Pathophysiological Mechanisms by which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers
Abstract
1. Introduction
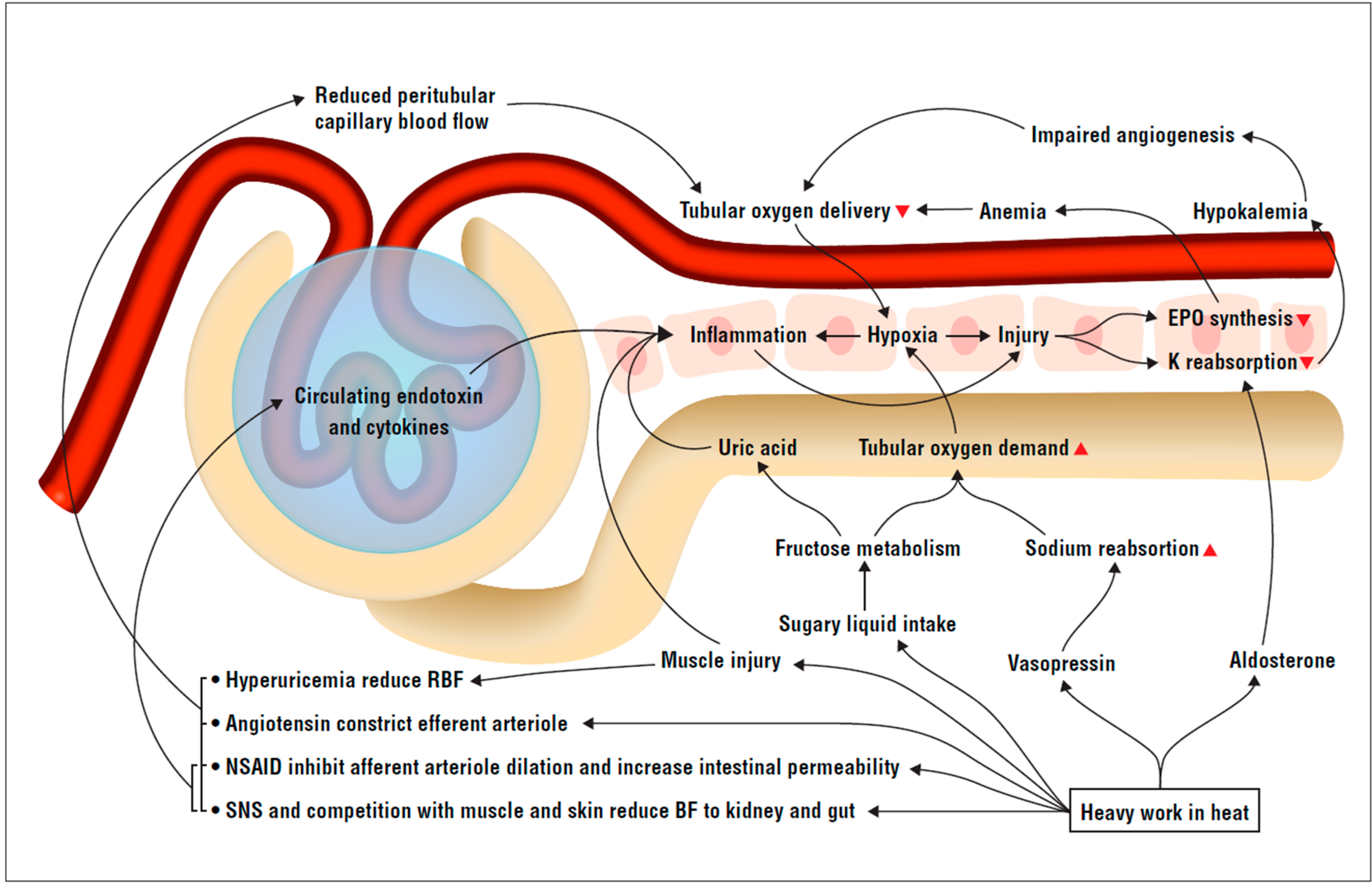
2. The Theoretical Framework
2.1. Systemic and Kidney Inflammation Triggers
2.1.1. Gut Permeability, Endotoxins, and Cytokines
2.1.2. Muscle Cellular Breakdown Byproducts and Cytokines
2.1.3. Sugar Intake, Fructose and Uric Acid
2.2. Kidney Blood Flow and Metabolism Mismatch
3. Empirical Findings in Sugarcane Workers
3.1. Material and Methods
3.1.1. Own Data
Overview
Laboratory Procedures
Outcome Definition
Covariate Definitions
Statistical Analysis
3.1.2. Ethical Approval
3.1.3. Other Published Data
4. Results with Discussion
4.1. Inflammation Coinciding with Kidney Injury
4.2. Systemic and Kidney Inflammation Triggers
4.2.1. Gut Permeability, Endotoxins, and Cytokines
4.2.2. Muscle Cellular Breakdown Byproducts and Cytokines
4.2.3. Sugar Intake, Fructose, and Uric Acid
4.3. Kidney Blood Flow and Metabolism Mismatch
Renin–Angiotensin–Aldosterone System, Vasopressin Activation, and Hypokalemia
5. Summary and Implications
5.1. Implications for Research
5.2. Implications for Workplaces and Intervention Studies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorensen, C.; Garcia-Trabanino, R. A New Era of Climate Medicine—Addressing Heat-Triggered Renal Disease. N. Engl. J. Med. 2019, 381, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; García-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Wesseling, C.; Newman, L.S. Chronic Kidney Disease of Unknown Cause in Agricultural Communities. N. Engl. J. Med. 2019, 380, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Ordunez, P.; Hoy, W. Case definitions and approaches for surveillance of chronic kidney disease in agricultural communities in Central America: Policy implications. Kidney Int. 2018, 93, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.; Crowe, J.; Hogstedt, C.; Jakobsson, K.; Lucas, R.A.I.; Wegman, D.H. Mesoamerican Nephropathy: Report from the First International Research Workshop on MeN; SALTRA/IRET-UNA: Heredia, Costa Rica, 2012. [Google Scholar]
- Hansson, E.; Glaser, J.; Weiss, I.; Ekström, U.; Apelqvist, J.; Hogstedt, C.; Peraza, S.; Lucas, R.; Jakobsson, K.; Wesseling, C.; et al. Workload and cross-harvest kidney injury in a Nicaraguan sugarcane worker cohort. Occup. Environ. Med. 2019, 76, 818–826. [Google Scholar] [CrossRef]
- Wesseling, C.; Glaser, J.; Rodríguez-Guzmán, J.; Weiss, I.; Lucas, R.; Peraza, S.; Da Silva, A.S.; Hansson, E.; Johnson, R.J.; Hogstedt, C.; et al. Chronic kidney disease of non-traditional origin in Mesoamerica: A disease primarily driven by occupational heat stress. Rev. Panam. Salud Pública 2020, 44. [Google Scholar] [CrossRef]
- Glaser, J.; Hansson, E.; Weiss, I.; Wesseling, C.; Jakobsson, K.; Ekstrom, U.; Apelqvist, J.; Lucas, R.; Monge, A.E.; Peraza, S.; et al. Preventing kidney injury among sugarcane workers: Promising evidence from enhanced workplace interventions. Occup. Environ. Med. 2020. [Google Scholar] [CrossRef]
- González-Quiroz, M.; Pearce, N.; Caplin, B.; Nitsch, R. What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis. Clin. Kidney J. 2017, 11, 496–506. [Google Scholar] [CrossRef]
- Laux, T.S.; Barnoya, J.; Guerrero, D.R.; Rothstein, M. Dialysis enrollment patterns in Guatemala: Evidence of the chronic kidney disease of non-traditional causes epidemic in Mesoamerica. BMC Nephrol. 2015, 16, 54. [Google Scholar] [CrossRef]
- Vandervort, D.R.; López, D.L.; Orantes, C.; Rodríguez, D.S. Spatial distribution of unspecified chronic kidney disease in El Salvador by crop area cultivated and ambient temperature. MEDICC Rev. 2014, 16, 31–38. [Google Scholar]
- Wesseling, C.; van Wendel de Joode, B.; Crowe, J.; Rittner, R.; Sanati, N.A.; Hogstedt, C.; Jakobsson, K. Mesoamerican nephropathy: Geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup. Environ. Med. 2015, 72, 714–721. [Google Scholar] [CrossRef]
- Kidney Disease Improving Global Outcomes. KDIGO Clinical Practice Guideline for Acute Kidney Injury. 2012. Available online: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf (accessed on 23 April 2020).
- Butler-Dawson, J.; Krisher, L.; Yoder, H.; Dally, M.; Sorensen, C.; Johnson, R.J.; Asensio, C.; Cruz, A.; Johnson, E.C.; Carlton, E.J.; et al. Evaluation of heat stress and cumulative incidence of acute kidney injury in sugarcane workers in Guatemala. Int. Arch. Occup. Environ. Health 2019, 92, 977–990. [Google Scholar] [CrossRef]
