutamine in conjunction with cancer therapy.
8. A Balanced Approach to Nutrition During Cancer Therapy
Given the need to balance the metabolic needs of cancer patients for energy to avoid fatigue with lipids and amino acids to heal, food choices can be confusing. Basic underlying principles for caregivers should address timing, both in relation to chemotherapy and/or radiation, what is sustainable, and what are important macro and micronutrients. Although a common perception is that damage from chemotherapy and radiation is immediate, there is ongoing inflammation and damage and a need for more nutrition for 1–2 weeks after each chemotherapy cycle or end of radiation. It is often helpful to ask to see the RT treatment plan (like a contour map). This helps one know which normal tissues are at highest risk of mucosal damage (e.g., mouth, oropharynx, and esophagus). If a mucosal surface is “in field”, then review with a dietician and plan how to maintain enteral calorie and protein intake to pre-empt malnutrition problems and provide an opportunity for less toxicity. Keeping this in mind, the plans to facilitate enteral nutrition should continue for two to four weeks after completion of RT to promote additional healing. For chemotherapy side effects, a review of potential side effects and reassessment and making improvements after each cycle should be the goal. If mucositis, esophagitis, or enteritis is an issue, supplementation with glutamine and disaccharide is a convenient and relatively easy means to ameliorate mucosal damage.
9. Summary and Conclusions
Topical oral swish and swallow glutamine and a disaccharide, such as trehalose, has potential to ameliorate not only OM, but also esophagitis and enteritis after cancer chemotherapy and radiation. If cancer patients and caregivers recognize that it is possible to increase mucosal glutamine absorption using disaccharides, there may be less mucosal damage experienced by cancer patients. A small amino acid intervention may make a difference and possibly contribute to better overall nutritional status, improved survival with fewer complications, and ultimately less sarcopenia and lymphopenia.
Author Contributions
P.M.A.: preparation, revision, formatting, and journal communication. R.V.L. contributed to revision of the manuscript. P.M.A. was responsible for tables, figures, and references. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by small, but important grants from the Hedberg family, Minnesota Vikings research grant and Minnesota Medical Foundation in the early 1990s (no research grant numbers for these grants are available) but much appreciation >25 years later. The preparation of the article was supported by Enlivity. The article processing charge (APC) was funded by the Pediatric Institute, Dept. of Cleveland Clinic Dept. of Pediatric Hematology/Oncology and Bone Marrow Transplantation Sarcoma Research Fund Grant Number T56428.
Acknowledgments
Anderson acknowledges Gillian Isabelle, Jill Tobacco, and Shemi Freytes from Enlivity for helpful discussions concerning amino acid supplementation, including Healios in cancer patients. Cassio Lynm from Amino Creative (Figure credit: ©Amino Creative, LLC, licensed under a CC-BY-NC-ND 4.0 International License cassio.lynm@aminocreative.com) was the medical illustrator for Figure 1 and Figure 2 (and support by Enlivity to create these figures was much appreciated). Anderson acknowledges dieticians including Sara Bewley RD and Christina DeTallo RD and pharmacists Mark Earl Pharm.D., Mike Stanton R.Ph., and Anthony Zembillas R.Ph. in promotion of enteral nutrition strategies and for applying principles of this article in real world settings in the pediatric oncology outpatient clinic and Cleveland Clinic Children’s Hospital.
Conflicts of Interest
Peter Anderson has a patent “glutamine and trehalose compositions” (US2015/0080331) and is on the scientific advisory board of Enlivity. Rajesh Lalla served as a consultant for Enlivity.
