Pasta Consumption and Connected Dietary Habits: Associations with Glucose Control, Adiposity Measures, and Cardiovascular Risk Factors in People with Type 2 Diabetes—TOSCA.IT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Dietary Information
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Lifestyle Management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Standard Italiani per la Cura del Diabete Mellito 2018. Available online: https://aemmedi.it/wp-content/uploads/2009/06/AMD-Standard-unico1.pdf (accessed on 16 October 2019).
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.K.; Graves, D.E.; Craven, T.E.; Lipkin, E.W.; Austin, M.; Margolis, K.L. Restricted-carbohydrate diets in patients with type 2 diabetes: A meta-analysis. J. Am. Diet Assoc. 2008, 108, 91–100. [Google Scholar] [CrossRef] [PubMed]
- The World Pasta Industry in 2011. Available online: http://www.internationalpasta.org/resources/extra/file/IPO%20AGM%202012/IPOreport2012fin2stat.pdf (accessed on 16 October 2019).
- Scazzina, F.; Dall’Asta, M.; Casiraghi, M.C.; Sieri, S.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Glycemic index and glycemic load of commercial Italian foods. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Kendall, C.W.C.; Braunstein, C.R.; Blanco Mejia, S.; Leiter, L.A.; Jenkins, D.J.A.; Sievenpiper, J.L. Effect of pasta in the context of low-glycaemic index dietary patterns on body weight and markers of adiposity: A systematic review and meta-analysis of randomised controlled trials in adults. BMJ Open 2018, 8, e019438. [Google Scholar] [CrossRef] [PubMed]
- Pounis, G.; Castelnuovo, A.D.; Costanzo, S.; Persichillo, M.; Bonaccio, M.; Bonanni, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Association of pasta consumption with body mass index and waist-to-hip ratio: Results from Moli-sani and INHES studies. Nutr. Diabetes 2016, 6, e218. [Google Scholar] [CrossRef] [PubMed]
- Shay, C.M.; Van Horn, L.; Stamler, J.; Dyer, A.R.; Brown, I.J.; Chan, Q.; Miura, K.; Zhao, L.; Okuda, N.; Daviglus, M.L.; et al. Food and nutrient intakes and their associations with lower BMI in middle-aged US adults: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am. J. Clin Nutr. 2012, 96, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Huang, M.; Li, J.; Ha, M.A.; Riccardi, G.; Liu, S. A systematic review on the relations between pasta consumption and cardio-metabolic risk factors. