Effects of a Ketogenic Diet on Muscle Fatigue in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial
Abstract
:1. Introduction
2. Materials and Methods
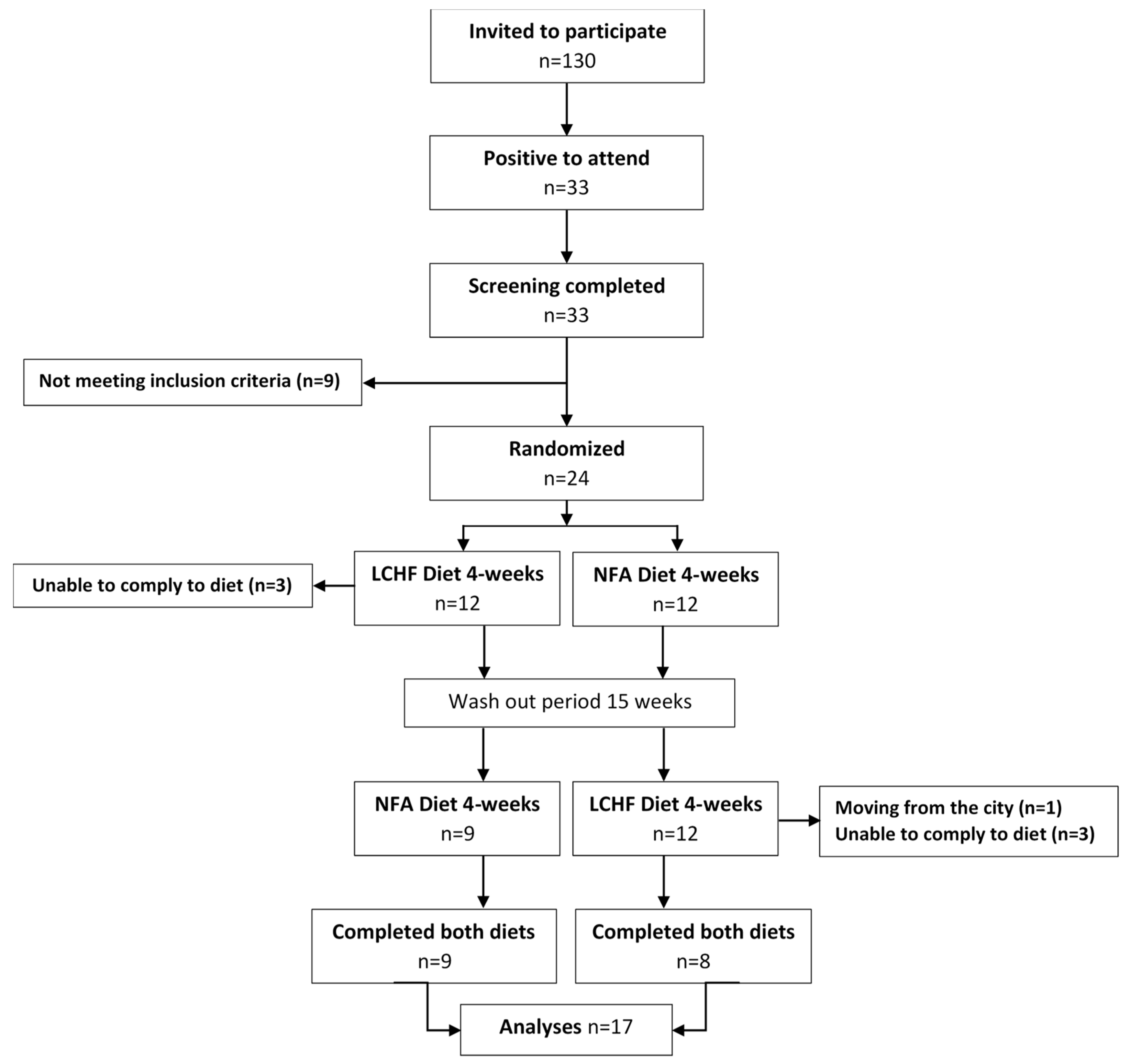
2.1. Study and Design Overview
2.2. Eligibility Criteria, Participants, and Study Setting
2.3. Allocation and Blinding
2.4. Study Diets
2.5. Dietary Adherence
2.6. Outcomes and Power Calculations
2.7. Daily Physical Activity Assessments
2.8. Fasting Blood Collection and Analysis of Ketone Bodies
2.9. Handgrip Force and Handgrip Time To Fatigue
2.10. Graded Incremental Exercise Test, Energy Substrate Utilization, and Blood Sampling
2.11. Statistical Analyses
3. Results
3.1. Dietary Intake and Compliance
3.2. Grip Strength
3.3. Graded Incremental Ergometer Cycling Test
3.4. Physical Activity During the Feeding Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, M.J.; Bangsbo, J.; Renaud, J.M. Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: Implications for fatigue. J. Appl. Physiol. 2008, 104, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knicker, A.J.; Renshaw, I.; Oldham, A.R.; Cairns, S.P. Interactive processes link the multiple symptoms of fatigue in sport competition. Sports Med. 2011, 41, 307–328. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary fat: From foe to friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef] [Green Version]
- Adam-Perrot, A.; Clifton, P.; Brouns, F. Low-carbohydrate diets: Nutritional and physiological aspects. Obes. Rev. 2006, 7, 49–58. [Google Scholar] [CrossRef]
- Noakes, T.D.; Windt, J. Evidence that supports the prescription of low-carbohydrate high-fat diets: A narrative review. Br. J. Sports. Med. 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D. The Art and Science of Low Carbohydrate Living; Beyond Obesity, LLC: Miami, FL, USA, 2011. [Google Scholar]
- Zinn, C.; Wood, M.; Williden, M.; Chatterton, S.; Maunder, E. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J. Int. Soc. Sports Nutr. 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports (Basel) 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.; Volek, J.S.; D’Agostino, D. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J. Strength Cond. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Nutrients 2014, 6, 2493–2508. [Google Scholar] [CrossRef]