References
- Souba, W.W.; Smith, R.J.; Wilmore, D.W. Glutamine metabolism by the intestinal tract. J. Parenter. Enter. Nutr. 1985, 9, 608–617. [Google Scholar] [CrossRef]
- Souba, W.W.; Klimberg, V.S.; Plumley, D.A.; Salloum, R.M.; Flynn, T.C.; Bland, K.I.; Copeland, E.M., 3rd. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J. Surg. Res. 1990, 48, 383–391. [Google Scholar] [CrossRef]
- Klimberg, V.S.; McClellan, J.L.; Organ, C.H., Jr. Honorary Lectureship. Glutamine, cancer, and its therapy. Am. J. Surg. 1996, 172, 418–424. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Souba, W.W.; Salloum, R.M.; Plumley, D.A.; Cohen, F.S.; Dolson, D.J.; Bland, K.I.; Copeland, E.M., 3rd. Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J. Surg. Res. 1990, 48, 319–323. [Google Scholar] [CrossRef]
- Smith, R.J.; Wilmore, D.W. Glutamine nutrition and requirements. J. Parenter. Enter. Nutr. 1990, 14, 94S–99S. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W.; Shabert, J.K. Role of glutamine in immunologic responses. Nutrition 1998, 14, 618–626. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Williams, G.R.; Nyrop, K.A.; Muss, H.B.; Shachar, S.S. Muscle composition and outcomes in patients with breast cancer: Meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Fahr, M.J.; Kornbluth, J.; Blossom, S.; Schaeffer, R.; Klimberg, V.S.; Harry, M. Vars Research Award. Glutamine enhances immunoregulation of tumor growth. J. Parenter. Enter. Nutr. 1994, 18, 471–476. [Google Scholar] [CrossRef]
- Lacey, J.M.; Wilmore, D.W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 1990, 48, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Stein, W.H.; Moore, S. The free amino acids of human blood plasma. J. Biol. Chem. 1954, 211, 15–26. [Google Scholar]
- Valencia, E.; Marin, A.; Hardy, G. Impact of oral L-glutamine on glutathione, glutamine, and glutamate blood levels in volunteers. Nutrition 2002, 18, 367–370. [Google Scholar] [CrossRef]
- Filho, J.C.; Bergström, J.; Stehle, P.; Fürst, P. Simultaneous measurements of free amino acid patterns of plasma, muscle and erythrocytes in healthy human subjects. Clin. Nutr. 1997, 16, 299–305. [Google Scholar] [CrossRef]
- Mcmenamy, R.H.; Lund, C.C.; Oncley, J.L. Unbound amino acid concentrations in human blood plasmas. J. Clin. Investig. 1957, 36, 1672–1679. [Google Scholar] [CrossRef]
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R. Emerging evidence on the pathobiology of mucositis. Support Care Cancer 2013, 21, 3233–3241. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef]
- Logan, R.M.; Al-Azri, A.R.; Bossi, P.; Stringer, A.M.; Joy, J.K.; Soga, Y.; Ranna, V.; Vaddi, A.; Raber-Durlacher, J.E.; Lalla, R.V. Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020, 28, 2485–2498. [Google Scholar] [CrossRef]
- Elad, S. The MASCC/ISOO mucositis guidelines 2019: The second set of articles and future directions. Support Care Cancer 2020, 28, 2445–2447. [Google Scholar] [CrossRef]
- Lalla, R.V.; Brennan, M.T.; Gordon, S.M.; Sonis, S.T.; Rosenthal, D.I.; Keefe, D.M. Oral Mucositis Due to High-Dose Chemotherapy and/or Head and Neck Radiation Therapy. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz011. [Google Scholar] [PubMed]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.M.; Schubert, M.M.; Elting, L.S.; Sonis, S.T.; Epstein, J.B.; Raber-Durlacher, J.E.; Migliorati, C.A.; McGuire, D.B.; Hutchins, R.D.; Peterson, D.E. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007, 109, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Anderson, P.M. Oral glutamine to prevent chemotherapy induced stomatitis: A pilot study. J. Lab. Clin. Med. 1996, 127, 223–228. [Google Scholar] [CrossRef]
- Anderson, P.M.; Ramsay, N.K.; Shu, X.O.; Rydholm, N.; Rogosheshke, J.; Nicklow, R.; Weisdorf, D.J.; Skubitz, K.M. Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transpl. 1998, 22, 339–344. [Google Scholar] [CrossRef]
- Anderson, P.M.; Schroeder, G.; Skubitz, K.M. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer 1998, 83, 1433–1439. [Google Scholar] [CrossRef]
