Non-Responsive Coeliac Disease: A Comprehensive Review from the NHS England National Centre for Refractory Coeliac Disease
Abstract
:1. Introduction
2. Defining Non-Responsive Coeliac Disease
3. Causes of Non-Responsive Coeliac Disease
3.1. An Alternative Primary Diagnosis
3.2. An Associated Condition
3.3. Dietary Indiscretion
3.4. Gluten Super-Sensitivity
3.5. Refractory Coeliac Disease
3.5.1. Overview
3.5.2. Diagnosis
3.5.3. Management
- (i)
- General Measures
- (ii)
- RCD1
- (iii)
- RCD2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 6, 70–81. [Google Scholar] [CrossRef]
- O’Mahony, S.; Howdle, P.D.; Losowsky, M.S. Review article: Management of patients with non-responsive coeliac disease. Aliment. Pharmacol. Ther. 1996, 10, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Baggus, E.M.R.; Hadjivassiliou, M.; Cross, S.; Penny, H.A. How to manage adult coeliac disease: Perspective from the NHS England rare diseases collaborative network for non-responsive and refractory coeliac disease. Frontline Gastroenterol. 2019. [Google Scholar] [CrossRef]
- Mooney, P.D.; Evans, K.E.; Singh, S.; Sanders, D.S. Treatment failure in coeliac disease: A practical guide to investigation and treatment of non-responsive and refractory coeliac disease. J. Gastrointest. Liver Dis. 2012, 21, 197–203. [Google Scholar]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewar, D.H.; Donnelly, S.C.; McLaughlin, S.D.; Johnson, M.W.; Ellis, H.J.; Ciclitira, P.J. Celiac disease: Management of persistent symptoms in patients on a gluten-free diet. World J. Gastroenterol. 2012, 18, 1348–1356. [Google Scholar] [CrossRef]
- Abdulkarim, A.S.; Burgart, L.J.; See, J.; Murray, J.A. Etiology of nonresponsive celiac disease: Results of a systematic approach. Am. J. Gastroenterol. 2002, 97, 2016–2021. [Google Scholar] [CrossRef]
- Hadithi, M.; Von Blomberg, B.M.E.; Crusius, J.B.A.; Bloemena, E.; Kostense, P.J.; Meijer, J.W.; Mulder, C.J.; Stehouwer, C.D.; Peña, A.S. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 2007, 147, 294–302. [Google Scholar] [CrossRef]
- Hill, I.D. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology 2005, 128, S25–S32. [Google Scholar] [CrossRef]
- Leffler, D.A.; Schuppan, D. Update on serologic testing in celiac disease. Am. J. Gastroenterol. 2010, 105, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; A Van Heel, D.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Peerally, M.F.; Barnes, J.H.; Kandasamy, V.; Whiteley, J.C.; Partridge, D.; Vergani, P.; Cross, S.S.; Green, P.H.; Sanders, D.S. The clinical and phenotypical assessment of seronegative villous atrophy; a prospective UK centre experience evaluating 200 adult cases over a 15-year period (2000–2015). Gut 2017, 66, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Sanders, D.S.; Biagi, F. Seronegative coeliac disease: Clearing the diagnostic dilemma. Curr. Opin. Gastroenterol. 2018, 34, 154–158. [Google Scholar] [CrossRef]