- Burke, L.M. Re-Examining High-Fat Diets for Sports Performance: Did We Call the ‘Nail in the Coffin’ Too Soon? Sports Med. 2015, 45 (Suppl. 1), S33–S49. [Google Scholar] [CrossRef] [Green Version]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.C., Jr. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McSwiney, F.T.; Doyle, L.; Plews, D.J.; Zinn, C. Impact of Ketogenic Diet on Athletes: Current Insights. Open Access J. Sports Med. 2019, 10, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Maunder, E.; Dulson, D.K. Exogenous Ketone Supplementation and Keto-Adaptation for Endurance Performance: Disentangling the Effects of Two Distinct Metabolic States. Sports Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A. Swifter, higher, stronger: What’s on the menu? Science 2018, 362, 781–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decroix, L.; De Pauw, K.; Foster, C.; Meeusen, R. Guidelines to Classify Female Subject Groups in Sport-Science Research. Int. J. Sports Physiol. Perform. 2016, 11, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014; p. 627. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, A.; Hellström, F.; Burén, J. Effects of a Ketogenic Low Carbohydrate High Fat Diet and a Diet Based on the Nordic Nutrition Recommendations: Study Protocol for an Explorative Randomized Controlled Trial with Crossover Design in Healthy Young Women. Available online: https://osf.io/t87yk/ (accessed on 12 December 2019).
- Scott, N.W.; McPherson, G.C.; Ramsay, C.R.; Campbell, M.K. The method of minimization for allocation to clinical trials: A review. Control. Clin. Trials 2002, 23, 662–674. [Google Scholar] [CrossRef]
- Treasure, T.; MacRae, K.D. Minimisation: The platinum standard for trials? Randomisation doesn’t guarantee similarity of groups; minimisation does. BMJ 1998, 317, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Margetts, B.M.; Nelson, M. Design Concepts in Nutritional Epidemiology, 2nd ed.; OUP Oxford: Oxford, UK, 1997. [Google Scholar]
- Lagerstrom, C.; Nordgren, B. Methods for measuring maximal isometric grip strength during short and sustained contractions, including intra-rater reliability. Ups. J. Med. Sci. 1996, 101, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Coldham, F.; Lewis, J.; Lee, H. The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J. Hand Ther. 2006, 19, 318–326. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Peronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar] [PubMed]
- Kenward, M.G.; Roger, J.H. The use of baseline covariates in crossover studies. Biostatistics 2010, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-137. 2018. Available online: https://CRAN.R-project.org/package=nlme (accessed on 6 October 2019).
