Early Morning Food Intake as a Risk Factor for Metabolic Dysregulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-In-Laboratory Procedures
2.2. In-Laboratory Procedures
2.3. Analysis
3. Results
3.1. Participant Characteristics
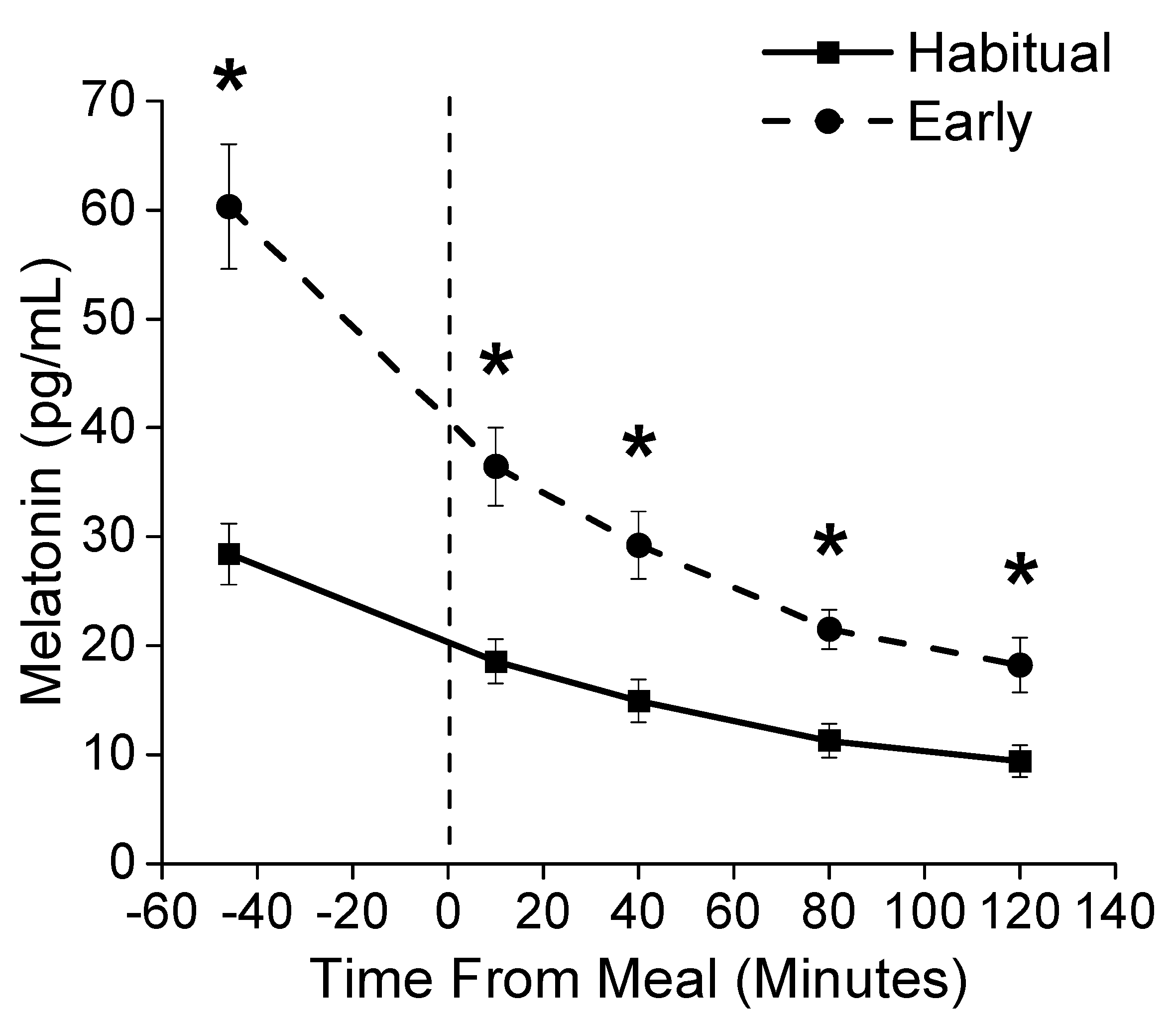
3.2. Sleep and Circadian Outcomes
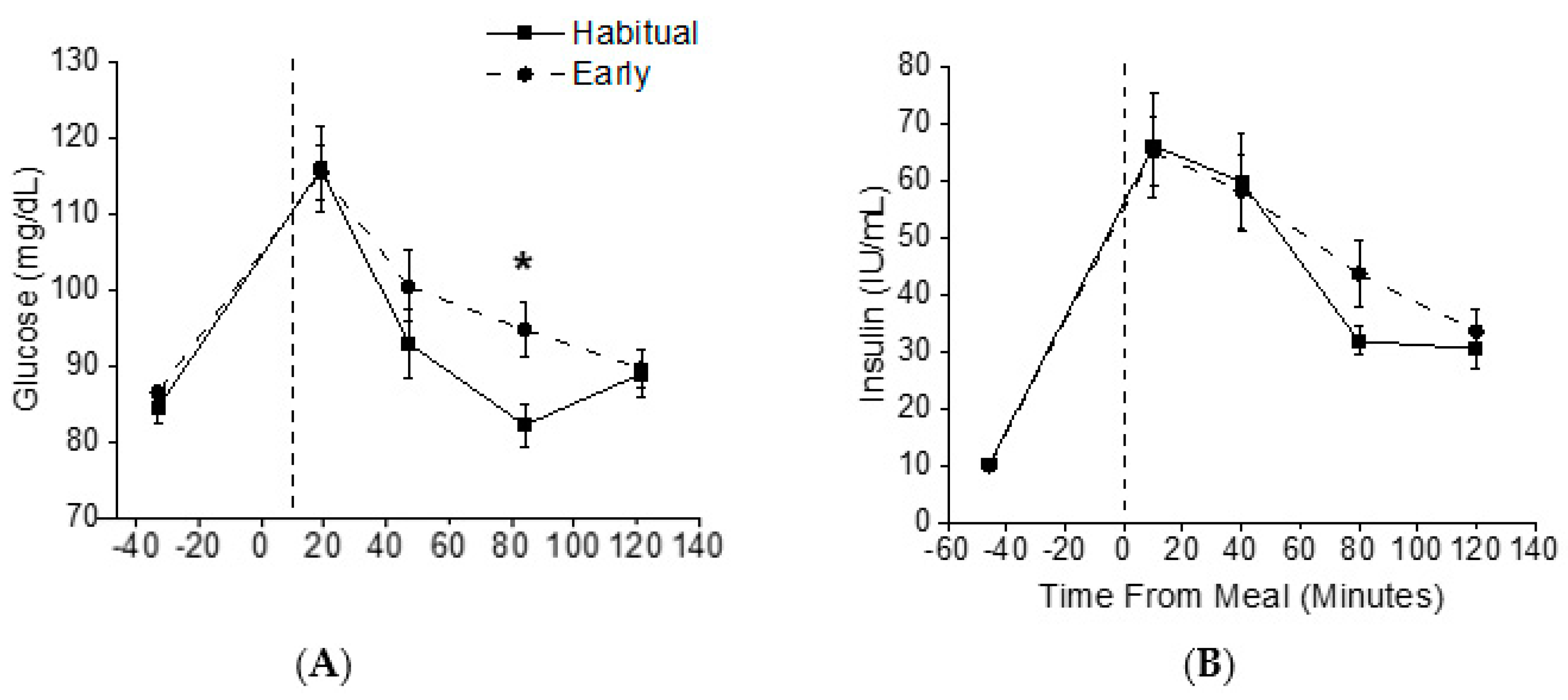
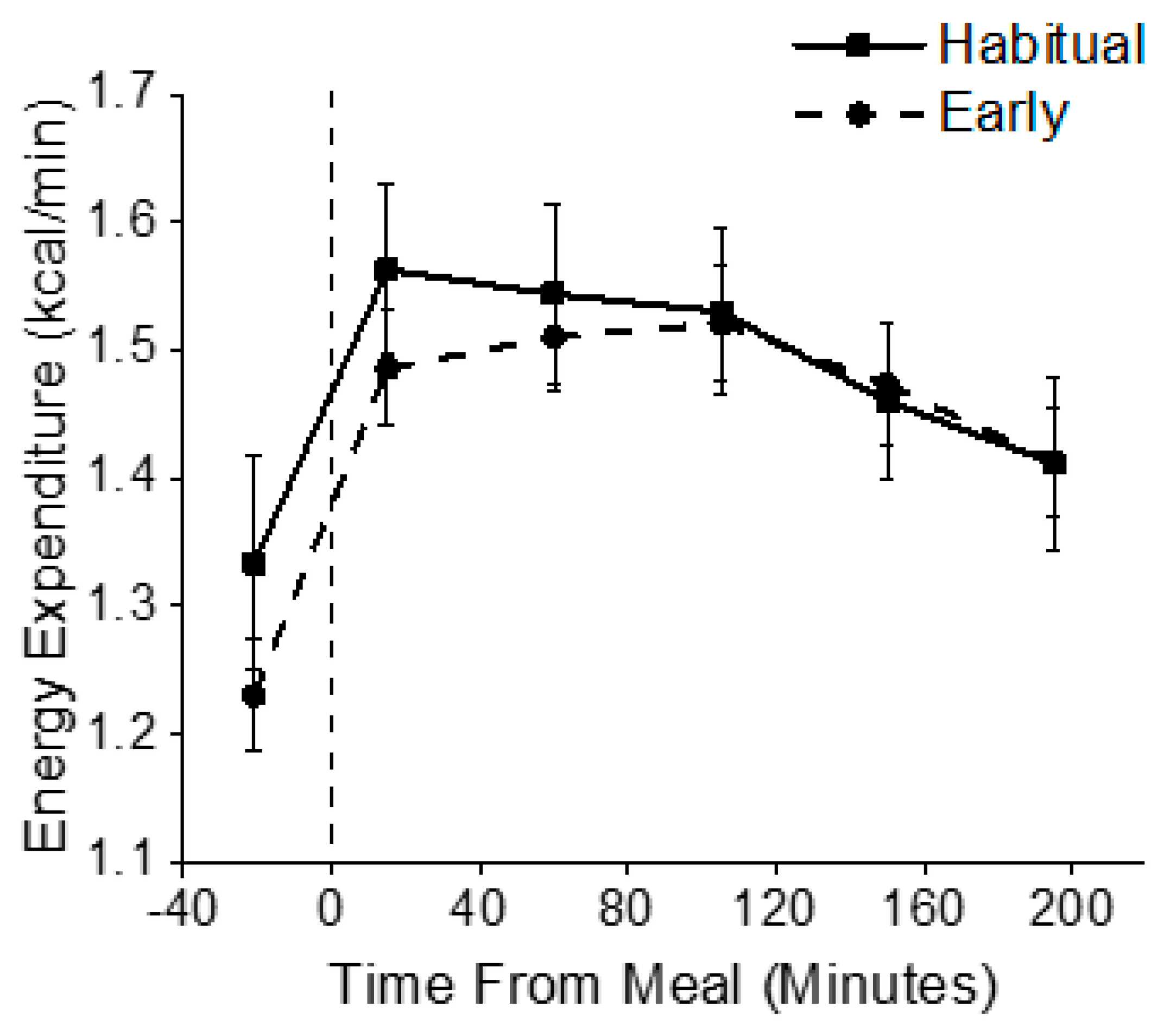
3.3. Metabolic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Beydoun, M.A.; Liang, L.; Caballero, B.; Kumanyika, S.K. Will All Americans Become Overweight or Obese? Estimating the Progression and Cost of the US Obesity Epidemic. Obesity 2008, 16, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and Quality of Sleep and Incidence of Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, R.H.; Depner, C.M.; Perreault, L.; Markwald, R.R.; Smith, M.R.; McHill, A.W.; Higgins, J.; Melanson, E.L.; Wright, K.P. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr. Biol. 2015, 25, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A.J.L. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef] [Green Version]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian Misalignment Augments Markers of Insulin Resistance and Inflammation, Independently of Sleep Loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [Green Version]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef] [Green Version]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Metabolic Consequences in Humans of Prolonged Sleep Restriction Combined with Circadian Disruption. Sci. Transl. Med. 2012, 4, 129ra43. [Google Scholar] [CrossRef] [Green Version]
- McHill, A.W.; Czeisler, C.A.; Phillips, A.J.K.; Keating, L.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Caloric and Macronutrient Intake Differ with Circadian Phase and between Lean and Overweight Young Adults. Nutrients 2019, 11, 587. [Google Scholar] [CrossRef] [Green Version]