- Lalla, R.V.; Peterson, D.E. Oral mucositis. Dent. Clin. N. Am. 2005, 49, 167–184. [Google Scholar] [CrossRef]
- Aquino, V.M.; Harvey, A.R.; Garvin, J.H.; Godder, K.T.; Nieder, M.L.; Adams, R.H.; Jackson, G.B.; Sandler, E.S. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: A pediatric blood and marrow transplant consortium study. Bone Marrow Transpl. 2005, 36, 611–616. [Google Scholar] [CrossRef]
- Cockerham, M.B.; Weinberger, B.B.; Lerchie, S.B. Oral glutamine for the prevention of oral mucositis associated with high-dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann. Pharmacother. 2000, 34, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Albertioni, F.; Rask, C.; Schroeder, H.; Peterson, C. Monitoring of methotrexate and 7-hydroxymethotrexate in saliva from children with acute lymphoblastic leukemia receiving high-dose consolidation treatment: Relation to oral mucositis. Anticancer Drugs 1997, 8, 119–124. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.B.; Altomonte, V.; Peterson, D.E.; Wingard, J.R.; Jones, R.J.; Grochow, L.B. Patterns of mucositis and pain in patients receiving preparative chemotherapy and bone marrow transplantation. Oncol. Nurs. Forum 1993, 20, 1493–1502. [Google Scholar] [PubMed]
- McGuire, D.B.; Peterson, D.B.; Muller, S.; Owen, D.C.; Slemmons, M.F.; Schubert, M.M. The 20 item oral mucositis index: Reliability and validity in bone marrow and stem cell transplant patients. Cancer Investig. 2002, 20, 893–903. [Google Scholar] [CrossRef]
- McGuire, D.B.; Yeager, K.A.; Dudley, W.N.; Peterson, D.E.; Owen, D.C.; Lin, L.S.; Wingard, J.R. Acute oral pain and mucositis in bone marrow transplant and leukemia patients: Data from a pilot study. Cancer Nurs. 1998, 21, 385–393. [Google Scholar] [CrossRef]
- Schloerb, P.R.; Skikne, B.S. Oral and parenteral glutamine in bone marrow transplantation: A randomized, double-blind study. J. Parenter. Enter. Nutr. 1999, 23, 117–122. [Google Scholar] [CrossRef]
- Wingard, J.R.; Niehaus, C.S.; Peterson, D.E.; Jones, R.J.; Piantadosi, S.; Levin, L.S.; Saral, R.; Santos, G.W. Oral mucositis after bone marrow transplantation. A marker of treatment toxicity and predictor of hepatic veno-occlusive disease. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 419–424. [Google Scholar] [CrossRef]
- Sonis, S.T.; Oster, G.; Fuchs, H.; Bellm, L.; Bradford, W.Z.; Edelsberg, J.; Hayden, V.; Eilers, J.; Epstein, J.B.; LeVeque, F.G. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J. Clin. Oncol. 2001, 19, 2201–2205. [Google Scholar] [CrossRef]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T.; et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004, 100 (Suppl. 9), 2026–2046. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Saunders, M.I.; Dische, S.; Bond, S.J. Radiotherapy-related early morbidity in head and neck cancer: Quantitative clinical radiobiology as deduced from the CHART trial. Radiother. Oncol. 2001, 60, 123–135. [Google Scholar] [CrossRef]
- Epstein, J.B.; Silverman, S.; Paggiarino, D.A., Jr.; Crockett, S.; Schubert, M.M.; Senzer, N.N.; Lockhart, P.B.; Gallagher, M.J.; Peterson, D.E.; Leveque, F.G. Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: Results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 2001, 92, 875–885. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leung, S.W.; Wang, C.J.; Chen, H.C.; Sun, L.M.; Fang, F.M.; Yeh, S.A.; Hsu, H.C.; Hsiung, C.Y. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 535–539. [Google Scholar] [CrossRef]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur. J. Oncol. Nurs. 2015, 19, 214–219. [Google Scholar] [CrossRef]
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef]
- Saunders, D.P.; Rouleau, T.; Cheng, K.; Yarom, N.; Kandwal, A.; Joy, J.; Bektas Kayhan, K.; van de Wetering, M.; Brito-Dellan, N.; Kataoka, T. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020, 28, 2473–2484. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, H.; Sun, X.; Xia, Z.; Li, Y.; Lin, X.; Guo, Y. Efficacy and safety of transdermal fentanyl for treatment of oral mucositis pain caused by chemotherapy. Expert. Opin. Pharmacother. 2008, 9, 3137–3144. [Google Scholar] [CrossRef]
