The Role of Preoperative Parenteral Nutrition
Abstract
1. Introduction
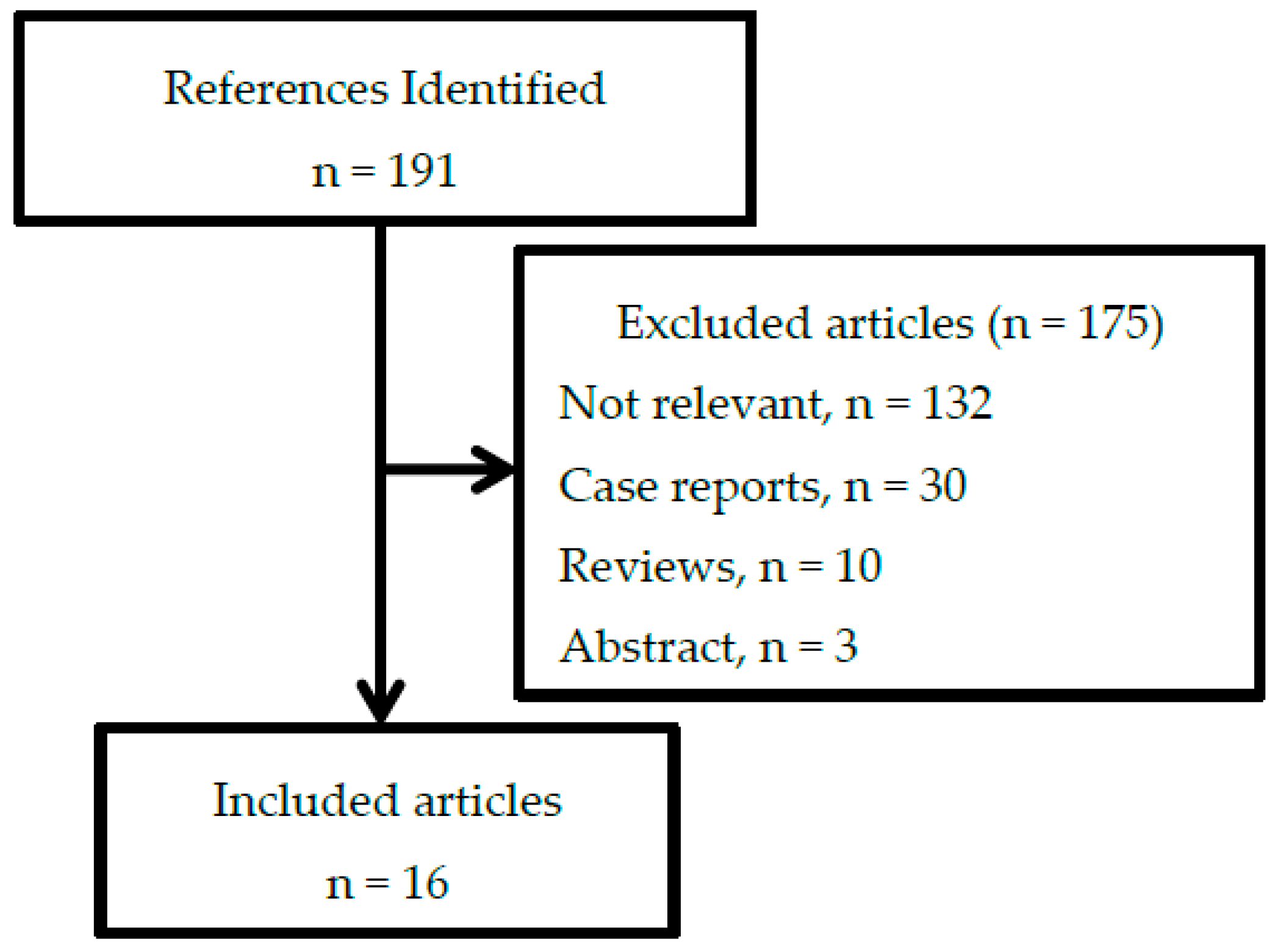
2. Materials and Methods
3. Results
3.1. Benefits of Preoperative Parenteral Nutrition
3.1.1. Postoperative Complications
3.1.2. Mortality
3.1.3. Length of Hospital Stay
3.1.4. Other Outcomes
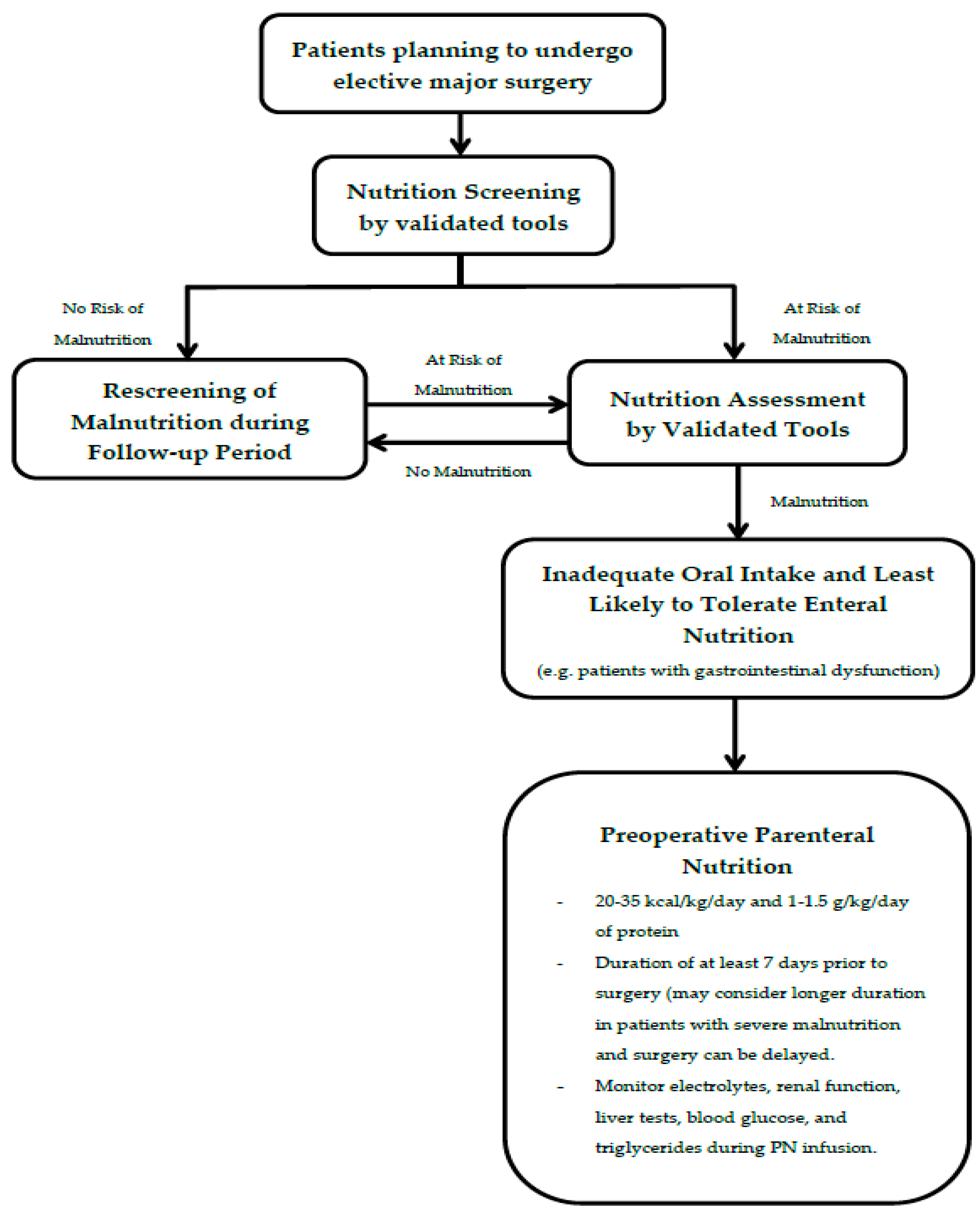
3.2. Preoperative Nutrition Assessment
3.3. Who May Benefit from Preoperative Parenteral Nutrition?
3.4. Dose and Duration of Preoperative Parenteral Nutrition
3.5. Complications and Monitoring of Preoperative Parenteral Nutrition
3.6. Preoperative Parenteral Nutrition: New Directions
3.7. Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozkalkanli, M.Y.; Ozkalkanli, D.T.; Katircioglu, K.; Savaci, S. Comparison of tools for nutrition assessment and screening for predicting the development of complications in orthopedic surgery. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2009, 24, 274–280. [Google Scholar] [CrossRef]
- Thomas, M.N.; Kufeldt, J.; Kisser, U.; Hornung, H.M.; Hoffmann, J.; Andraschko, M.; Werner, J.; Rittler, P. Effects of malnutrition on complication rates, length of hospital stay, and revenue in elective surgical patients in the G-DRG-system. Nutrition 2016, 32, 249–254. [Google Scholar] [CrossRef]
- Abunnaja, S.; Cuviello, A.; Sanchez, J.A. Enteral and parenteral nutrition in the perioperative period: State of the art. Nutrients 2013, 5, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Traynor, C.; Hall, G.M. Endocrine and metabolic changes during surgery: Anaesthetic implications. Br. J. Anaesth. 1981, 53, 153–160. [Google Scholar] [CrossRef]
- Yeh, D.D.; Fuentes, E.; Quraishi, S.A.; Cropano, C.; Kaafarani, H.; Lee, J.; King, D.R.; DeMoya, M.; Fagenholz, P.; Butler, K.; et al. Adequate Nutrition May Get You Home: Effect of Caloric/Protein Deficits on the Discharge Destination of Critically Ill Surgical Patients. JPEN J. Parenter. Enter. Nutr. 2016, 40, 37–44. [Google Scholar] [CrossRef]
- Evans, D.C.; Martindale, R.G.; Kiraly, L.N.; Jones, C.M. Nutrition optimization prior to surgery. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2014, 29, 10–21. [Google Scholar] [CrossRef]