- Sateia, M.J. International Classification of Sleep Disorders-Third Edition. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, T.M. A time to work: Recent trends in shift work and flexible schedules. Mon. Labor Rev. 2007, 130, 3. [Google Scholar]
- Di Lorenzo, L.; De Pergola, G.; Zocchetti, C.; L’Abbate, N.; Basso, A.; Pannacciulli, N.; Cignarelli, M.; Giorgino, R.; Soleo, L. Effect of shift work on body mass index: Results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int. J. Obes. 2003, 27, 1353–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilha, H.G.; Crispim, C.A.; Zimberg, I.Z.; Folkard, S.; Tufik, S.; Mello, M.T. de Metabolic Responses on the Early Shift. Chronobiol. Int. 2010, 27, 1080–1092. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Bakeman, R. Recommended effect size statistics for repeated measures designs. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef]
- Burke, T.M.; Scheer, F.A.J.L.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J. Sleep Res. 2015, 24, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Olejnik, S.; Algina, J. Generalized Eta and Omega Squared Statistics: Measures of Effect Size for Some Common Research Designs. Psychol. Methods 2003, 8, 434–447. [Google Scholar] [CrossRef] [Green Version]
- Kecklund, G.; Åkerstedt, T.; Lowden, A. Morning Work: Effects of Early Rising on Sleep and Alertness. Sleep 1997, 20, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czeisler, C.A.; Zimmerman, J.C.; Ronda, J.M.; Moore-Ede, M.C.; Weitzman, E.D. Timing of REM Sleep is Coupled to the Circadian Rhythm of Body Temperature in Man. Sleep 1980, 2, 329–346. [Google Scholar]