- Arguelles-Grande, C.; Tennyson, C.A.; Lewis, S.K.; Green, P.H.; Bhagat, G. Variability in small bowel histopathology reporting between different pathology practice settings: Impact on the diagnosis of coeliac disease. J. Clin. Pathol. 2012, 65, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, A.; Nikkels, P.; Houwen, R.; Kate, F.T. Reproducibility of the histological diagnosis of celiac disease. Scand. J. Gastroenterol. 2011, 46, 1065–1073. [Google Scholar] [CrossRef]
- Anderson, R.P.; Henry, M.J.; Taylor, R.; Duncan, E.L.; Danoy, P.; Costa, M.J.; Addison, K.; Tye-Din, J.A.; Kotowicz, M.A.; Knight, R.E.; et al. A novel serogenetic approach determines the community prevalence of celiac disease and informs improved diagnostic pathways. BMC Med. 2013, 11, 188. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Mearin, M.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Leffler, D.A.; Dennis, M.; Hyett, B.; Kelly, E.; Schuppan, D.; Kelly, C.P. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin. Gastroenterol. Hepatol. 2007, 5, 445–450. [Google Scholar] [CrossRef]
- van Wanrooij, R.L.J.; Bouma, G.; Bontkes, H.J.; Neefjes-Borst, A.; van Grieken, N.C.; von Blomberg, B.M.E.; Mulder, C.J.J. Outcome of referrals for non-responsive celiac disease in a tertiary center: Low incidence of refractory celiac disease in The Netherlands. Clin. Transl. Gastroenterol. 2017, 8, e218. [Google Scholar] [CrossRef]
- Oxford, E.C.; Nguyen, D.D.; Sauk, J.; Korzenik, J.; Yajnik, V.; Friedman, S.; Ananthakrishnan, A. Impact of coexistent celiac disease on phenotype and natural history of inflammatory bowel diseases. Am. J. Gastroenterol. 2013, 108, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fine, K.D.; Meyer, R.L.; Lee, E.L. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology 1997, 112, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.M.; Leeds, J.S.; Robinson, K.; Shah, P.; Lobo, A.J.; Mcalindon, M.E.; Sanders, D.S. Reflux and irritable bowel syndrome are negative predictors of quality of life in coeliac disease and inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, V.P.; Raguseo, L.; Tripaldi, M.; Indrio, F.; Gentile, A.; De Toma, M.; Cristofori, F. Increased prevalence of abdominal pain-functional gastrointestinal disorders in pediatric celiac patients. Dig. Liver Dis. 2017, 49, e267. [Google Scholar] [CrossRef]
- Testa, A.; Imperatore, N.; Rispo, A.; Rea, M.; Tortora, R.; Nardone, O.M.; Lucci, L.; Accarino, G.; Caporaso, N.; Castiglione, F. Beyond irritable bowel syndrome: The efficacy of the low fodmap diet for improving symptoms in inflammatory bowel diseases and celiac disease. Dig. Dis. 2018, 36, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, L.; Bascuñán, K.A.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Branchi, F.; Ferretti, F.; Dell’Osso, B.; Montanari, V.; Bardella, M.T.; et al. A low FODMAP gluten-free diet improves functional gastrointestinal disorders and overall mental health of celiac disease patients: A randomized controlled trial. Nutrients 2018, 10, 1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francavilla, R.; Piccolo, M.; Francavilla, A.; Polimeno, L.; Semeraro, F.; Cristofori, F.; Castellaneta, S.; Barone, M.; Indrio, F.; Gobbetti, M.; et al. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent IBS-type symptoms: A randomized, double-blind, placebo-controlled, multicenter trial. J. Clin. Gastroenterol. 2019, 53, e117–e125. [Google Scholar] [CrossRef] [Green Version]