- Sorensen, C.J.; Butler-Dawson, J.; Dally, M.; Krisher, L.; Griffin, B.; Johnson, R.J.; Lemery, J.; Asensio, C.; Tenney, L.; Newman, L.S. Risk Factors and Mechanisms Underlying Cross-Shift Decline in Kidney Function in Guatemalan Sugarcane Workers. J. Occup. Environ. Med. 2019, 61, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Santos, U.P.; Zanetta, D.M.T.; Terra-Filho, M.; Burdmann, E. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015, 87, 792–799. [Google Scholar] [CrossRef] [PubMed]
- García-Trabanino, R.; Jarquin, E.; Wesseling, C.; Johnson, R.J.; Gonzalez-Quiroz, M.; Weiss, I.; Glaser, J.; Vindell, J.J.; Stockfelt, L.; Roncal-Jimenez, C.A.; et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador—A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ. Res. 2015, 142, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, D.H.; Apelqvist, J.; Bottai, M.; Ekström, U.; García-Trabanino, R.; Glaser, J.; Hogstedt, C.; Jakobsson, K.; Jarquin, E.; Lucas, R.A.; et al. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand. J. Work. Environ. Heal. 2017, 44, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.; Aragón, A.; González, M.; Weiss, I.; Glaser, J.; Bobadilla, N.A.; Roncal-Jimenez, C.; Correa-Rotter, R.; Johnson, R.J.; Barregard, L.; et al. Kidney function in sugarcane cutters in Nicaragua—A longitudinal study of workers at risk of Mesoamerican nephropathy. Environ. Res. 2016, 147, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Laws, R.L.; Brooks, D.; Amador, J.J.; Weiner, D.E.; Kaufman, J.S.; Ramirez-Rubio, O.; Riefkohl, A.; Scammell, M.K.; López-Pilarte, D.; Sánchez, J.M.; et al. Biomarkers of Kidney Injury Among Nicaraguan Sugarcane Workers. Am. J. Kidney Dis. 2015, 67, 209–217. [Google Scholar] [CrossRef]
- Laws, R.L.; Brooks, D.; Amador, J.J.; Weiner, D.E.; Kaufman, J.S.; Ramirez-Rubio, O.; Riefkohl, A.; Scammell, M.K.; López-Pilarte, D.; Sánchez, J.M.; et al. Changes in kidney function among Nicaraguan sugarcane workers. Int. J. Occup. Environ. Heal. 2015, 21, 241–250. [Google Scholar] [CrossRef]
- Kupferman, J.; Ramírez-Rubio, O.; Amador, J.J.; López-Pilarte, D.; Wilker, E.H.; Laws, R.L.; Sennett, C.; Robles, N.V.; Lau, J.L.; Salinas, A.J.; et al. Acute Kidney Injury in Sugarcane Workers at Risk for Mesoamerican Nephropathy. Am. J. Kidney Dis. 2018, 72, 475–482. [Google Scholar] [CrossRef]
- Gonzalez-Quiroz, M.; Smpokou, E.T.; Silverwood, R.; Camacho, A.; Faber, D.; Garcia, B.L.R.; Oomatia, A.; Hill, M.; Glaser, J.; Le Blond, J.; et al. Decline in Kidney Function among Apparently Healthy Young Adults at Risk of Mesoamerican Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2200–2212. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Ruiz, L.; Sennett, C.M.; Sánchez-Delgado, M.; García-Urbina, A.; Gámez-Altamirano, T.; Basra, K.; Laws, R.L.; Amador, J.J.; Lopez-Pilarte, D.; Tripodis, Y.; et al. Prevalence and Risk Factors for CKD Among Brickmaking Workers in La Paz Centro, Nicaragua. Am. J. Kidney Dis. 2019, 74, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C.; Abrahams, C.; Seftel, H.C. Chronic Interstitial Nephritis As A Consequence Of Heatstroke. Q. J. Med. 1970, 39. [Google Scholar] [CrossRef]
- McCullough, P.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Colar, J.M.; Geddes, T.; Gold, J.M.; Trivax, J.E. Changes in renal markers and acute kidney injury after marathon running. Nephrology 2011, 16, 194–199. [Google Scholar] [CrossRef]
- Mansour, S.G.; Martin, T.G.; Obeid, W.; Pata, R.W.; Myrick, K.M.; Kukova, L.; Jia, Y.; Bjornstad, P.; El-Khoury, J.M.; Parikh, C.R. The Role of Volume Regulation and Thermoregulation in AKI during Marathon Running. Clin. J. Am. Soc. Nephrol. 2019, 14, 1297–1305. [Google Scholar] [CrossRef]
- Mansour, S.G.; Verma, G.; Pata, R.W.; Martin, T.G.; Perazella, M.A.; Parikh, C.R. Kidney Injury and Repair Biomarkers in Marathon Runners. Am. J. Kidney Dis. 2017, 70, 252–261. [Google Scholar] [CrossRef]
- Fischer, R.S.; Vangala, C.; Truong, L.; Mandayam, S.; Chavarria, D.; Llanes, O.M.G.; Laguna, M.U.F.; Baez, A.G.; Garcia, F.; García-Trabanino, R.; et al. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int. 2017, 93, 681–690. [Google Scholar] [CrossRef]
- Fischer, R.S.B.; Vangala, C.; Mandayam, S.; Chavarria, D.; García-Trabanino, R.; Garcia, F.; Garcia, L.L.; Murray, K.O. Clinical markers to predict progression from acute to chronic kidney disease in Mesoamerican nephropathy. Kidney Int. 2018, 94, 1205–1216. [Google Scholar] [CrossRef]
- Fischer, R.S.B.; Mandayam, S.; Chavarria, D.; Vangala, C.; Garcia, M.N.; Garcia, L.L.; Palma, L.; Garcia, F.; García-Trabanino, R.; Murray, K.O. Clinical Evidence of Acute Mesoamerican Nephropathy. Am. J. Trop. Med. Hyg. 2017, 97, 1247–1256. [Google Scholar] [CrossRef]