- Benloucif, S.; Burgess, H.J.; Klerman, E.B.; Lewy, A.J.; Middleton, B.; Murphy, P.J.; Parry, B.L.; Revell, V.L. Measuring melatonin in humans. J. Clin. Sleep Med. 2008, 4, 66–69. [Google Scholar] [CrossRef]
- Wright, K.P.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. CB 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stothard, E.R.; McHill, A.W.; Depner, C.M.; Birks, B.R.; Moehlman, T.M.; Ritchie, H.K.; Guzzetti, J.R.; Chinoy, E.D.; LeBourgeois, M.K.; Axelsson, J.; et al. Circadian Entrainment to the Natural Light-Dark Cycle across Seasons and the Weekend. Curr. Biol. 2017, 27, 508–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, C.; Dashti, H.S.; Lane, J.M.; Anderson, S.G.; Schernhammer, E.S.; Rutter, M.K.; Saxena, R.; Scheer, F.A.J.L. Night Shift Work, Genetic Risk, and Type 2 Diabetes in the UK Biobank. Diabetes Care 2018, 41, 762–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canuto, R.; Garcez, A.S.; Olinto, M.T.A. Metabolic syndrome and shift work: A systematic review. Sleep Med. Rev. 2013, 17, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Huang, X.; Rong, Y.; He, M.; Wang, Y.; Yuan, J.; Wu, T.; Chen, W. The Effects of Shift Work on Sleeping Quality, Hypertension and Diabetes in Retired Workers. PLoS ONE 2013, 8, e71107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bøggild, H.; Knutsson, A. Shift work, risk factors and cardiovascular disease. Scand. J. Work. Environ. Health 1999, 25, 85–99. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Scheer, F.A.J.L. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J. Clin. Endocrinol. Metab. 2016, 101, 1066–1074. [Google Scholar] [CrossRef]
- Heath, G.; Coates, A.; Sargent, C.; Dorrian, J. Sleep Duration and Chronic Fatigue Are Differently Associated with the Dietary Profile of Shift Workers. Nutrients 2016, 8, 771. [Google Scholar] [CrossRef]