- Hall, N.J.; Rubin, G.; Charnock, A. Systematic review: Adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharm. Ther. 2009, 30, 315–330. [Google Scholar] [CrossRef]
- Lerner, B.A.; Phan Vo, L.T.; Yates, S.; Rundle, A.G.; Green, P.H.; Lebwohl, B. Detection of gluten in gluten-free labeled restaurant food: Analysis of crowd-sourced data. Am. J. Gastroenterol. 2019, 114, 792–797. [Google Scholar] [CrossRef]
- Bottaro, G.; Cataldo, F.; Rotolo, N.; Spina, M.; Corazza, G.R. The clinical pattern of subclinical/silent celiac disease: An analysis on 1026 consecutive cases. Am. J. Gastroenterol. 1999, 94, 691–696. [Google Scholar] [CrossRef]
- Silvester, J.A.; Kurada, S.; Szwajcer, A.; Kelly, C.P.; Leffler, D.A.; Duerksen, DR. Tests for serum transglutaminase and endomysial antibodies do not detect most patients with celiac disease and persistent villous atrophy on gluten-free diets: A meta-analysis. Gastroenterology. 2017, 153, 689.e1–701.e1. [Google Scholar] [CrossRef] [PubMed]
- Pekki, H.; Kurppa, K.; Maki, M.; Huhtala, H.; Sievänen, H.; Laurila, K.; Collin, P.; Kaukinen, K. Predictors and significance of incomplete mucosal recovery in celiac disease after 1 year on a gluten-free diet. Am. J. Gastroenterol. 2015, 110, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.M.; Corbett, G.; Currie, E.; Lee, J.; Sweeney, N.; Woodward, J.M. Optimising delivery of care in coeliac disease—Comparison of the benefits of repeat biopsy and serological follow-up. Aliment. Pharm. Ther. 2013, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.T.; Murray, J.A. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahab, P.J.; Meijer, J.W.; Mulder, C.J. Histologic follow-up of people with celiac disease on a gluten-free diet: Slow and incomplete recovery. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Grefte, J.M.; Bouman, J.G.; Grond, J.; Jansen, W.; Kleibeuker, JH. Slow and incomplete histological and functional recovery in adult gluten sensitive enteropathy. J. Clin. Pathol. 1988, 41, 886–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.F.; Sugai, E.; Temprano, M.P.; Niveloni, S.I.; Vázquez, H.G.; Moreno, M.L.; Domínguez-Flores, M.R.; Muñoz-Suano, A.; Smecuol, E.; Stefanolo, J.P.; et al. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J. Gastroenterol. 2019, 25, 1409–1420. [Google Scholar] [CrossRef]
- Moreno, M.L.; Cebolla, A.; Munoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, A.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Lau, M.S.; Mooney, P.D.; White, W.L.; Rees, M.A.; Wong, S.H.; Kurien, M.; Trott, N.; Leffler, D.A.; Hadjivassiliou, M.; Sanders, D.S. The role of an IgA/IgG-deamidated gliadin peptide point-of-care test in predicting persistent villous atrophy in patients with celiac disease on a gluten-free diet. Am. J. Gastroenterol. 2017, 112, 1859–1867. [Google Scholar] [CrossRef]