- Wijkström, J. Chronic Kidney Disease of Unknown Etiology in Central America and Sri Lanka—Renal Morphology and Clinical Characteristics; Karolinska Institutet: Stockholm, Sweden, 2017. [Google Scholar]
- Pearce, N.; Caplin, B. Let’s take the heat out of the CKDu debate: More evidence is needed. Occup. Environ. Med. 2019, 76, 357–359. [Google Scholar] [CrossRef]
- Arici, M.; Walls, J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: Is C-reactive protein the missing link? Kidney Int. 2001, 59, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, E.; Parkhideh, S.; Ahmadi, F.; Khashayar, P. Serum CRP Levels in Pre-Dialysis Patients. Ren. Fail. 2008, 30, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Schlader, Z.; Hostler, D.; Parker, M.D.; Pryor, R.R.; Lohr, J.W.; Johnson, B.; Chapman, C. The Potential for Renal Injury Elicited by Physical Work in the Heat. Nutrients 2019, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Junglee, N.A.; Di Felice, U.; Dolci, A.; Fortes, M.B.; Jibani, M.M.; Lemmey, A.B.; Walsh, N.P.; Macdonald, J.H. Exercising in a hot environment with muscle damage: Effects on acute kidney injury biomarkers and kidney function. Am. J. Physiol. Physiol. 2013, 305, F813–F820. [Google Scholar] [CrossRef] [PubMed]
- Kasapis, C.; Thompson, P.D. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Kreher, J.B.; Schwartz, J.B. Overtraining Syndrome: A practical guide. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.G.; Wilson, T.E. Human cardiovascular responses to passive heat stress. Compr. Physiol. 2015, 5, 17–43. [Google Scholar] [CrossRef]
- Gil, S.M.; Yazaki, E.; Evans, D.F. Aetiology of Running-Related Gastrointestinal Dysfunction. Sports Med. 1998, 26, 365–378. [Google Scholar] [CrossRef]
- Van Wijck, K.; Lenaerts, K.; Van Loon, L.J.C.; Peters, W.H.M.; Buurman, W.A.; DeJong, C.H.C. Exercise-Induced Splanchnic Hypoperfusion Results in Gut Dysfunction in Healthy Men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [PubMed]
- Pals, K.L.; Chang, R.T.; Ryan, A.J.; Gisolfi, C.V. Effect of running intensity on intestinal permeability. J. Appl. Physiol. 1997, 82, 571–576. [Google Scholar] [CrossRef]
- Bösenberg, A.T.; Brock-Utne, J.G.; Gaffin, S.L.; Wells, M.T.; Blake, G.T. Strenuous exercise causes systemic endotoxemia. J. Appl. Physiol. 1988, 65, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2012, 48, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Rowell, L.B.; Detry, J.R.; Profant, G.R.; Wyss, C. Splanchnic vasoconstriction in hyperthermic man--role of falling blood pressure. J. Appl. Physiol. 1971, 31, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.J.; Law, L.Y.L.; Lim, C.L. Gastrointestinal response and endotoxemia during intense exercise in hot and cool environments. Eur. J. Appl. Physiol. 2013, 113, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L. Heat Sepsis Precedes Heat Toxicity in the Pathophysiology of Heat Stroke—A New Paradigm on an Ancient Disease. Antioxidants 2018, 7, 149. [Google Scholar] [CrossRef]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef]
- Leon, L.R.; Bouchama, A. Heat stroke. Compr. Physiol. 2015, 5, 611–647. [Google Scholar]
- Goldmann, L.S.; Andrew, I. Cecil Medicine; Elsevier Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Johnson, R.J.; Sánchez-Lozada, L.G.; Newman, L.S.; Lanaspa, M.A.; Diaz, H.F.; Lemery, J.; Rodriguez-Iturbe, B.; Tolan, D.R.; Butler-Dawson, J.; Sato, Y.; et al. Climate Change and the Kidney. Ann. Nutr. Metab. 2019, 74 (Suppl. S3), 38–44. [Google Scholar] [CrossRef]
- Johnson, R.J. Pro: Heat stress as a potential etiology of Mesoamerican and Sri Lankan nephropathy: A late night consult with Sherlock Holmes. Nephrol. Dial. Transplant. 2017, 32, 598–602. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Quercia, A.D.; Dellepiane, S.; Ferrario, S.; Camussi, G.; Biancone, L. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol. Dial. Transplant. 2014, 29, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.S.; Ramos, C.I.; Cuppari, L. The cross-talk between the kidney and the gut: Implications for chronic kidney disease. Nutrire 2017, 42, 27. [Google Scholar] [CrossRef]
- Zhang, J.; Ankawi, G.; Sun, J.; Digvijay, K.; Yin, Y.; Rosner, M.; Ronco, C. Gut-kidney crosstalk in septic acute kidney injury. Crit. Care 2018, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Medenwald, D.; Girndt, M.; Loppnow, H.; Kluttig, A.; Nuding, S.; Tiller, D.; Thiery, J.J.; Greiser, K.H.; Haerting, J.; Werdan, K. Inflammation and Renal Function after a Four-Year Follow-Up in Subjects with Unimpaired Glomerular Filtration Rate: Results from the Observational, Population-Based CARLA Cohort. PLoS ONE 2014, 9, e108427. [Google Scholar] [CrossRef]