- Burke, L.M.; Angus, D.J.; Cox, G.R.; Cummings, N.K.; Febbraio, M.A.; Gawthorn, K.; Hawley, J.A.; Minehan, M.; Martin, D.T.; Hargreaves, M. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J. Appl. Physiol. 2000, 89, 2413–2421. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef] [Green Version]
- Phinney, S.D. Ketogenic diets and physical performance. Nutr. Metab. 2004, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Kilbom, S.; Armstrong, T.; Buckle, P.; Fine, L.; Hagberg, M.; Haring-Sweeney, M.; Martin, B.; Punnett, L.; Silverstein, B.; Sjøgaard, G.; et al. Musculoskeletal Disorders: Work-related Risk Factors and Prevention. Int. J. Occup. Environ. Health 1996, 2, 239–246. [Google Scholar] [CrossRef]
- Sjøgaard, G.; Savard, G.; Juel, C. Muscle blood flow during isometric activity and its relation to muscle fatigue. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 57, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Bonde-Petersen, F.; Mork, A.L.; Nielsen, E. Local muscle blood flow and sustained contractions of human arm and back muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1975, 34, 43–50. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C.J.; Allen, M.D.; Olympico, E.; Shoemaker, J.K.; Rice, C.L. Blood flow and muscle oxygenation during low, moderate, and maximal sustained isometric contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R475–R481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadamoto, T.; Bonde-Petersen, F.; Suzuki, Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 51, 395–408. [Google Scholar] [CrossRef]
- Urbain, P.; Strom, L.; Morawski, L.; Wehrle, A.; Deibert, P.; Bertz, H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr. Metab. (Lond) 2017, 14, 17. [Google Scholar] [CrossRef] [Green Version]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [Green Version]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [Green Version]
- Hawley, J.A.; Burke, L.M.; Phillips, S.M.; Spriet, L.L. Nutritional modulation of training-induced skeletal muscle adaptations. J. Appl. Physiol. 2011, 110, 834–845. [Google Scholar] [CrossRef]
- Spriet, L.L. Nutritional Support for Athletic Performance. Sports Med. 2015, 45 (Suppl. 1), S3–S4. [Google Scholar] [CrossRef] [Green Version]
- Hultman, E.; Spriet, L.L.; Söderlund, K. Biochemistry of muscle fatigue. Biomed. Biochim. Acta 1986, 45, S97–S106. [Google Scholar]