- Keller, H.; Laur, C.; Atkins, M.; Bernier, P.; Butterworth, D.; Davidson, B.; Hotson, B.; Nasser, R.; Laporte, M.; Marcell, C.; et al. Update on the Integrated Nutrition Pathway for Acute Care (INPAC): Post implementation tailoring and toolkit to support practice improvements. Nutr. J. 2018, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Ward, N. Nutrition support to patients undergoing gastrointestinal surgery. Nutr. J. 2003, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Iresjo, B.M.; Engstrom, C.; Lundholm, K. Preoperative overnight parenteral nutrition (TPN) improves skeletal muscle protein metabolism indicated by microarray algorithm analyses in a randomized trial. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Iresjo, B.M.; Engstrom, C.; Smedh, U.; Lundholm, K. Overnight Steady-State Infusions of Parenteral Nutrition on Myosin Heavy Chain Transcripts in Rectus Abdominis Muscle Related to Amino Acid Transporters, Insulin-like Growth Factor 1, and Blood Amino Acids in Patients Aimed at Major Surgery. JPEN J. Parenter. Enter. Nutr. 2019, 43, 497–507. [Google Scholar] [CrossRef]
- Celaya Perez, S.; Navarro, M.; Roman, A.; Salinas, J.C.; Larrad, L.; Lasierra, M.P.; Lozano Mantecon, R. The effect of preoperative parenteral nutrition on the capacity of the immune response in malnourished patients (preoperative parenteral nutrition and immunity). Nutr. Hosp. 1989, 4, 145–148. [Google Scholar] [PubMed]
- Ooi, S.E.; Chen, G.W.; Chou, C.T. Adequate nourishment through total parenteral nutrition treatment may augment immune function in patients with colon cancer. Arch. Med. Res. 2004, 35, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Pache, B.; Martin, D.; Hahnloser, D.; Demartines, N.; Hubner, M. Preoperative Nutritional Conditioning of Crohn’s Patients-Systematic Review of Current Evidence and Practice. Nutrients 2017, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.T.; Ha, I.; Hogan, C.; Nguyen, E.; Jamal, M.M.; Bechtold, M.L.; Nguyen, D.L. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn’s disease patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 997–1002. [Google Scholar] [CrossRef]
- Heyland, D.K.; Montalvo, M.; MacDonald, S.; Keefe, L.; Su, X.Y.; Drover, J.W. Total parenteral nutrition in the surgical patient: A meta-analysis. Can. J. Surg. J. Can. Chir. 2001, 44, 102–111. [Google Scholar]
- Burden, S.; Todd, C.; Hill, J.; Lal, S. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst. Rev. 2012, 11, CD008879. [Google Scholar] [CrossRef]
- Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N. Engl. J. Med. 1991, 325, 525–532. [Google Scholar] [CrossRef]
- Von Meyenfeldt, M.F.; Meijerink, W.J.; Rouflart, M.M.; Builmaassen, M.T.; Soeters, P.B. Perioperative nutritional support: A randomised clinical trial. Clin. Nutr. 1992, 11, 180–186. [Google Scholar] [CrossRef]