- Hollon, J.R.; Cureton, P.A.; Martin, M.L.; Puppa, E.L.; Fasano, A. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet-adherent non-responsive celiac disease patients. BMC Gastroenterol. 2013, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Leonard, M.; Cureton, P.; Fasano, A. Indications and use of the gluten contamination elimination diet for patients with nonresponsive celiac disease. Nutrients 2017, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, R.W.; Løvik, A.; Tollefsen, S.; Andresen, P.A.; Vatn, M.H.; de Lange, T.; Bratlie, J.; Brandtzaeg, P.; Farstad, I.N.; Lundin, K.E.A. Effect of elemental diet on mucosal immunopathology and clinical symptoms in type 1 refractory celiac disease. Clin. Gastroenterol. Hepatol. 2005, 3, 875–885. [Google Scholar] [CrossRef]
- Mandal, A.; Mayberry, J. Elemental diet in the treatment of refractory coeliac disease. Eur. J. Gastroenterol. Hepatol. 2001, 13, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; Lundin, K.E.A.; Murray, J.A.; Sanders, D.S.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Kelly, D.G.; Lahr, B.D.; Dogan, A.; Wu, T.T.; Murray, J.A. Clinical staging and survival in refractory celiac disease: A single center experience. Gastroenterology 2009, 136, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malamut, G.; Afchain, P.; Verkarre, V.; Lecomte, T.; Amiot, A.; Damotte, D.; Bouhnik, Y.; Colombel, J.-F.; Delchier, J.-C.; Allez, M.; et al. Presentation and long-term follow-up of refractory celiac disease: Comparison of type I with type II. Gastroenterology 2009, 136, 81–90. [Google Scholar] [CrossRef]
- Roshan, B.; Leffler, D.A.; Jamma, S.; Dennis, M.D.; Sheth, S.; Falchuk, K.R.; Najarian, R.M.; Goldsmith, J.; Tariq, S.; Schuppan, D.; et al. The incidence and clinical spectrum of refractory celiac disease in a north american referral center. Am. J. Gastroenterol. 2011, 106, 923–928. [Google Scholar] [CrossRef]
- Rowinski, S.A.; Christensen, E. Epidemiologic and therapeutic aspects of refractory coeliac disease—A systematic review. Dan. Med. J. 2016, 63, A5307. [Google Scholar]
- Cellier, C.; Delabesse, E.; Helmer, C.; Mende, S.; Seegebarth, A.; Siegmund, B.; Hennig, S.; Todorova, K.; Rosenwald, A.; Daum, S.; et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. Lancet 2000, 356, 203–208. [Google Scholar] [CrossRef]
- Al-Toma, A.; Verbeek, W.H.; Mulder, C.J. Update on the management of refractory coeliac disease. J. Gastrointestin. Liver Dis. 2007, 16, 57–63. [Google Scholar]
- Al-Toma, A.; Verbeek, W.H.; Hadithi, M.; von Blomberg, B.M.; Mulder, C.J. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: Retrospective evaluation of single-centre experience. Gut 2007, 56, 1373–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daum, S.; Ipczynski, R.; Schumann, M.; Wahnschaffe, U.; Zeitz, M.; Ullrich, R. High rates of complications and substantial mortality in both types of refractory sprue. Eur. J. Gastroenterol. Hepatol. 2009, 21, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Cellier, C.; Patey, N.; Mauvieux, L.; Jabri, B.; Delabesse, E.; Cervoni, J-P.; Burtin, M-L.; Guy-Grand, D.; Bouhnik, Y.; Modigliani, R.; et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 1998, 114, 471–481. [Google Scholar] [CrossRef]
- Ettersperger, J.; Montcuquet, N.; Malamut, G.; Guegan, N.; Lopez-Lastra, S.; Gayraud, S.; Reimann, C.; Vidal, E.; Cagnard, N.; Villarese, P.; et al. Interleukin-15-dependent T-cell-like innate intraepithelial lymphocytes develop in the intestine and transform into lymphomas in celiac disease. Immunity 2016, 45, 610–625. [Google Scholar] [CrossRef]