- Salimi, S.; Shardell, M.; Seliger, S.L.; Bandinelli, S.; Guralnik, J.; Ferrucci, L. Inflammation and Trajectory of Renal Function in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2018, 66, 804–811. [Google Scholar] [CrossRef]
- Naito, M.; Bomsztyk, K.; Zager, R.A. Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure. J. Am. Soc. Nephrol. 2008, 19, 1321–1330. [Google Scholar] [CrossRef]
- Zager, R.A.; Johnson, A.C.; Hanson, S.; Lund, S. Acute nephrotoxic and obstructive injury primes the kidney to endotoxin-driven cytokine/chemokine production. Kidney Int. 2006, 69, 1181–1188. [Google Scholar] [CrossRef]
- Zager, R.A.; Johnson, A.C.M.; Hanson, S.Y.; Lund, S. Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-α generation and systemic release. Am. J. Physiol. Physiol. 2005, 289, F289–F297. [Google Scholar] [CrossRef]
- Zager, R.A.; Johnson, A.C.; Lund, S. ‘Endotoxin tolerance’: TNF-α hyper-reactivity and tubular cytoresistance in a renal cholesterol loading state. Kidney Int. 2007, 71, 496–503. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.-F.; Wang, J.-Y.; Chou, T. Vasoactive mediators and renal haemodynamics in exertional heat stroke complicated by acute renal failure. QJM 2003, 96, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Roncal-Jimenez, C.; García-Trabanino, R.; Barregard, L.; Lanaspa, M.A.; Wesseling, C.; Harra, T.; Aragón, A.; Grases, F.; Jarquin, E.R.; Gonzalez-Quiroz, M.; et al. Heat Stress Nephropathy From Exercise-Induced Uric Acid Crystalluria: A Perspective on Mesoamerican Nephropathy. Am. J. Kidney Dis. 2016, 67, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Milagres, T.; Arroyo, F.E.G.; Lanaspa, M.A.; Garcia, G.; Ishimoto, T.; Andres-Hernando, A.; Kuwabara, M.; Jensen, T.; Sato, Y.; Glaser, J.; et al. Rehydration with fructose worsens dehydration-induced renal damage. BMC Nephrol. 2018, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- García-Arroyo, F.E.; Tapia, E.; Blas-Marron, M.G.; Gonzaga, G.; Silverio, O.; Cristóbal, M.; Osorio-Alonso, H.; Arellano-Buendía, A.S.; Zazueta, C.; Aparicio-Trejo, O.E.; et al. Vasopressin Mediates the Renal Damage Induced by Limited Fructose Rehydration in Recurrently Dehydrated Rats. Int. J. Biol. Sci. 2017, 13, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.L.; Johnson, B.; Sackett, J.R.; Parker, M.D.; Schlader, Z.J. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R189–R198. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J. Am. Soc. Nephrol. 2009, 20, 545–553. [Google Scholar] [CrossRef]
- Cotter, J.D.; Thornton, S.N.; Lee, J.K.W.; Laursen, P.B. Are we being drowned in hydration advice? Thirsty for more? Extrem. Physiol. Med. 2014, 3, 18. [Google Scholar] [CrossRef]
- Cuddy, J.S.; Ruby, B.C. High Work Output Combined With High Ambient Temperatures Caused Heat Exhaustion in a Wildland Firefighter Despite High Fluid Intake. Wilderness Environ. Med. 2011, 22, 122–125. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Bross, T.L.; Hamilton, R.T. Are we being drowned by overhydration advice on the Internet? Physician Sportsmed. 2016, 44, 343–348. [Google Scholar] [CrossRef]
- Åstrand, P.O.; Rodahl, K.; Dahl, H.A.; Strømme, S.B. Textbook of Work Physiology: Physiological Bases of Exercise; Human Kinetics: Windsor, ON, Canada, 2003. [Google Scholar]
- Kawakami, S.; Yasuno, T.; Matsuda, T.; Fujimi, K.; Ito, A.; Yoshimura, S.; Uehara, Y.; Tanaka, H.; Saito, T.; Higaki, Y. Association between exercise intensity and renal blood flow evaluated using ultrasound echo. Clin. Exp. Nephrol. 2018, 22, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Grimby, G. Renal clearances during prolonged supine exercise at different loads. J. Appl. Physiol. 1965, 20, 1294–1298. [Google Scholar] [CrossRef]
- Wyler, F.; Stalder, G.; Kaeslin, M.; Hof, R.P. Hemodynamics in Experimental Hypernatremic Dehydration with Special Reference to Individual Organ Blood Flow in Shock and after Rehydration. Pediatr. Res. 1983, 17, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Volianitis, S.; Secher, N.H. Cardiovascular control during whole body exercise. J. Appl. Physiol. 2016, 121, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Low, D.A.; Keller, D.M.; Wingo, J.E.; Brothers, R.M.; Crandall, C.G. Sympathetic nerve activity and whole body heat stress in humans. J. Appl. Physiol. 2011, 111, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S.A. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef]