- Huang, H.Q.; Cai, Q.Q.; Lin, X.B.; Wang, B.F.; Bu, Q.; Gao, Y.; Peng, Y.L. Transdermal fentanyl in treating severe painful mucositis caused by autologous hematopoietic stem cell transplantation. Ai Zheng 2007, 26, 390–393. [Google Scholar]
- Sobue, T.; Bertolini, M.; Thompson, A.; Peterson, D.E.; Diaz, P.I.; Dongari-Bagtzoglou, A. Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol. Oral Microbiol. 2018, 33, 212–223. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or radiation-induced oral mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.E.P.; Cheng, K.K.F.; Chiang, K.; Kandwal, A.; Loprinzi, C.L.; Mori, T.; Potting, C.; Rouleau, T.; Toro, J.J.; Ranna, V.; et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 28, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, M.; Eriksen, J.G.; Bensadoun, R.J.; Boers-Doets, C.B.; Lalla, R.V.; Peterson, D.E. Oral Mucosal Injury Caused by Targeted Cancer Therapies. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz012. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Srivastava, R.; Lalla, R.V. Oral mucosal injury in oncology patients: Perspectives on maturation of a field. Oral Dis. 2015, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Pathobiology of mucositis. Semin. Oncol. Nurs. 2004, 20, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support Oncol. 2007, 5 (Suppl. 4), 3–11. [Google Scholar]
- Li, H.L.; Lu, L.; Wang, X.S.; Qin, L.Y.; Wang, P.; Qiu, S.P.; Wu, H.; Huang, F.; Zhang, B.B.; Shi, H.L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother. Pharmacol. 2009, 63, 239–251. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef]
- Anderson, P.; Kaye, L. The therapeutic alliance: Adapting to the unthinkable with better information. Health Commun. 2009, 24, 775–778. [Google Scholar] [CrossRef]
- Vlassara, H.; Brownlee, M.; Manogue, K.R.; Dinarello, C.A.; Pasagian, A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: Role in normal tissue remodeling. Science 1988, 240, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Mahoney, J.; Le Trang, N.; Pekala, P.; Cerami, A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J. Exp. Med. 1985, 161, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.P.; Epstein, J.B.; Elad, S.; Allemano, J.; Bossi, P.; van de Wetering, M.D.; Rao, N.G.; Potting, C.; Cheng, K.K.; Freidank, A.; et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients. Support Care Cancer 2013, 21, 3191–3207. [Google Scholar] [CrossRef]
- Peterson, D.E.; Ohrn, K.; Bowen, J.; Fliedner, M.; Lees, J.; Loprinzi, C.; Mori, T.; Osaguona, A.; Weikel, D.S.; Elad, S.; et al. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer 2013, 21, 327–332. [Google Scholar] [CrossRef]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: Vitamins, minerals, and nutritional supplements. Support Care Cancer 2019, 27, 3997–4010. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Saha, A.; Azam, M.; Mukherjee, A.; Sur, P.K. Role of oral glutamine in alleviation and prevention of radiation-induced oral mucositis: A prospective randomized study. South Asian J. Cancer 2014, 3, 8–12. [Google Scholar]
- Tsujimoto, T.; Yamamoto, Y.; Wasa, M.; Takenaka, Y.; Nakahara, S.; Takagi, T.; Tsugane, M.; Hayashi, N.; Maeda, K.; Inohara, H.; et al. L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: A double-blind, randomized, placebo-controlled trial. Oncol. Rep. 2015, 33, 33–39. [Google Scholar] [CrossRef]
- Peterson, D.E.; Jones, J.B.; Petit, R.G., 2nd. Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer 2007, 109, 322–331. [Google Scholar] [CrossRef]