- Bagdi, E.; Diss, T.C.; Munson, P.; Isaacson, P.G. Mucosal intra-epithelial lymphocytes in enteropathy-associated T-cell lymphoma, ulcerative jejunitis, and refractory celiac disease constitute a neoplastic population. Blood 1999, 94, 260–264. [Google Scholar] [CrossRef]
- Liu, H.; Brais, R.; Lavergne-Slove, A.; Jeng, Q.; Payne, K.; Ye, H.; Liu, Z.; Carreras, J.; Huang, Y.; Bacon, C.M.; et al. Continual monitoring of intraepithelial lymphocyte immunophenotype and clonality is more important than snapshot analysis in the surveillance of refractory coeliac disease. Gut 2010, 59, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Celli, R.; Hui, P.; Triscott, H.; Bogardus, S.; Gibson, J.; Hwang, M.; Robert, M.E. Clinical insignficance of monoclonal T-cell populations and duodenal intraepithelial T-cell phenotypes in celiac and nonceliac patients. Am. J. Surg. Pathol. 2019, 43, 151–160. [Google Scholar] [CrossRef]
- Verbeek, W.H.; Goerres, M.S.; von Blomberg, B.M.; Oudejans, J.J.; Scholten, P.E.; Hadithi, M.; Al-Toma, A.; Schreurs, M.W.; Mulder, C.J. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in refractory celiac disease. Clin. Immunol. 2008, 126, 48–56. [Google Scholar] [CrossRef]
- Leon, F. Flow cytometry of intestinal intraepithelial lymphocytes in celiac disease. J. Immunol. Methods 2011, 363, 177–186. [Google Scholar] [CrossRef]
- Woodward, J. Improving outcomes of refractory celiac disease—Current and emerging treatment strategies. Clin. Exp. Gastroenterol. 2016, 9, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Cheminant, M.; Bruneau, J.; Malamut, G.; Sibon, D.; Guegan, N.; van Gils, T.; Cording, S.; Trinquand, A.; Verkarre, V.; Lhermitte, L.; et al. NKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointestinal T-cell lymphoproliferative diseases: A CELAC study. Gut 2019, 68, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; O’Connell, S.; Hawkins, J.; Dalley, C.; Jack, A.; Mannari, D.; McNamara, C.; Scott, M.; Shenton, G.; Soilleux, E.; et al. Haematological cancers: Improving outcomes. A summary of updated NICE service guidance in relation to Specialist Integrated Haematological Malignancy Diagnostic Services (SIHMDS). J. Clin. Pathol. 2017, 70, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Murray, J.A. Classification and management of refractory coeliac disease. Gut 2010, 59, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Brar, P.; Lee, S.; Lewis, S.; Egbuna, I.; Bhagat, G.; Green, P.H. Budesonide in the treatment of refractory celiac disease. Am. J. Gastroenterol. 2007, 102, 2265–2269. [Google Scholar] [CrossRef]
- Edsbacker, S.; Larsson, P.; Wollmer, P. Gut delivery of budesonide, a locally active corticosteroid, from plain and controlled-release capsules. Eur. J. Gastroenterol. Hepatol. 2002, 14, 1357–1362. [Google Scholar] [CrossRef]
- Mukewar, S.S.; Sharma, A.; Rubio-Tapia, A.; Wu, T.T.; Jabri, B.; Murray, J.A. Open-capsule budesonide for refractory celiac disease. Am. J. Gastroenterol. 2017, 112, 959–967. [Google Scholar] [CrossRef]