- Garcia-Arroyo, F.E.; Cristóbal, M.; Arellano-Buendía, A.S.; Osorio, H.; Tapia, E.; Soto, V.; Madero, M.; Lanaspa, M.A.; Roncal-Jimenez, C.A.; Bankir, L.; et al. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R57–R65. [Google Scholar] [CrossRef]
- Chapman, C.; Grigoryan, T.; Vargas, N.T.; Reed, E.L.; Kueck, P.J.; Pietrafesa, L.D.; Bloomfield, A.C.; Johnson, B.; Schlader, Z. High-fructose corn syrup-sweetened soft drink consumption increases vascular resistance in the kidneys at rest and during sympathetic activation. Am. J. Physiol. Physiol. 2020, 318, F1053–F1065. [Google Scholar] [CrossRef]
- Bragadottir, G.; Redfors, B.; Nygren, A.; Sellgren, J.; Ricksten, S. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol. Scand. 2009, 53, 1052–1059. [Google Scholar] [CrossRef]
- Yalamanchili, H.B.; Calp-Inal, S.; Zhou, X.J.; Choudhury, D. Hypokalemic Nephropathy. Kidney Int. Rep. 2018, 3, 1482–1488. [Google Scholar] [CrossRef]
- Elitok, S.; Bieringer, M.; Schneider, W.; Luft, F.C. Kaliopenic nephropathy revisited. Clin. Kidney J. 2016, 9, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Anutrakulchai, S.; Roncal, C.A.; Sato, W.; Glushakova, O.Y.; Croker, B.P.; Suga, S.I.; Ouyang, X.; Tungsanga, K.; Nakagawa, T.; Johnson, R.J.; et al. Hypokalemic nephropathy is associated with impaired angiogenesis. J. Am. Soc. Nephrol. 2008, 19, 125–134. [Google Scholar] [CrossRef]
- Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am. J. Med. 1999, 106, 13–24. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef]
- Ramirez-Rubio, O.; Brooks, D.; Amador, J.J.; Kaufman, J.S.; Weiner, D.E.; Scammell, M.K. Chronic kidney disease in Nicaragua: A qualitative analysis of semi-structured interviews with physicians and pharmacists. BMC Public Health 2013, 13, 350. [Google Scholar] [CrossRef]
- Walker, R.J.; Fawcett, J.P.; Flannery, E.M.; Gerrard, D.F. Indomethacin potentiates exercise-induced reduction in renal hemodynamics in athletes. Med. Sci. Sports Exerc. 1994, 26, 1302. [Google Scholar] [CrossRef]
- Ejaz, A.A.; Mu, W.; Kang, D.-H.; Roncal, C.; Sautin, Y.Y.; Henderson, G.; Tabah-Fisch, I.; Keller, B.; Beaver, T.M.; Nakagawa, T.; et al. Could Uric Acid Have a Role in Acute Renal Failure? Clin. J. Am. Soc. Nephrol. 2006, 2, 16–21. [Google Scholar] [CrossRef]
- Arakawa, K.; Hosono, A.; Shibata, K.; Ghadimi, R.; Fuku, M.; Goto, C.; Imaeda, N.; Tokudome, Y.; Hoshino, H.; Marumoto, M.; et al. Changes in blood biochemical markers before, during, and after a 2-day ultramarathon. Open Access J. Sports Med. 2016, 7, 43–50. [Google Scholar] [CrossRef][Green Version]
- Kratz, A.; Lewandrowski, K.; Siegel, A.J.; Chun, K.Y.; Flood, J.G.; Van Cott, E.M.; Lee-Lewandrowski, E. Effect of Marathon Running on Hematologic and Biochemical Laboratory Parameters, Including Cardiac Markers. Am. J. Clin. Pathol. 2002, 118, 856–863. [Google Scholar] [CrossRef]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.; Surriano, R.; Bishop, D.J.; Quod, M.J.; Lee, H.; Martin, D.T.; et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef]
- Sano, M.; Takei, M.; Shiraishi, Y.; Suzuki, Y. Increased Hematocrit During Sodium-Glucose Cotransporter 2 Inhibitor Therapy Indicates Recovery of Tubulointerstitial Function in Diabetic Kidneys. J. Clin. Med. Res. 2016, 8, 844–847. [Google Scholar] [CrossRef]
- Devarajan, P. Update on Mechanisms of Ischemic Acute Kidney Injury. J. Am. Soc. Nephrol. 2006, 17, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Bodin, T.; García-Trabanino, R.; Weiss, I.; Jarquín, E.; Glaser, J.; Jakobsson, K.; Lucas, R.A.I.; Wesseling, C.; Hogstedt, C.; Wegmann, D.H.; et al. Intervention to reduce heat stress and improve efficiency among sugarcane workers in El Salvador: Phase 1. Occup. Environ. Med. 2016, 73, 409–416. [Google Scholar] [CrossRef]
- Ekström, U.; Apelqvist, J.; Hansson, E.; Bodin, T.; Wegman, D.H.; Abrahamson, M.; Jakobsson, K. Insufficient mixing of thawed serum samples leading to erroneous results - experience from a field study and use of a correction procedure. Scand. J. Clin. Lab. Investig. 2019, 80, 99–105. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Lente, F.V.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtraion Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease Improving Global Outcomes. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease (CKD). 2012. Available online: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf (accessed on 23 April 2020).