- Persson, M.; Mårtensson, J. Situations influencing habits in diet and exercise among nurses working night shift. J. Nurs. Manag. 2006, 14, 414–423. [Google Scholar] [CrossRef]
- Zitting, K.-M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human Resting Energy Expenditure Varies with Circadian Phase. Curr. Biol. CB 2018, 28, 3685–3690.e3. [Google Scholar] [CrossRef] [Green Version]
- Krauchi, K.; Wirz-Justice, A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 267, R819–R829. [Google Scholar] [CrossRef] [PubMed]
- Spengler, C.M.; Czeisler, C.A.; Shea, S.A. An endogenous circadian rhythm of respiratory control in humans. J. Physiol. 2000, 526, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Cayanan, E.A.; Eyre, N.A.B.; Lao, V.; Comas, M.; Hoyos, C.M.; Marshall, N.S.; Phillips, C.L.; Shiao, J.S.C.; Guo, Y.-L.L.; Gordon, C.J. Is 24-hour energy intake greater during night shift compared to non-night shift patterns? A systematic review. Chronobiol. Int. 2019, 36, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Dorrian, J.; Coates, A.M.; Leung, G.K.W.; Davis, R.; Rosbotham, E.; Warnock, R.; Huggins, C.E.; Bonham, M.P. Temporal pattern of eating in night shift workers. Chronobiol. Int. 2019, 36, 1613–1625. [Google Scholar] [CrossRef]
- Romon, M.; Edme, J.L.; Boulenguez, C.; Lescroart, J.L.; Frimat, P. Circadian variation of diet-induced thermogenesis. Am. J. Clin. Nutr. 1993, 57, 476–480. [Google Scholar] [CrossRef]
- Morris, C.J.; Garcia, J.I.; Myers, S.; Yang, J.N.; Trienekens, N.; Scheer, F.A.J.L. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity 2015, 23, 2053–2058. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Morris, C.J.; Caputo, R.; Wang, W.; Garaulet, M.; Scheer, F.A.J.L. Sex differences in the circadian misalignment effects on energy regulation. Proc. Natl. Acad. Sci. USA 2019, 116, 23806–23812. [Google Scholar] [CrossRef]