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Masulli, M.; Cocozza, S.; Anichini, R.; Babini, A.C.; Boemi, M.; Bonora, E.; Buzzetti, R.; Carpinteri, R.; Caselli, C.; et al. Sex differences in food choices, adherence to dietary recommendations and plasma lipid profile in type 2 diabetes—The TOSCA.IT study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Rivellese, A.A.; Boemi, M.; Cavalot, F.; Costagliola, L.; De Feo, P.; Miccoli, R.; Patti, L.; Trovati, M.; Vaccaro, O.; Zavaroni, I.; et al. Dietary habits in type II diabetes mellitus: How is adherence to dietary recommendations? Eur. J. Clin Nutr. 2008, 62, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, O.; Masulli, M.; Nicolucci, A.; Bonora, E.; Del Prato, S.; Maggioni, A.P.; Rivellese, A.A.; Squatrito, S.; Giorda, C.B.; Sesti, G.; et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): A randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017, 5, 887–897. [Google Scholar] [CrossRef]
- Vaccaro, O.; Masulli, M.; Bonora, E.; Del Prato, S.; Giorda, C.B.; Maggioni, A.P.; Mocarelli, P.; Nicolucci, A.; Rivellese, A.A.; Squatrito, S.; et al. Addition of either pioglitazone or a sulfonylurea in type 2 diabetic patients inadequately controlled with metformin alone: Impact on cardiovascular events. A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori 2003, 89, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S152–S160. [Google Scholar] [CrossRef]
- Salvini, S.; Parpinel, M.; Gnagnarella, P.; Maisonneuve, P.; Turrini, A. Banca Dati di Composizione Degli Alimenti per Studi Epidemiologici in Italia; Istituto Europeo di Oncologia: Milan, Italy, 1998. [Google Scholar]
- Carnovale, E.; Marletta, L. Tabella di Composizione degli Alimenti; Crea-Nut (ex INRAN): Rome, Italy, 2000. [Google Scholar]
- Vitale, M.; Masulli, M.; Rivellese, A.A.; Babini, A.C.; Boemi, M.; Bonora, E.; Buzzetti, R.; Ciano, O.; Cignarelli, M.; Cigolini, M.; et al. Influence of dietary fat and carbohydrates proportions on plasma lipids, glucose control and low-grade inflammation in patients with type 2 diabetes-The TOSCA.IT Study. Eur. J. Nutr. 2016, 55, 1645–1651. [Google Scholar] [CrossRef]
- Augustin, L.S.; Kendall, C.W.; Jenkins, D.J.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Giacco, R.; Brighenti, F.; Parillo, M.; Capuano, M.; Ciardullo, A.V.; Rivieccio, A.; Rivellese, A.A.; Riccardi, G. Characteristics of some wheat-based foods of the Italian diet in relation to their influence on postprandial glucose metabolism in patients with type 2 diabetes. Br. J. Nutr. 2001, 85, 33–40. [Google Scholar] [CrossRef][Green Version]
- Kristensen, M.; Jensen, M.G.; Riboldi, G.; Petronio, M.; Bügel, S.; Toubro, S.; Tetens, I.; Astrup, A. Wholegrain vs. refined wheat bread and pasta. Effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite 2010, 54, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gæde, P.; Oellgaard, J.; Carstensen, B.; Rossing, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016, 59, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, N.; Vinknes, K.J.; Veierød, M.B.; Retterstøl, K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Smith, D.A.; Mehta, A.E.; Ganda, O.; Handelsman, Y.; Rodbard, H.W.; Shepherd, M.D.; Seibel, J.A. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr. Pract. 2012, 18 (Suppl. 1), 1–78. [Google Scholar] [CrossRef]