- McClean, M.; Amador, J.J.; Laws, R.; Kaufman, J.S.; Weiner, D.E.; Sanchez-Rodriguez, J.M.; Brooks, D. Biological Sampling Report: Investigating Biomarkers of Kidney Injury and Chronic Kidney Disease among Workers in Western Nicaragua; Boston University School of Public Health: Boston, MA, USA, 2012. [Google Scholar]
- Fischer, R.S.B. Lack of evidence regarding an Infectious Etiology of Mesoamerican Nephropathy make it unlikely. In Proceedings of the Third International Workshop on Chronic Kidney Diseases of Uncertain/Non-traditional Etiology in Mesoamerica and Other Regions, San José, Costa Rica, 20–22 March 2019. [Google Scholar]
- Riefkohl, A.; Ramírez-Rubio, O.; Laws, R.L.; McClean, M.D.; Weiner, D.E.; Kaufman, J.S.; Galloway, R.L.; Shadomy, S.V.; Guerra, M.; Amador, J.J.; et al. Leptospira seropositivity as a risk factor for Mesoamerican Nephropathy. Int. J. Occup. Environ. Health 2017, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yih, W.K.; Kulldorff, M.; Friedman, D.J.; Leibler, J.H.; Amador, J.J.; López-Pilarte, D.; Galloway, R.L.; Ramírez-Rubio, O.; Riefkohl, A.; Brooks, D.R. Investigating Possible Infectious Causes of Chronic Kidney Disease of Unknown Etiology in a Nicaraguan Mining Community. Am. J. Trop. Med. Hyg. 2019, 101, 676–683. [Google Scholar] [CrossRef]
- Lambert, G.P.; Broussard, L.J.; Mason, B.L.; Mauermann, W.J.; Gisolfi, C.V. Gastrointestinal permeability during exercise: Effects of aspirin and energy-containing beverages. J. Appl. Physiol. 2001, 90, 2075–2080. [Google Scholar] [CrossRef]
- Gill, S.K.; Teixeira, A.; Rama, L.; Prestes, J.; Rosado, F.; Hankey, J.; Scheer, V.; Hemmings, K.; Ansley-Robson, P.; Costa, R.J. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc. Immunol. Rev. 2015, 21, 114–128. [Google Scholar]
- Tiller, N.B.; Chiesa, S.T.; Roberts, J.D.; Turner, L.A.; Jones, S.; Romer, L.M. Physiological and Pathophysiological Consequences of a 25-Day Ultra-Endurance Exercise Challenge. Front. Physiol. 2019, 10, 589. [Google Scholar] [CrossRef]
- Lucas, R.A.; Bodin, T.; García-Trabanino, R.; Wesseling, C.; Glaser, J.; Weiss, I.; Jarquin, E.; Jakobsson, K.; Wegman, D.H. Heat stress and workload associated with sugarcane cutting—An excessively strenuous occupation! Extrem. Physiol. Med. 2015, 4, A23. [Google Scholar] [CrossRef]
- Sato, Y.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Jensen, T.; Tolan, D.R.; Sanchez-Lozada, L.-G.; Newman, L.S.; Butler-Dawson, J.; Sorensen, C.; Glaser, J.; et al. Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure. Am. J. Physiol. Physiol. 2019, 317, F1111–F1121. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Qi, D.; Sun, J.-F.; Li, P.; Fan, H.-Y. Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities. Sci. Rep. 2015, 5, 11822. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Livingston, M.; Hao, J.; Li, L.; Mei, C.; Dong, Z. Autophagy is activated to protect against endotoxic acute kidney injury. Sci. Rep. 2016, 6, 22171. [Google Scholar] [CrossRef]
- Krisher, L. A Total Worker Health® approach to assessing kidney health in sugarcane workers in Guatemala: An opportunity for nutrition intervention. In Proceedings of the Third International Workshop on Chronic Kidney Diseases of Uncertain/Non-Traditional Etiology in Mesoamerica and Other Regions, San José, Costa Rica, 20–22 March 2019. [Google Scholar]
- Wijkström, J.; Gonzalez-Quiroz, M.; Hernandez, M.; Trujillo, Z.; Hultenby, K.; Ring, A.; Söderberg, M.; Aragón, A.; Elinder, C.-G.; Wernerson, A. Renal Morphology, Clinical Findings, and Progression Rate in Mesoamerican Nephropathy. Am. J. Kidney Dis. 2017, 69, 626–636. [Google Scholar] [CrossRef]
- Wesseling, C.; Aragón, A.; Gonzalez-Quiroz, M.; Weiss, I.; Glaser, J.; Rivard, C.J.; Roncal-Jiménez, C.; Correa-Rotter, R.; Johnson, R.J. Heat stress, hydration and uric acid: A cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 2016, 6, e011034. [Google Scholar] [CrossRef]
- Kupferman, J.; Amador, J.J.; Lynch, K.E.; Laws, R.L.; López-Pilarte, D.; Ramírez-Rubio, O.; Kaufman, J.S.; Lau, J.L.; Weiner, D.E.; Robles, N.V.; et al. Characterization of Mesoamerican Nephropathy in a Kidney Failure Hotspot in Nicaragua. Am. J. Kidney Dis. 2016, 68, 716–725. [Google Scholar] [CrossRef]
- Raines, N.; González, M.; Wyatt, C.; Kurzrok, M.; Pool, C.; Lemma, T.; Weiss, I.; Marín, C.; Prado, V.; Marcas, E.; et al. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev. 2014, 16, 16–22. [Google Scholar]
- Brezis, M.; Rosen, S. Hypoxia of the Renal Medulla—Its Implications for Disease. N. Engl. J. Med. 1995, 332, 647–655. [Google Scholar] [CrossRef]