| Parameter Minutes of Recording Time | Habitual Sleep | Simulated Early Morning Shiftwork | p Value |
|---|---|---|---|
| Stage 1 | 16.3 ± 1.7 | 15.2 ± 1.6 | 0.63 |
| Stage 2 | 235.4 ± 5.0 | 182.3 ± 8.9 | p < 0.0001 |
| Stage 3/4 (SWS) | 76.9 ± 6.9 | 84.8 ± 7.9 | 0.11 |
| REM | 107.3 ± 4.6 | 68.4 ± 3.6 | p < 0.0001 |
| Total Sleep Time (TST) | 435.8 ± 5.8 | 350.7 ± 6.0 | p < 0.0001 |
| Sleep Efficiency (SE) | 90.8 ± 1.2 | 88.7 ± 1.0 | 0.14 |
| SOL 1.5 min | 16.3 ± 3.3 | 16.4 ± 2.6 | 0.97 |
| SOL 10 min | 17.9 ± 3.3 | 19.2 ± 2.8 | 0.77 |
| WASO from SOL 1.5 min | 27.9 ± 4.1 | 28.3 ± 4.3 | 0.94 |
| REML from SOL 1.5 min | 109.7 ± 9.6 | 95.5 ± 9.0 | 0.33 |
| SWSL from SOL 1.5 min | 15.2 ± 1.5 | 14.0 ± 1.7 | 0.39 |
| Number of Awakenings | 21.4 ± 2.0 | 19.1 ± 1.9 | 0.24 |
| Avg Duration of Awakenings | 1.3 ± 0.2 | 1.5 ± 0.2 | 0.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stothard, E.R.; Ritchie, H.K.; Birks, B.R.; Eckel, R.H.; Higgins, J.; Melanson, E.L.; Wright Jr., K.P.; McHill, A.W. Early Morning Food Intake as a Risk Factor for Metabolic Dysregulation. Nutrients 2020, 12, 756. https://doi.org/10.3390/nu12030756
Stothard ER, Ritchie HK, Birks BR, Eckel RH, Higgins J, Melanson EL, Wright Jr. KP, McHill AW. Early Morning Food Intake as a Risk Factor for Metabolic Dysregulation. Nutrients. 2020; 12(3):756. https://doi.org/10.3390/nu12030756
Chicago/Turabian StyleStothard, Ellen R., Hannah K. Ritchie, Brian R. Birks, Robert H. Eckel, Janine Higgins, Edward L. Melanson, Kenneth P. Wright Jr., and Andrew W. McHill. 2020. "Early Morning Food Intake as a Risk Factor for Metabolic Dysregulation" Nutrients 12, no. 3: 756. https://doi.org/10.3390/nu12030756