- Hultman, E. Fuel selection, muscle fibre. Proc. Nutr. Soc. 1995, 54, 107–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, K.; Beaver, W.L.; Davis, J.A.; Pu, J.Z.; Heber, D.; Whipp, B.J. Lactate, pyruvate, and lactate-to-pyruvate ratio during exercise and recovery. J. Appl. Physiol. 1985, 59, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Phinney, S.D.; Bistrian, B.R.; Wolfe, R.R.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Physical and biochemical adaptation. Metabolism 1983, 32, 757–768. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellingwerff, T.; Spriet, L.L.; Watt, M.J.; Kimber, N.E.; Hargreaves, M.; Hawley, J.A.; Burke, L.M. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E380–E388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, W.K.; Paton, C.D.; Garnham, A.P.; Burke, L.M.; Carey, A.L.; Hawley, J.A. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J. Appl. Physiol. 2008, 105, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Hills, A.P.; Street, S.J.; Byrne, N.M. Physical Activity and Health: “What is Old is New Again”. Adv. Food Nutr. Res. 2015, 75, 77–95. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Colley, R.C.; Garriguet, D.; Janssen, I.; Craig, C.L.; Clarke, J.; Tremblay, M.S. Physical activity of Canadian adults: Accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011, 22, 7–14. [Google Scholar]
- Sims, S.T.; Heather, A.K. Myths and Methodologies: Reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp. Physiol. 2018, 103, 1309–1317. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Mean ± SD |
|---|---|
| Age (years) | 23.7 ± 2.0 |
| Body weight (kg) | 61.6 ± 5.4 |
| Body height (cm) | 168 ± 5 |
| BMI (kg/m2) | 21.9 ± 1.3 |
| Systolic BP (mmHg) | 105 ± 8 |
| Diastolic BP (mmHg) | 64 ± 7 |
| Parameter | LCHF Diet | NFA Diet | ||
|---|---|---|---|---|
| Mean ± SD | E% | Mean ± SD | E% | |
| Energy (kJ) | 9988 ± 372 | 9938 ± 613 | ||
| Energy (kcal) | 2387 ± 89 | 2375 ± 146 | ||
| Carbohydrate (g) | 24 ± 4 | 4 | 259 ± 31 | 44 |
| Dietary fiber (g) | 9 ± 4 | 1 | 40 ± 6 | 3 |
| Total fat (g) | 206 ± 10 | 76 | 88 ± 17 | 33 |
| -SFA (g) | 88 ± 11 | 33 | 27 ± 5 | 10 |
| -MUFA (g) | 70 ± 8 | 26 | 33 ± 7 | 12 |
| -PUFA (g) | 31 ± 9 | 11 | 20 ± 6 | 7 |
| Cholesterol (mg) | 1070 ± 271 | 375 ± 104 | ||
| Protein (g) | 111 ± 8 | 19 | 115 ± 11 | 20 |
| Protein (g/kg body weight) | 1.8 ± 0.2 | 1.9 ± 0.2 | ||
| Parameter | Baseline (Mean ± SD) | Treatment Effect (95% CI) | p-value |
|---|---|---|---|
| HG Time to fatigue (s) | 202 ± 85 | −15 [−40;10] | 0.26 |
| HG MVIFmean (N) | 212 ± 33 | 2 [−10;13] | 0.77 |