- Bozzetti, F.; Gavazzi, C.; Miceli, R.; Rossi, N.; Mariani, L.; Cozzaglio, L.; Bonfanti, G.; Piacenza, S. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: A randomized, clinical trial. JPEN J. Parenter. Enter. Nutr. 2000, 24, 7–14. [Google Scholar] [CrossRef]
- Yao, G.X.; Wang, X.R.; Jiang, Z.M.; Zhang, S.Y.; Ni, A.P. Role of perioperative parenteral nutrition in severely malnourished patients with Crohn’s disease. World J. Gastroenterol. 2005, 11, 5732–5734. [Google Scholar] [CrossRef]
- Wu, G.H.; Liu, Z.H.; Wu, Z.H.; Wu, Z.G. Perioperative artificial nutrition in malnourished gastrointestinal cancer patients. World J. Gastroenterol. 2006, 12, 2441–2444. [Google Scholar] [CrossRef] [PubMed]
- Grivceva Stardelova, K.; Misevska, P.; Zdravkovska, M.; Trajkov, D.; Serafimoski, V. Total parenteral nutrition in treatment of patients with inflammatory bowel disease. Prilozi 2008, 29, 21–43. [Google Scholar] [PubMed]
- Wu, M.H.; Lin, M.T.; Chen, W.J. Effect of perioperative parenteral nutritional support for gastric cancer patients undergoing gastrectomy. Hepato-Gastroenterol. 2008, 55, 799–802. [Google Scholar]
- Jacobson, S. Early postoperative complications in patients with Crohn’s disease given and not given preoperative total parenteral nutrition. Scand. J. Gastroenterol. 2012, 47, 170–177. [Google Scholar] [CrossRef]
- Salinas, H.; Dursun, A.; Konstantinidis, I.; Nguyen, D.; Shellito, P.; Hodin, R.; Bordeianou, L. Does preoperative total parenteral nutrition in patients with ulcerative colitis produce better outcomes? Int. J. Colorectal Dis. 2012, 27, 1479–1483. [Google Scholar] [CrossRef]
- Kirkil, C.; Bulbuller, N.; Aygen, E.; Basbug, M.; Ayten, R.; Ilhan, N.; Ilhan, Y.S.; Akbulut, S. The effect of preoperative nutritional supports on patients with gastrointestinal cancer: Prospective randomized study. Hepato-Gastroenterol. 2012, 59, 86–89. [Google Scholar] [CrossRef]
- Jie, B.; Jiang, Z.M.; Nolan, M.T.; Zhu, S.N.; Yu, K.; Kondrup, J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012, 28, 1022–1027. [Google Scholar] [CrossRef]
- Ganaie, A.R.; Itoo, M.S.; Bhat, G.M. Effects of perioperative parenteral nutrition on wound healing and hospital stay in surgical patients: A randomized controlled study. Int. J. Res. Med. Sci. 2015, 3, 3156–3160. [Google Scholar] [CrossRef]