- Pytlik, R.; Benes, P.; Patorkova, M.; Chocenska, E.; Gregora, E.; Prochazka, B.; Kozak, T. Standardized parenteral alanyl-glutamine dipeptide supplementation is not beneficial in autologous transplant patients: A randomized, double-blind, placebo controlled study. Bone Marrow Transplant 2002, 30, 953–961. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Souba, W.W. The importance of intestinal glutamine metabolism in maintaining a healthy gastrointestinal tract and supporting the body’s response to injury and illness. Surg. Annu. 1990, 22, 61–76. [Google Scholar]
- Anderson, P. Predicting and facilitating survival of pediatric cancer patients: The ALC story. Pediatr. Blood Cancer 2010, 55, 1041–1042. [Google Scholar] [CrossRef]
- Anderson, P.M. Immune Therapy for Sarcomas. Adv. Exp. Med. Biol. 2017, 995, 127–140. [Google Scholar]
- De Angulo, G.; Yuen, C.; Palla, S.L.; Anderson, P.M.; Zweidler-McKay, P.A. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: Implications for risk stratification and future studies. Cancer 2008, 112, 407–415. [Google Scholar] [CrossRef]
- Moore, C.; Eslin, D.; Levy, A.; Roberson, J.; Giusti, V.; Sutphin, R. Prognostic significance of early lymphocyte recovery in pediatric osteosarcoma. Pediatr. Blood Cancer 2010, 55, 1096–1102. [Google Scholar] [CrossRef]
- Vasquez, L.; Leon, E.; Beltran, B.; Maza, I.; Oscanoa, M.; Geronimo, J. Pretreatment Neutrophil-to-Lymphocyte Ratio and Lymphocyte Recovery: Independent Prognostic Factors for Survival in Pediatric Sarcomas. J. Pediatr. Hematol. Oncol. 2017, 39, 538–546. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Campbell, P.; Zhou, Y.; Sandlund, J.T.; Jeha, S.; Ribeiro, R.C.; Inaba, H.; Bhojwani, D.; Relling, M.V.; Howard, S.C.; et al. Prognostic impact of absolute lymphocyte counts at the end of remission induction in childhood acute lymphoblastic leukemia. Cancer 2013, 119, 2061–2066. [Google Scholar] [CrossRef]
- DuBois, S.G.; Elterman, K.; Grier, H.E. Early lymphocyte recovery in Ewing sarcoma. J. Pediatr. Hematol. Oncol. 2007, 29, 351–352. [Google Scholar] [CrossRef]
- Souba, W.W.; Klimberg, V.S.; Copeland, E.M. Oral glutamine reduces bacterial translocation following abdominal radiation. J. Surg. Res. 1990, 48, 1–5. [Google Scholar] [CrossRef]
- Klimberg, S. Prevention of radiogenic side effects using glutamine-enriched elemental diets. Recent. Results Cancer Res. 1991, 121, 283–285. [Google Scholar]
- Klimberg, V.S.; Salloum, R.M.; Kasper, M.; Plumley, D.A.; Dolson, D.J.; Hautamaki, R.D.; Mendenhall, W.R.; Bova, F.C.; Bland, K.I.; Copeland, E.M.; et al. Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch. Surg. 1990, 125, 1040–1045. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Souba, W.W.; Dolson, D.J.; Salloum, R.M.; Hautamaki, R.D.; Plumley, D.A.; Mendenhall, W.M.; Bova, F.J.; Khan, S.R.; Hackett, R.L.; et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer 1990, 66, 62–68. [Google Scholar] [CrossRef]
- Rubio, I.; Suva, L.J.; Todorova, V.; Bhattacharyya, S.; Kaufmann, Y.; Maners, A.; Smith, M.; Klimberg, V.S. Oral glutamine reduces radiation morbidity in breast conservation surgery. J. Parenter. Enter. Nutr. 2013, 37, 623–630. [Google Scholar] [CrossRef]
- Souba, W.W.; Klimberg, V.S.; Copeland, E.M., 3rd. Glutamine nutrition in the management of radiation enteritis. J. Parenter. Enter. Nutr. 1990, 14 (Suppl. 4), 106S–108S. [Google Scholar]
- Cao, Y.; Kennedy, R.; Klimberg, V.S. Glutamine protects against doxorubicin-induced cardiotoxicity. J. Surg. Res. 1999, 85, 178–182. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Klimberg, V.S. Effect of glutamine on gut glutathione fractional release in the implanted tumor model. Nutr. Cancer 2007, 59, 199–206. [Google Scholar] [CrossRef]
- Lim, V.; Korourian, S.; Todorova, V.K.; Kaufmann, Y.; Klimberg, V.S. Glutamine prevents DMBA-induced squamous cell cancer. Oral Oncol. 2009, 45, 148–155. [Google Scholar] [CrossRef]
- Rouse, K.; Nwokedi, E.; Woodliff, J.E.; Epstein, J.; Klimberg, V.S. Glutamine enhances selectivity of chemotherapy through changes in glutathione metabolism. Ann. Surg. 1995, 221, 420–426. [Google Scholar] [CrossRef]