- Goerres, M.S.; Meijer, J.W.; Wahab, P.J.; Kerckhaert, J.A.; Groenen, P.J.; Van Krieken, J.H.; Mulder, C.J. Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment. Pharmacol. Ther. 2003, 18, 487–494. [Google Scholar] [CrossRef]
- Tack, G.J.; van Asseldonk, D.P.; van Wanrooij, R.L.; van Bodegraven, A.A.; Mulder, C.J. Tioguanine in the treatment of refractory coeliac disease—A single centre experience. Aliment. Pharmacol. Ther. 2012, 36, 274–281. [Google Scholar] [CrossRef]
- Costantino, G.; della Torre, A.; Lo Presti, M.A.; Caruso, R.; Mazzon, E.; Fries, W. Treatment of life-threatening type I refractory coeliac disease with long-term infliximab. Dig. Liver Dis. 2008, 40, 74. [Google Scholar] [CrossRef]
- Gillett, H.R.; Arnott, I.D.; McIntyre, M.; Campbell, S.; Dahele, A.; Priest, M.; Jackson, R.; Ghosh, S. Successful infliximab treatment for steroid-refractory celiac disease: A case report. Gastroenterology 2002, 122, 800–805. [Google Scholar] [CrossRef]
- Jamma, S.; Leffler, D.A.; Dennis, M.; Najarian, R.M.; Schuppan, D.B.; Sheth, S.; Kelly, C.P. Small intestinal release mesalamine for the treatment of refractory celiac disease type I. J. Clin. Gastroenterol. 2011, 45, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Rondonotti, E.; Lundin, K.E.; Spada, C.; Keuchel, M.; Kaukinen, K.; de Franchis, R.; Jacobs, M.A.; Villa, F.; Mulder, C.J. Video capsule endoscopy in celiac disease: Current clinical practice. J. Dig. Dis. 2012, 13, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hadithi, M.; Mallant, M.; Oudejans, J.; van Waesberghe, J.H.; Mulder, C.J.; Comans, E.F. 18F-FDG PET versus CT for the detection of enteropathy-associated T-cell lymphoma in refractory celiac disease. J. Nucl. Med. 2006, 47, 1622–1627. [Google Scholar] [PubMed]
- Van Weyenberg, S.J.; Meijerink, M.R.; Jacobs, M.A.; van Kuijk, C.; Mulder, C.J.; van Waesberghe, J.H. MR enteroclysis in refractory celiac disease: Proposal and validation of a severity scoring system. Radiology 2011, 259, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bawardy, B.; Barlow, J.M.; Vasconcelos, R.N.; Kim, S.T.; Bruining, D.H.; Hansel, S.L.; Sheedy, S.P.; Murray, J.A.; Rubio-Tapia, A.; Rajan, E.; et al. Cross-sectional imaging in refractory celiac disease. Abdom. Radiol. (NY) 2017, 42, 389–395. [Google Scholar] [CrossRef]
- Hadithi, M.; Al-toma, A.; Oudejans, J.; van Bodegraven, A.A.; Mulder, C.J.; Jacobs, M. The Value of double-balloon enteroscopy in patients with refractory coeliac disease. Am. J. Gastroenterol. 2007, 102, 987–996. [Google Scholar] [CrossRef]
- Mallant, M.; Hadithi, M.; Al-toma, A.; Kater, M.; Jacobs, M.; Manoliu, R.; Mulder, C.; van Waesberghe, J.H. Abdominal computed tomography in refractory coeliac disease and enteropathy associated T-cell lymphoma. World J. Gastroenterol. 2007, 13, 1696–1700. [Google Scholar] [CrossRef] [Green Version]
- Daum, S.; Wahnschaffe, U.; Glasenapp, R.; Borchert, M.; Ullrich, R.; Zeitz, M.; Faiss, S. Capsule endoscopy in refractory celiac disease. Endoscopy 2007, 39, 455–458. [Google Scholar] [CrossRef]