- Investigators, N.-S.S.; Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Marcason, W. Should Albumin and Prealbumin Be Used as Indicators for Malnutrition? J. Acad. Nutr. Diet. 2017, 117, 1144. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Brown, M.P.; Slish, J.H.; Rosenthal, M.D. Serum Levels of Prealbumin and Albumin for Preoperative Risk Stratification. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2019, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Van Stijn, M.F.; Korkic-Halilovic, I.; Bakker, M.S.; van der Ploeg, T.; van Leeuwen, P.A.; Houdijk, A.P. Preoperative nutrition status and postoperative outcome in elderly general surgery patients: A systematic review. JPEN J. Parenter. Enter. Nutr. 2013, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.H.; McCullough, J.; Davidson, B.; Vesnaver, E.; Laporte, M.; Gramlich, L.; Allard, J.; Bernier, P.; Duerksen, D.; Jeejeebhoy, K. The Integrated Nutrition Pathway for Acute Care (INPAC): Building consensus with a modified Delphi. Nutr. J. 2015, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, C.L.; Levy, P.; Sheean, P.M.; Wang, X. Enteral compared with parenteral nutrition: A meta-analysis. Am. J. Clin. Nutr. 2001, 74, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Gianotti, L.; Gentilini, O.; Parisi, V.; Salis, C.; Di Carlo, V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit. Care Med. 2001, 29, 242–248. [Google Scholar] [CrossRef]
- Tian, F.; Heighes, P.T.; Allingstrup, M.J.; Doig, G.S. Early Enteral Nutrition Provided Within 24 Hours of ICU Admission: A Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2018, 46, 1049–1056. [Google Scholar] [CrossRef]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Bellingan, G.; Leonard, R.; Mythen, M.G.; Rowan, K.M.; et al. Trial of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef]
- Jankowski, M.; Las-Jankowska, M.; Sousak, M.; Zegarski, W. Contemporary enteral and parenteral nutrition before surgery for gastrointestinal cancers: A literature review. World J. Surg. Oncol. 2018, 16, 94. [Google Scholar] [CrossRef]
- Bozzetti, F. Nutritional support in oncologic patients: Where we are and where we are going. Clin. Nutr. 2011, 30, 714–717. [Google Scholar] [CrossRef]
- Bozzetti, F.; Braga, M.; Gianotti, L.; Gavazzi, C.; Mariani, L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: A randomised multicentre trial. Lancet 2001, 358, 1487–1492. [Google Scholar] [CrossRef]