- Todorova, V.K.; Harms, S.A.; Kaufmann, Y.; Luo, S.; Luo, K.Q.; Babb, K.; Klimberg, V.S. Effect of dietary glutamine on tumor glutathione levels and apoptosis-related proteins in DMBA-induced breast cancer of rats. Breast Cancer Res. Treat. 2004, 88, 247–256. [Google Scholar] [CrossRef]
- Todorova, V.K.; Harms, S.A.; Luo, S.; Kaufmann, Y.; Babb, K.B.; Klimberg, V.S. Oral glutamine (AES-14) supplementation inhibits PI-3k/Akt signaling in experimental breast cancer. J. Parenter. Enter. Nutr. 2003, 27, 404–410. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Petit, R.G., 2nd; Shinal, E.; French, C. AES-14 facilitates rapid intracellular transport of high levels of L-glutamine in mucosal epithelial cells. Proc. Am. Soc. Clin. Oncol. 2000, 2002, 261b. [Google Scholar]
- Mayers, J.R.; Vander Heiden, M.G. Famine versus feast: Understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 2015, 40, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M. Supportive care after chemotherapy and radiation with a swish and swallow glutamine+ disaccharide nutritional supplement to reduce mucositis and improve enteral nutrition. In Proceedings of the 46th Congress of The International Society of Paediatric Oncology (SIOP), Toronto, ON, Canada, 22–25 October 2014. [Google Scholar]
- Todorova, V.; Vanderpool, D.; Blossom, S.; Nwokedi, E.; Hennings, L.; Mrak, R.; Klimberg, V.S. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition 2009, 25, 812–817. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Nwokedi, E.; Hutchins, L.F.; Pappas, A.A.; Lang, N.P.; Broadwater, J.R.; Read, R.C.; Westbrook, K.C. Glutamine facilitates chemotherapy while reducing toxicity. J. Parenter. Enter. Nutr. 1992, 16 (Suppl. 6), 83S–87S. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Pappas, A.A.; Nwokedi, E.; Jensen, J.C.; Broadwater, J.R.; Lang, N.P.; Westbrook, K.C. Effect of supplemental dietary glutamine on methotrexate concentrations in tumors. Arch. Surg. 1992, 127, 1317–1320. [Google Scholar] [CrossRef]
- Rubio, I.T.; Cao, Y.; Hutchins, L.F.; Westbrook, K.C.; Klimberg, V.S. Effect of glutamine on methotrexate efficacy and toxicity. Ann. Surg. 1998, 227, 772–778. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 749. [Google Scholar] [CrossRef]
- Souba, W.W.; Strebel, F.R.; Bull, J.M.; Copeland, E.M.; Teagtmeyer, H.; Cleary, K. Interorgan glutamine metabolism in the tumor-bearing rat. J. Surg. Res. 1988, 44, 720–726. [Google Scholar] [CrossRef]
- Labow, B.I.; Souba, W.W. Glutamine. World J. Surg. 2000, 24, 1503–1513. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef]
- Asselin, B.; Rizzari, C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk. Lymphoma 2015, 56, 2273–2280. [Google Scholar] [CrossRef]
- Covini, D.; Tardito, S.; Bussolati, O.; Chiarelli, L.R.; Pasquetto, M.V.; Digilio, R.; Valentini, G.; Scotti, C. Expanding targets for a metabolic therapy of cancer: L-asparaginase. Recent Pat. Anticancer Drug Discov. 2012, 7, 4–13. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Su, Y.; Zhang, J.Y.; Antanasijevic, A.; Caffrey, M.; Schalk, A.M.; Liu, L.; Rondelli, D.; Oh, A.; Mahmud, D.L.; et al. A Novel l-Asparaginase with low l-Glutaminase Coactivity Is Highly Efficacious against Both T- and B-cell Acute Lymphoblastic Leukemias In Vivo. Cancer Res. 2018, 78, 1549–1560. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Kornbluth, J.; Feng, Z.; Fahr, M.; Schaefer, R.F.; Klimberg, V.S. Effect of glutamine on the initiation and promotion phases of DMBA-induced mammary tumor development. J. Parenter. Enter. Nutr. 2003, 27, 411–418. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Luo, S.; Johnson, A.; Babb, K.; Klimberg, V.S. Timing of oral glutamine on DMBA-induced tumorigenesis. J. Surg. Res. 2003, 111, 158–165. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Spring, P.; Klimberg, V.S. Oral glutamine prevents DMBA-induced mammary carcinogenesis via upregulation of glutathione production. Nutrition 2008, 24, 462–469. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).