- Tack, G.J.; Verbeek, W.H.; Al-Toma, A.; Kuik, D.J.; Schreurs, M.W.J.; Visser, O.; Mulder, C.J.J. Evaluation of cladribine treatment in refractory celiac disease type II. World J. Gastroenterol. 2011, 17, 506–513. [Google Scholar] [CrossRef]
- Al-Toma, A.; Goerres, M.S.; Meijer, J.W.; von Blomberg, B.M.; Wahab, P.J.; Kerckhaert, J.A.; Mulder, C.J. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin. Gastroenterol. Hepatol. 2006, 4, 1322–1327. [Google Scholar] [CrossRef]
- Schmidt, C.; Kasim, E.; Schlake, W.; Gerken, G.; Giese, T.; Stallmach, A. TNF-alpha antibody treatment in refractory collagenous sprue: Report of a case and review of the literature. Z. Gastroenterol. 2009, 47, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Vivas, S.; Ruiz de Morales, J.M.; Ramos, F.; Suarez-Vilela, D. Alemtuzumab for refractory celiac disease in a patient at risk for enteropathy-associated T-cell lymphoma. N. Engl. J. Med. 2006, 354, 2514–2515. [Google Scholar] [CrossRef] [PubMed]
- Wahab, P.J.; Crusius, J.B.; Meijer, J.W.; Uil, J.J.; Mulder, C.J. Cyclosporin in the treatment of adults with refractory coeliac disease—An open pilot study. Aliment. Pharmacol. Ther. 2000, 14, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.J.; Wahab, P.J.; Meijer, J.W.; Metselaar, E. A pilot study of recombinant human interleukin-10 in adults with refractory coeliac disease. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Cellier, C.; Bouma, G.; van Gils, T.; Khater, S.; Malamut, G.; Crespo, L.; Collin, P.; Green, P.H.R.; Crowe, S.E.; Tsuji, W.; et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: A phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol. Hepatol. 2019, 4, 960–970. [Google Scholar] [CrossRef]
- Snowden, J.A.; Badoglio, M.; Labopin, M.; Giebel, S.; McGrath, E.; Marjanovic, Z.; Burman, J.; Moore, J.; Rovira, M.; Wulffraat, N.M.; et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune disease. Blood Adv. 2017, 1, 2742–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snowden, J.A.; Badoglio, M.; Tobias Alexander, T. The rise of autologous HCT for autoimmune diseases: What is behind it and what does it mean for the future of treatment? An update on behalf of the EBMT Autoimmune Diseases Working Party. Expert Rev. Clin. Immunol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Duarte, R.F.; Labopin, M.; Bader, P.; Basak, G.W.; Bonini, C.; Chabannon, C.; Corbacioglu, S.; Dreger, P.; Dufour, C.; Gennery, A.R.; et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2019. Bone Marrow Transplant. 2019, 54, 1525–1552. [Google Scholar] [CrossRef]
- Al-Toma, A.; Visser, O.J.; van Roessel, H.M.; von Blomberg, B.M.; Verbeek, W.H.; Scholten, P.E.; Ossenkoppele, G.J.; Huijgens, P.C.; Mulder, C.J. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood 2007, 109, 2243–2249. [Google Scholar] [CrossRef] [Green Version]
- Al-Toma, A.; Verbeek, W.H.; Visser, O.J.; Kuijpers, K.C.; Oudejans, J.J.; Kluin-Nelemans, H.C.; Mulder, C.J.; Huijgens, P.C. Disappointing outcome of autologous stem cell transplantation for enteropathy-associated T-cell lymphoma. Dig. Liver Dis. 2007, 39, 634–641. [Google Scholar] [CrossRef]