- Braga, M.; Ljungqvist, O.; Soeters, P.; Fearon, K.; Weimann, A.; Bozzetti, F.; ESPEN. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin. Nutr. 2009, 28, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.L. Impact of nutritional support on the clinical outcome of the surgical patient. Clin. Nutr. 1994, 13, 331–340. [Google Scholar] [CrossRef]
- Torgersen, Z.; Balters, M. Perioperative nutrition. Surg. Clin. N. Am. 2015, 95, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Ayers, P.; Adams, S.; Boullata, J.; Gervasio, J.; Holcombe, B.; Kraft, M.D.; Marshall, N.; Neal, A.; Sacks, G.; Seres, D.S.; et al. A.S.P.E.N. parenteral nutrition safety consensus recommendations. JPEN J. Parenter. Enter. Nutr. 2014, 38, 296–333. [Google Scholar] [CrossRef] [PubMed]
- Dronge, A.S.; Perkal, M.F.; Kancir, S.; Concato, J.; Aslan, M.; Rosenthal, R.A. Long-term glycemic control and postoperative infectious complications. Arch. Surg. 2006, 141, 375–380; discussion 380. [Google Scholar] [CrossRef]
- Marin Caro, M.M.; Laviano, A.; Pichard, C. Impact of nutrition on quality of life during cancer. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 480–487. [Google Scholar] [CrossRef]
- Maillard, J.; Elia, N.; Haller, C.S.; Delhumeau, C.; Walder, B. Preoperative and early postoperative quality of life after major surgery—A prospective observational study. Health Qual. Life Outcomes 2015, 13, 12. [Google Scholar] [CrossRef]
- Hasenberg, T.; Essenbreis, M.; Herold, A.; Post, S.; Shang, E. Early supplementation of parenteral nutrition is capable of improving quality of life, chemotherapy-related toxicity and body composition in patients with advanced colorectal carcinoma undergoing palliative treatment: Results from a prospective, randomized clinical trial. Colorectal Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2010, 12, e190–e199. [Google Scholar] [CrossRef]
- Jin, Y.; Yong, C.; Ren, K.; Li, D.; Yuan, H. Effects of Post-Surgical Parenteral Nutrition on Patients with Gastric Cancer. Cell. Physiol. Biochem. 2018, 49, 1320–1328. [Google Scholar] [CrossRef]
| Study, Year | Study Design | Patients (Number) | Nutrition Status | Intervention | Postoperative Complications | Mortality | LOS (Days) | Remark | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | p-Value | Intervention | Control | p-Value | Intervention | Control | p-Value | |||||