- Wijkström, J.; Leiva, R.; Elinder, C.-G.; Leiva, S.; Trujillo, Z.; Trujillo, L.; Söderberg, M.; Hultenby, K.; Wernerson, A. Clinical and Pathological Characterization of Mesoamerican Nephropathy: A New Kidney Disease in Central America. Am. J. Kidney Dis. 2013, 62, 908–918. [Google Scholar] [CrossRef]
- Valdés, R.H.; Orantes, C.M.; Almaguer, M.; Alfonso, P.; Bayarre, H.D.; Leiva, I.M.; Smith, M.J.; Cubias, R.A.; Torres, C.G.; Almendárez, W.O.; et al. Clinical characteristics of chronic kidney disease of nontraditional causes in Salvadoran farming communities. MEDICC Rev. 2014, 16, 39–48. [Google Scholar]
- Pelliccia, A.; Solberg, E.; Papadakis, M.; Adami, P.E.; Biffi, A.; Caselli, S.; La Gerche, A.; Niebauer, J.; Pressler, A.; Schmied, C.M.; et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2018, 40, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Kiel, R.J.; Smith, F.E.; Chason, J.; Khatib, R.; Reyes, M.P. Coxsackievirus B3 myocarditis in C3H/HeJ mice: Description of an inbred model and the effect of exercise on virulence. Eur. J. Epidemiol. 1989, 5, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Gnauck, A.; Lentle, R.G.; Kruger, M.C. Chasing a ghost?—Issues with the determination of circulating levels of endotoxin in human blood. Crit. Rev. Clin. Lab. Sci. 2016, 53, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.L.; Johnson, B.D.; Vargas, N.T.; Hostler, D.; Parker, M.D.; Schlader, Z.J. Hyperthermia and Dehydration During Physical Work in the Heat Both Contribute to the Risk of Acute Kidney Injury. J. Appl. Physiol. 2020, 128. [Google Scholar] [CrossRef]
- Alhadad, S.B.; Tan, P.M.S.; Lee, J.K.W. Efficacy of Heat Mitigation Strategies on Core Temperature and Endurance Exercise: A Meta-Analysis. Front. Physiol. 2019, 10, 71. [Google Scholar] [CrossRef]
| Nicaragua (Adelante Cohort) | El Salvador (IEA Cohort) | |||||||
|---|---|---|---|---|---|---|---|---|
| Worker-Harvests | Worker Follow-up Occasions | |||||||
| Total | IKI | Incidence Ratio (IR) (95% CI) | IR Adjusted for Baseline eGFR (95% CI) | Total | IKI | Odds Ratio (OR) (95% CI) | OR Adjusted for Baseline eGFR (95% CI) | |
| Age (years) | ||||||||
| 18–30 | 320 | 32 (10%) | ref | Ref | 289 | 22 (8%) | Ref | Ref |
| 31–40 | 155 | 16 (10%) | 1.0 (0.6–1.9) | 0.9 (0.5–1.6) | 179 | 32 (18%) | 5.7 (1.3–25) | 1.1 (0.3–3.8) |
| 41–50 | 43 | 5 (12%) | 1.2 (0.5–3.0) | 0.9 (0.3–2.3) | 108 | 20 (19%) | 6.2 (1.1–33) | 0.6 (0.1–2.4) |
| >50 | 14 | 0 (0%) | NA | NA | 108 | 27 (25%) | 17.5 (3.2–95) | 0.8 (0.2–3.1) |
| eGFR, baseline (mL/min/1.73 m2) | ||||||||
| >90 | 358 | 29 (8%) | Ref | Not meaningful | 398 | 16 (4%) | Ref | Not meaningful |
| 90–60 | 163 | 23 (14%) | 1.7 (1.0–3.0) | 136 | 15 (11%) | 5.0 (1.4–18) | ||
| 45–60 | 10 | 1 (10%) | 1.2 (0.2–9.1) | 57 | 19 (33%) | 43 (8.6–211) | ||
| <45 | 1 | 0 (0%) | NA | 85 | 51 (60%) | 290 (51–1663) | ||
| CRP, baseline (mg/L) | ||||||||
| <3 | 423 | 40 (9%) | Ref | Ref | 482 | 69 (14%) | Ref | Ref |
| 3–10 | 85 | 12 (14%) | 1.5 (0.8–2.8) | 1.4 (0.7–2.6) | 153 | 21 (14%) | 1.2 (0.3–4.7) | 0.6 (0.2–1.7) |
| 10–20 | 12 | 0 (0%) | NA | NA | 28 | 10 (36%) | 22 (1.3–384) | 0.9 (0.1–5.8) |
| >20 | 12 | 1 (8%) | 0.9 (0.1–6.4) | 0.7 (0.1–5.4) | 13 | 1 (8%) | 0.3 (0.01–62) | 0.1 (0.01–3.5) |
| CRP, follow-up (mg/L) | ||||||||
| <3 | 340 | 10 (3%) | Ref | Ref | 434 | 25 (6%) | Ref | Ref |
| 3–10 | 142 | 14 (10%) | 3.4 (1.5–7.5) | 3.3 (1.5–7.5) | 173 | 37 (21%) | 8.7 (3.3–23) | 5.0 (1.9–12.9) |
| 10–20 | 24 | 9 (38%) | 13 (5–31) | 13 (5.1–31) | 43 | 21 (49%) | 58 (14–249) | 21 (5.3–82) |
| >20 | 26 | 20 (77%) | 26 (12–56) | 25 (12–54) | 34 | 18 (53%) | 71 (15–337) | 19 (4.7–76) |
| NSAID use at least once per week † | ||||||||
| No | 444 | 37 (8%) | Ref | Ref | 647 | 92 (14%) | Ref | Ref |
| Yes | 88 | 16 (18%) | 2.2 (1.2–3.9) | 2.1 (1.2–3.8) | 25 | 7 (28%) | 2.9 (0.5–16) | 2.8 (0.6–14) |
| Incident fever in past week(s) †† | ||||||||
| No | 463 | 40 (9%) | Ref | Ref | 384 | 41 (11%) | Ref | Ref |
| Yes | 43 | 11 (26%) | 3.0 (1.5–5.8) | 3.1 (1.6–6.1) | 287 | 58 (20%) | 5.6 (1.9–16) | 2.1 (0.9–5.0) |
| Sugary drink intake (L) | Not available | |||||||
| <0.2 | 64 | 2 (3%) | Ref | Ref | ||||
| 0.2–1 | 312 | 30 (10%) | 3.1 (0.7–13) | 3.1 (0.7–13) | ||||
| >1 | 155 | 21 (14%) | 4.3 (1.0–18) | 4.4 (1.0–19) | ||||
| Morning boli intake (N of 300 mL sachets) | ||||||||
| 0 | 125 | 18 (14%) | Ref | Ref | ||||
| 0–3 | 214 | 23 (11%) | 0.7 (0.4–1.4) | 0.8 (0.4–1.5) | ||||
| ≥3 | 180 | 12 (7%) | 0.4 (0.2–0.9) | 0.5 (0.2–0.9) | ||||
| Morning water intake (L) | ||||||||
| 0–2 | 76 | 12 (16%) | Ref | Ref | ||||
| 2–5 | 209 | 18 (9%) | 0.5 (0.3–1.1) | 0.6 (0.3–1.2) | ||||
| >5 | 246 | 23 (9%) | 0.6 (0.3–1.2) | 0.6 (0.3–1.3) | ||||
| Mg, baseline (mmol/L) | ||||||||
| ≥0.7 | Not collected | 455 | 63 (14%) | Ref | Ref | |||
| <0.7 | 67 | 29 (43%) | 22 (3.7–124) | 1.6 (0.4–6.1) | ||||