- Nijeboer, P.; van Wanrooij, R.L.J.; van Gils, T.; Wierdsma, N.J.; Tack, G.J.; Witte, B.I.; Bontkes, H.J.; Visser, O.; Mulder, C.; Bouma, G. Lymphoma development and survival in refractory coeliac disease type II: Histological response as prognostic factor. United Eur. Gastroenterol. J. 2017, 5, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Beurden, Y.H.; van Gils, T.; van Gils, N.A.; Kassam, Z.; Mulder, C.J.; Aparicio-Pages, N. Serendipity in refractory celiac disease: Full recovery of duodenal villi and clinical symptoms after fecal microbiota transfer. J. Gastrointestin. Liver Dis. 2016, 25, 385–388. [Google Scholar] [PubMed]
- Girbovan, A.; Sur, G.; Samasca, G.; Lupan, I. Dysbiosis a risk factor for celiac disease. Med. Microbiol. Immunol. 2017, 206, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Stepankova, R.; Tlaskalova-Hogenova, H.; Sinkora, J.; Jodl, J.; Fric, P. Changes in jejunal mucosa after long-term feeding of germfree rats with gluten. Scand. J. Gastroenterol. 1996, 31, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Donat, E.; Ribes-Koninckx, C.; Fernandez-Murga, M.L.; Sanz, Y. Duodenal-mucosal bacteria associated with celiac disease in children. Appl. Environ. Microbiol. 2013, 79, 5472–5479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, C.H.; Holm, T.L.; Krych, L.; Andresen, L.; Nielsen, D.S.; Rune, I.; Hansen, A.K.; Skov, S. Gut microbiota regulates NKG2D ligand expression on intestinal epithelial cells. Eur. J. Immunol. 2013, 43, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Schippa, S.; Iebba, V.; Barbato, M.; Di Nardo, G.; Totino, V.; Checchi, M.P.; Longhi, C.; Maiella, G.; Cucchiara, S.; Conte, M.P. A distinctive ‘microbial signature’ in celiac pediatric patients. BMC Microbiol. 2010, 10, 175. [Google Scholar] [CrossRef]
- Galipeau, H.J.; Verdu, E.F. Gut microbes and adverse food reactions: Focus on gluten related disorders. Gut Microbes 2014, 5, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Wacklin, P.; Kaukinen, K.; Tuovinen, E.; Collin, P.; Lindfors, K.; Partanen, J.; Mäki, M.; Mättö, J. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm. Bowel. Dis. 2013, 19, 934–941. [Google Scholar] [CrossRef] [Green Version]
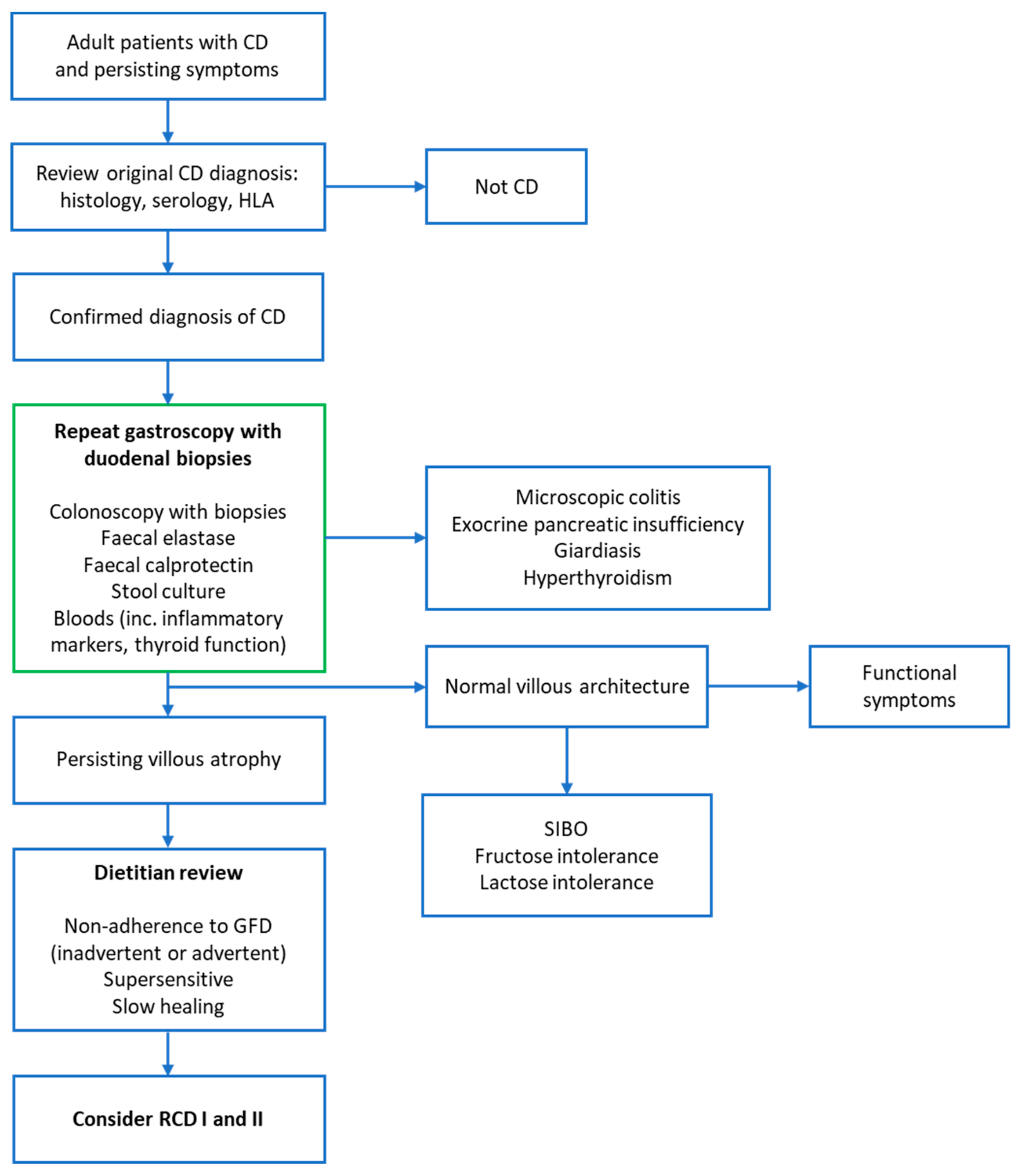
| Pancreatic insufficiency |
| Inflammatory bowel disease |
| Lactose and/or fructose intolerance |
| Small intestinal bacterial overgrowth |
| Microscopic colitis |
| Irritable bowel syndrome |
| Functional dysmotility |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penny, H.A.; Baggus, E.M.R.; Rej, A.; Snowden, J.A.; Sanders, D.S. Non-Responsive Coeliac Disease: A Comprehensive Review from the NHS England National Centre for Refractory Coeliac Disease. Nutrients 2020, 12, 216. https://doi.org/10.3390/nu12010216
Penny HA, Baggus EMR, Rej A, Snowden JA, Sanders DS. Non-Responsive Coeliac Disease: A Comprehensive Review from the NHS England National Centre for Refractory Coeliac Disease. Nutrients. 2020; 12(1):216. https://doi.org/10.3390/nu12010216
Chicago/Turabian StylePenny, Hugo A., Elisabeth M. R. Baggus, Anupam Rej, John A. Snowden, and David S. Sanders. 2020. "Non-Responsive Coeliac Disease: A Comprehensive Review from the NHS England National Centre for Refractory Coeliac Disease" Nutrients 12, no. 1: 216. https://doi.org/10.3390/nu12010216