| 1. Veterans Affairs Total Cooperative Study Group 1991 | Randomized Control Trial | Thoracoabdominal surgery (395) | 100% Malnutrition * | 7–15 days preop and 3 days postop PN (with lipid) | No PN | 49/192 (25.5%) | 50/203 (24.6%) | >0.05 | 31/231 (13.4%) | 24/228 (10.5%) | >0.05 | N/A | Lower noninfectious complications in PN group (5% vs. 43%, p = 0.03) in patients with severe malnutrition (SGA C or NRI < 83.5) | ||
| 2. Von Meyenfeldt et al. (1992) | Randomized Control Trial | Gastric cancer (29) Colorectal cancer (72) | 29% Malnutrition (Nutrition index <1.31) | 10 days preop PN (with lipid) | No PN | 6/51 (11.8%) | 7/50 (14%) | >0.05 | 2/51 (3.9%) | 2/50 (4%) | >0.05 | Mean total (SD) = 36.3 (17.7) | Mean total (SD) = 31.7 (22.1) | >0.05 | Lower septic complication in PN group (5.6% vs. 81.8%, p < 0.05) in patients >10% weight loss |
| 3. Bozzetti et al. 2000 | Randomized Control Trial | Gastric cancer (74) Colorectal cancer (16) | 100% Malnutrition (>10% weight loss in 6 months) | 10 days preop and 9 days postop PN (with lipid) | No preop PN and 9 days postop PN (940 kcal + 85 g protein) | 16/43 (37.2%) | 27/47 (57.4%) | 0.03 | 0/43 (0%) | 5/47 (10.6%) | 0.05 | Median total (range) = 33 (18–161) | Median total (range) = 27 (15–103) | <0.001 | |
| Median postop (range) = 14 (7–143) | Median postop (range) = 14 (6–59) | 0.98 | |||||||||||||
| 4. Yao et al. 2005 | Prospective Study | Crohn’s disease (32) | 100% Severe Malnutrition (BMI < 15 kg/m2) | 1 week preop and 3 weeks postop PN (with lipid) | No PN | 6/16 (37.5%) | 7/16 (43.8%) | 0.86 | N/A | N/A | - BMI increased significantly in PN group (13.9 ± 0.6 to 15.3 ± 0.7 kg/m2 (p = 0.02)) - Serum IgM decreased significantly in PN group (133 ± 16 mg/dL to 105 ± 29 mg/dL, p = 0.02) | ||||
| 5. Wu et al. 2006 | Prospective study | Gastric cancer (253) Colorectal cancer (215) | 100% Malnutrition (SGA B or C) | 7 days preop and 7 days postop PN (with lipid) (68%) or EN (32%) | Preop standard oral diet and postop hypocaloric PN | 31/235 (13.2%) | 64/233 (27.5%) | 0.012 | 5/235 (2.1%) | 14/233 (6%) | 0.003 | Median total = 34 | Median total = 52 | 0.014 | |
| 6. Grivceva et al. 2008 | Retrospective Study | Severe Crohn’s disease (63) and severe ulcerative colitis (27) | 22.2% Malnutrition (BMI < 18.5 kg/m2) | Mean (SD) = 12.5 (5) days preop PN | No PN | N/A | N/A | Mean total (SD) = 18.9 (8.9) | Mean total (SD) = 18.9 (6.5) | 0.98 | |||||
| 7. Wu et al. 2008 | Retrospective study | Gastric cancer underwent TG (40) and SG (78) | 100% Malnutrition (weight loss >10% in 6 months or albumin < 3 g/dL) | At least 5 days preop and postop PN until can eat normally (with lipid) | No PN | 4/25 (16%) TG | 10/15 (66.7%) TG | 0.002 | 1/25 (4%) TG | 4/15 (26.7%) TG | 0.056 | Mean postop (SD) = 21.3 (12.3) | Mean postop (SD) = 35.2 (25.1) | 0.024 | |