| HG MVIFpeak (N) | 220 ± 35 | 4 [−7;16] | 0.48 |
| Parameter | Baseline (Mean ± SD) | Treatment Effect (95% CI) | P value |
|---|---|---|---|
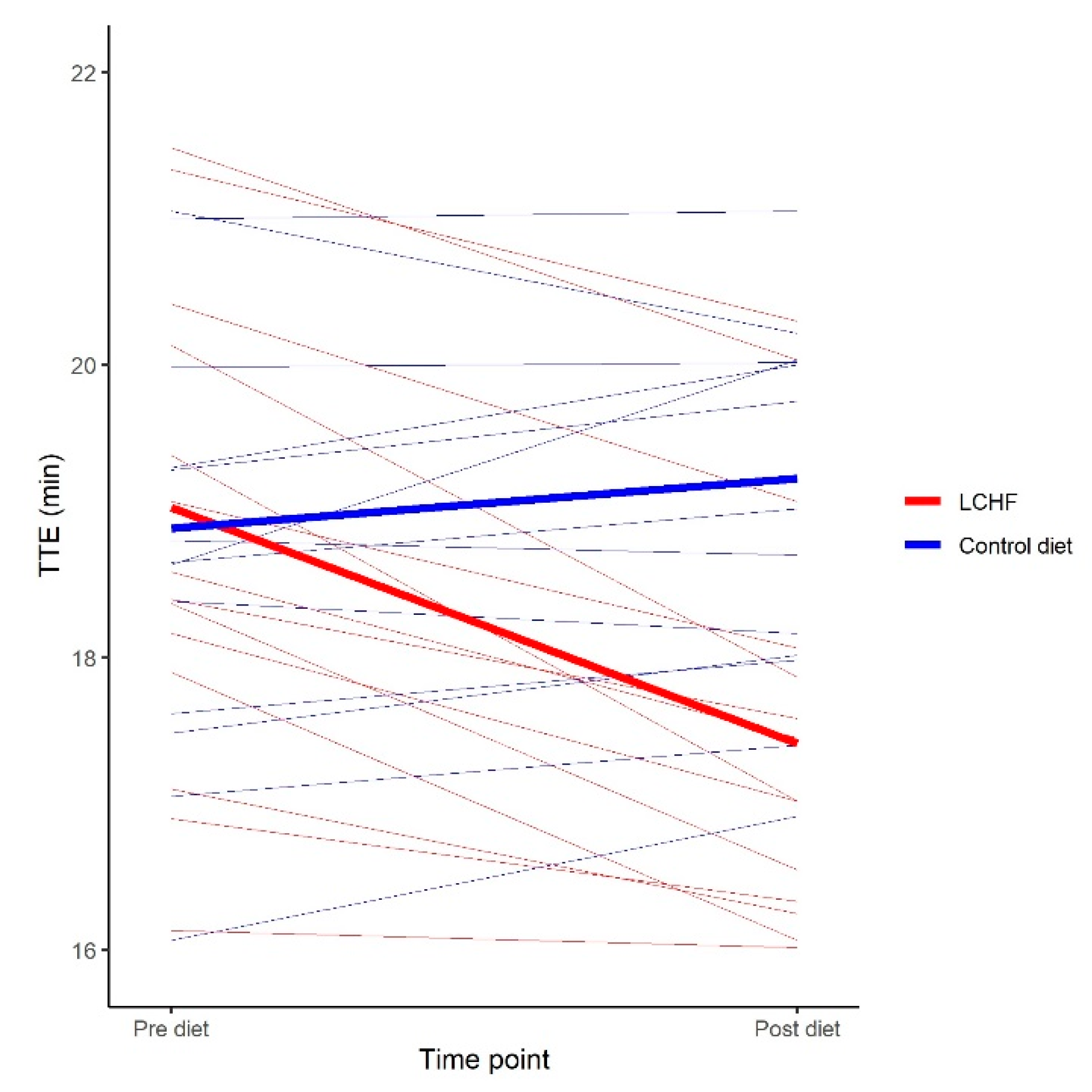
| TTE (min) | 18.9 ± 1.6 | −1.85 [−2.30; −1.40] | <0.001 |
| VO2max (L/min) | 2.73 ± 0.29 | −0.21 [−0.37; −0.05] | 0.029 |
| VO2max (mL/kg/min) | 44.24 ± 4.41 | −1.49 [−3.83;0.84] | 0.266 |
| Incremental Cycling test 60 W ~40% VO2max | |||
| HR (beats/min) | 115 ± 11 | 11 [6;17] | 0.004 |
| RER | 0.80 ± 0.06 | −0.10 [−0.12; −0.08] | <0.001 |
| Fat ox (g/min) | 0.33 ± 0.09 | 0.17 [0.14;0.21] | <0.001 |
| CHO ox (g/min) | 0.37 ± 0.23 | −0.45 [−0.54; −0.36] | <0.001 |
| Lactate (mmol/L) | 1.07 ± 0.31 | −0.30 [−0.48; −0.12] | 0.008 |
| RPE (1-20 scale) | 8.7 ± 1.2 | 0 [0;1] | 0.631 |
| Incremental Cycling test 90 W ~50% VO2max | |||
| HR (beats/min) | 135 ± 11 | 14 [9;18] | <0.001 |
| RER | 0.88 ± 0.05 | −0.13 [−0.15; −0.12] | <0.001 |
| Fat ox (g/min) | 0.25 ± 0.12 | 0.32 [0.28;0.37] | <0.001 |
| CHO ox (g/min) | 1.12 ± 0.29 | −0.78 [−0.87; −0.68] | <0.001 |
| Lactate (mmol/L) | 1.26 ± 0.40 | −0.25 [−0.41; −0.08] | 0.012 |
| RPE (1-20 scale) | 11.5 ± 1.6 | 2 [1;3] | 0.005 |
| Incremental Cycling test 120 W ~65% VO2max | |||
| HR (beats/min) | 156 ± 14 | 17 [10;24] | 0.001 |
| RER | 0.94 ± 0.05 | −0.11 [−0.13; −0.09] | <0.001 |
| Fat ox (g/min) | 0.17 ± 0.14 | 0.34 [0.29;0.40] | <0.001 |
| CHO ox (g/min) | 1.80 ± 0.31 | −0.78 [−0.92; −0.65] | <0.001 |
| Lactate (mmol/L) | 1.81 ± 0.62 | −0.13 [−0.28;0.02] | 0.118 |
| RPE (1-20 scale) | 13.8 ± 1.4 | 2 [1;2] | <0.001 |
| End of Incremental Cycling test | |||
| Lactate (mmol/L) | 6.72 ± 1.65 | −1.71 [−2.79; −0.64] | 0.012 |
| RPE (1-20 scale) | 19.5 ± 0.5 | 0 [0;0] | 0.515 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sjödin, A.; Hellström, F.; Sehlstedt, E.; Svensson, M.; Burén, J. Effects of a Ketogenic Diet on Muscle Fatigue in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2020, 12, 955. https://doi.org/10.3390/nu12040955
Sjödin A, Hellström F, Sehlstedt E, Svensson M, Burén J. Effects of a Ketogenic Diet on Muscle Fatigue in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients. 2020; 12(4):955. https://doi.org/10.3390/nu12040955
Chicago/Turabian StyleSjödin, Anna, Fredrik Hellström, EwaCarin Sehlstedt, Michael Svensson, and Jonas Burén. 2020. "Effects of a Ketogenic Diet on Muscle Fatigue in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial" Nutrients 12, no. 4: 955. https://doi.org/10.3390/nu12040955