| K, baseline (mmol/L) | ||||||||
| ≥3.5 | Not collected | 496 | 82 (17%) | Ref | Ref | |||
| <3.5 | 26 | 10 (38%) | 11 (0.9–138) | 5.2 (0.7–38) | ||||
| K, follow-up (mmol/L) | ||||||||
| ≥3.5 | Not collected | 482 | 71 (15%) | Ref | Ref | |||
| <3.5 | 47 | 21 (45%) | 13 (3.1–57) | 7.2 (2.1–25) | ||||
| Na, follow-up (mmol/L) | ||||||||
| ≥137 | 283 | 20 (7%) | Not possible | 153 | 9 (6%) | Not possible | ||
| <137 | 15 | 1 (7%) | 1 | 0 (0%) | ||||
| Incident biochemical changes * | eGFR coefficient (ml/min/1.73 m2) compared to baseline | |||||||
| Hemoglobin <125 g/L | ||||||||
| No | Not collected | 138 | 3 (2%) | Ref | ||||
| Yes | 16 | 3 (19%) | −9 (−12, −5) | |||||
| Dipstick hematuria | ||||||||
| No | Not collected | 105 | 2 (2%) | Ref | ||||
| Yes | 16 | 3 (19%) | −5 (−8, −1) | |||||
| Dipstick proteinuria | ||||||||
| No | Not collected | 141 | 5 (4%) | Ref | ||||
| Yes | 9 | 4 (44%) | −10 (−15, −5) | |||||
| Uric acid crystals | ||||||||
| No | Not collected | 142 | 7 (5%) | Ref | ||||
| Yes | 10 | 2 (20%) | −6 (−11, −1) | |||||
| Non-hyaline cylinders | ||||||||
| No | Not collected | 128 | 6 (5%) | Ref | ||||
| Yes | 9 | 2 (22%) | −7 (−13, −1) | |||||
| Microscopy leukocyturia | ||||||||
| <25/fov | Not collected | 146 | 4 (3%) | Ref | ||||
| ≥25/fov | 7 | 5 (71%) | −26 (−31, −21) | |||||
| Country | Year | Biochemical parameter | Median (interquartile Range) | Regression coefficients (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| El Salvador † | Baseline | During harvest | ||||||
| Non IKI day | IKI day | IKI day vs. non IKI day regression coefficient | ||||||
| 1 | Worker-visits | 74 | 145 | 9 | Unadjusted | eGFR-adjusted | ||
| WBC | 109/L | 8.2 (7.2–9.0) | 7.7 (6.6–8.5) | 9.5 (9.3–10) | 2.2 (1.3–3.1) | 1.6 (0.6–2.7) | ||
| Neutrophils | 4.7 (3.9–5.5) | 3.9 (3.3–4.5) | 5.5 (5.3–5.8) | 2.0 (1.3–2.8) | 1.7 (0.8–2.6) | |||
| Lymphocytes | 2.6 (2.2–3.0) | 2.9 (2.5–3.2) | 3.0 (2.7–3.3) | 0.1 (–0.3–0.4) | 0.1 (–0.4–0.5) | |||
| Monocytes | 0.7 (0.6–0.9) | 0.7 (0.5–1.0) | 0.9 (0.8–1.0) | 0.2 (–0.1–0.5) | 0.0 (–0.4–0.3) | |||
| Urine–pH | – | 6 (5.5–6.5) | 6 (5.5–6.5) | 5.5 (5.5–6.0) | –0.4 (–0.8–0.0) | –0.3 (–0.7–0.2) | ||
| Hemoglobin | g/L | 148 (143–153) | 142 (135–148) | 125 (123–130) | –9 (–16––2) | 3 (–4–9) | ||
| Sodium | mmol/L | 141 (139–141) | 140 (139–141) | 140 (139–141) | 0 (–1–1) | 0 (–1–1) | ||
| 1 + 2 | Worker–visits | 345 | 580 | 101 | ||||
| CRP | mg/L | 1.4 (0.7–3.3) | 1.5 (0.7–4.0) | 7.5 (3.0–13) | 7.6 (5.7–9.5) | 3.3 (1.0–5.7) | ||
| Uric acid | mmol/L | 361 (310–443) | 328 (281–389) | 586 (477–672) | 117 (98–135) | 32 (14–50) | ||
| CPK | µkat/L | 2.4 (1.8–3.4) | 2.8 (2.2–3.9) | 3.8 (2.6–5.1) | 0.8 (0.3–1.4) | 0.7 (0.0–1.3) | ||
| 2 | Worker–visits | 213 | 429 | 92 | ||||
| Potassium | mmol/L | 3.9 (3.5–4.1) | 3.9 (3.5–4.2) | 3.4 (3.0–3.9) | –0.3 (–0.5––0.2) | –0.1 (–0.3–0) | ||
| Nicaragua †† | Baseline | At end–harvest | ||||||
| No IKI | IKI | Cross–harvest trend difference between IKI and non–IKI workers | ||||||
| 1 + 2 | Workers | 533 | 480 | 53 | Unadjusted | eGFR–adjusted | ||
| CRP | mg/L | 1.1 (LoQ–2.3) | 1.6 (0.8–3.7) | 12 (5.8–24) | 15 (12–17) | 12 (9–14) | ||
| Uric acid | mmol/L | 324 (277–374) | 326 (282–378) | 428 (345–499) | 61 (47–74) | –5 (–19–9) | ||
| CPK | µkat/L | 2.6 (2.0–3.4) | 3.5 (2.6–4.8) | 3.4 (2.6–5.2) | 0.3 (–0.2–0.8) | 0.0 (–0.6–0.6) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansson, E.; Glaser, J.; Jakobsson, K.; Weiss, I.; Wesseling, C.; Lucas, R.A.I.; Wei, J.L.K.; Ekström, U.; Wijkström, J.; Bodin, T.; et al. Pathophysiological Mechanisms by which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers. Nutrients 2020, 12, 1639. https://doi.org/10.3390/nu12061639
Hansson E, Glaser J, Jakobsson K, Weiss I, Wesseling C, Lucas RAI, Wei JLK, Ekström U, Wijkström J, Bodin T, et al. Pathophysiological Mechanisms by which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers. Nutrients. 2020; 12(6):1639. https://doi.org/10.3390/nu12061639
Chicago/Turabian StyleHansson, Erik, Jason Glaser, Kristina Jakobsson, Ilana Weiss, Catarina Wesseling, Rebekah A. I. Lucas, Jason Lee Kai Wei, Ulf Ekström, Julia Wijkström, Theo Bodin, and et al. 2020. "Pathophysiological Mechanisms by which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers" Nutrients 12, no. 6: 1639. https://doi.org/10.3390/nu12061639
APA StyleHansson, E., Glaser, J., Jakobsson, K., Weiss, I., Wesseling, C., Lucas, R. A. I., Wei, J. L. K., Ekström, U., Wijkström, J., Bodin, T., Johnson, R. J., & Wegman, D. H. (2020). Pathophysiological Mechanisms by which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers. Nutrients, 12(6), 1639. https://doi.org/10.3390/nu12061639