| 10/46 (21.7%) SG | 14/32 (43.8%) SG | 0.048 | 2/46 (4.4%) SG | 4/32 (12.5%) SG | 0.221 | Mean postop (SD) = 14.5 (4.3) | Mean postop (SD) = 13.4 (2.9) | 0.261 | |||||||
| 8. Jacobson et al. 2012 | Prospective Study | Moderate to severe Crohn’s disease (120) | N/A | 18–90 days (mean 46 days) preop PN (with lipid) | No PN | 0/15 (0%) | 29/105 (27.6%) | <0.05 | N/A | Mean postop (SD) = 17 (7) | N/A | ||||
| 9. Salinas et al. 2012 | Retrospective Study | Ulcerative colitis (235) | N/A | 7–28 days (median 9 days) preop PN | No PN | 28/56 (50%) | 63/179 (35.2%) | 0.047 | 1/56 (1.8%) | 0/179 (0%) | 0.238 | N/A | |||
| 10. Kirkil et al. 2012 | Randomized Control Trial | Gastric cancer (35) Colorectal cancer (40) | 100% Malnutrition (SGA B or C) | 7days preop PN (with lipid) | Immune-enhancing EN, Standard EN, and No EN/PN | N/A | N/A | N/A | The mean total antioxidant capacity significantly increased in immune-enhancing EN and EN groups. | ||||||
| 11. Jie et al. 2012 | Prospective study | Intra-abdominal surgery (512) | 100% Malnutrition (NRS ≥ 5) | 7 days preop and 7 days postop PN (with lipid) (73.4%) or EN (26.6%) | No EN and PN | 11/43 (25.6%) | 39/77 (50.6%) | 0.008 | 0/43 (0%) | 2/77 (2.6%) | 0.536 | Mean total = 26.2 (10.1) | Mean total LOS = 25.7 (12.7) | 0.806 | |
| Mean postop = 13.7 (7.9) | Mean postop LOS = 17.9 (11.3) | 0.018 | |||||||||||||
| 12. Ganaie et al. 2015 | Randomized Control Trial | Various major surgical procedures (100) | 100% Malnutrition ** | Preop and postop PN | No PN | 8/50 (16%) | 15/50 (30%) | <0.05 | 3/50 (6%) | 3/50 (6%) | Mean postop LOS = 20 (11) | Mean postop LOS = 26.52 (13.78) | <0.05 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakananurak, N.; Gramlich, L. The Role of Preoperative Parenteral Nutrition. Nutrients 2020, 12, 1320. https://doi.org/10.3390/nu12051320
Lakananurak N, Gramlich L. The Role of Preoperative Parenteral Nutrition. Nutrients. 2020; 12(5):1320. https://doi.org/10.3390/nu12051320
Chicago/Turabian StyleLakananurak, Narisorn, and Leah Gramlich. 2020. "The Role of Preoperative Parenteral Nutrition" Nutrients 12, no. 5: 1320. https://doi.org/10.3390/nu12051320
APA StyleLakananurak, N., & Gramlich, L. (2020). The Role of Preoperative Parenteral Nutrition. Nutrients, 12(5), 1320. https://doi.org/10.3390/nu12051320