- Byun, S.S.; Mayat, Z.K.; Aggarwal, B.; Parekh, N.; Makarem, N. Quantity, Quality, and Timing of Carbohydrate Intake and Blood Pressure. Curr. Nutr. Rep. 2019, 8, 270–280. [Google Scholar] [CrossRef]
- Piccinelli, R.; Arcella, D.; Buonocore, P.; Capriotti, M.; D’Addezio, L.; Le Donne, C.; Mistura, L.; Pettinelli, A.; Sette, S.; Turrini, A.; et al. L’Indagine Nazionale sui Consumi Alimentari in Italia INRAN-SCAI 2005–2006. I Consumi Alimentari in Termini di Alimenti. Osservatorio Consumi Alimentari. INRAN: Rome, Italy, 2011. [Google Scholar]
- Fulgoni, V.L.; Bailey, R. Association of Pasta Consumption with Diet Quality and Nutrients of Public Health Concern in Adults: National Health and Nutrition Examination Survey 2009–2012. Curr. Dev. Nutr. 2017, 1, e001271. [Google Scholar] [CrossRef]
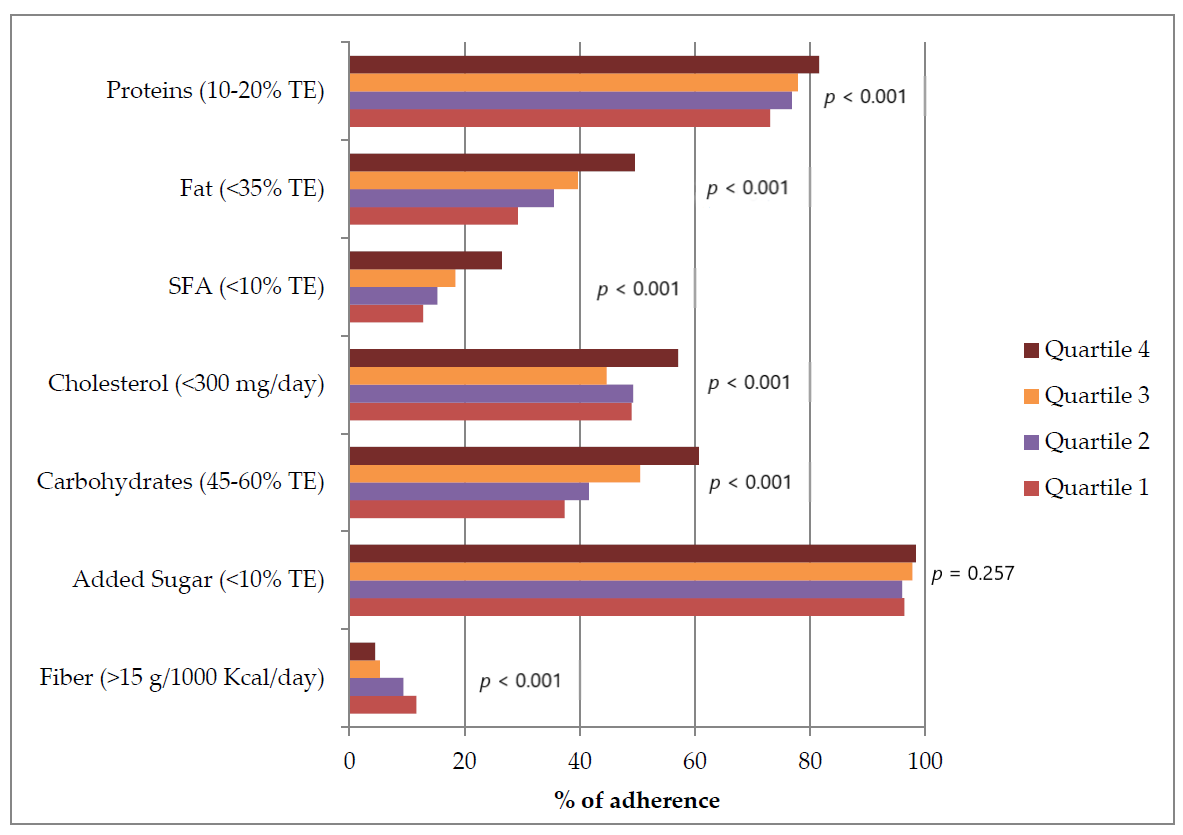
| Quartile 1 (n = 640) | Quartile 2 (n = 641) | Quartile 3 (n = 641) | Quartile 4 (n = 640) | p-Value | |
|---|---|---|---|---|---|
| Pasta consumption (g/1000 Kcal) | 7.5 ± 4.4 (0.0–15.8) | 19.4 ± 4.2 (15.9–27.4) | 30.8 ± 5.0 (27.5–41.1) | 52.4 ± 14.0 (41.2–120.0) | |
| Age (years) | 62.0 ± 6.5 | 62.3 ± 6.5 | 62.0 ± 6.5 | 62.1 ± 6.4 | 0.842 |
| Diabetes Duration (years) | 8.5 ± 5.8 | 8.3 ± 5.4 | 8.7 ± 6.0 | 8.5 ± 5.6 | 0.658 |
| Smoking status (%) a | |||||
| Current Smokers | 125 (19.5) | 114 (17.8) | 97 (15.1) | 106 (16.6) | 0.339 |
| Educational status (%) | |||||
| None | 194 (30.4) | 197 (30.7) | 180 (28.1) | 218 (34.1) | 0.204 |
| Primary | 226 (35.4) | 248 (38.6) | 250 (39.0) | 231 (36.1) | |
| Secondary | 185 (29.0) | 160 (24.9) | 174 (27.1) | 169 (26.4) | |
| University | 34 (5.3) | 36 (5.8) | 37 (5.8) | 22 (3.4) | |
| Geographical area (%) | |||||
| North | 224 (34.9) | 216 (33.8) | 218 (34.0) | 233 (36.4) | 0.681 |
| Center | 156 (24.6) | 182 (28.3) | 176 (27.5) | 159 (24.8) | |
| South | 260 (40.5) | 243 (37.9) | 247 (38.5) | 248 (38.8) | |
| Quartile 1 (n = 640) | Quartile 2 (n = 641) | Quartile 3 (n = 641) | Quartile 4 (n = 640) | p for Anova | p for Trend | |
|---|---|---|---|---|---|---|
| Pasta (g/1000 Kcal) | 7.5 ± 4.4 (0.0–15.8) | 19.4 ± 4.2 (15.9–27.4) | 30.8 ± 5.0 (27.5–41.1) | 52.4 ± 14.0 (41.2–120.0) | ||
| Energy (Kcal) | 1841 ± 820 | 1962 ± 881 | 1941 ± 683 | 1852 ± 673 | 0.067 | 0.519 |
| Protein (% of TE) | 18.6 ± 2.8 | 18.3 ± 2.5 | 18.3 ± 2.4 | 17.9 ± 2.5 | <0.0001 | <0.0001 |
| Total Fat (% of TE) | 38.3 ± 6.4 | 37.1 ± 6.1 | 36.3 ± 5.8 | 35.1 ± 5.7 | <0.0001 | <0.0001 |
| SFA (% of TE) | 12.8 ± 2.7 | 12.4 ± 2.5 | 12.1 ± 2.4 | 11.4 ± 2.3 | <0.0001 | <0.0001 |
| MUFA (% of TE) | 18.4 ± 4.1 | 18.0 ± 3.9 | 17.8 ± 3.6 | 17.5 ± 3.7 | 0.002 | <0.0001 |
| PUFA (% of TE) | 4.7 ± 1.3 | 4.5 ± 1.0 | 4.3 ± 1.0 | 4.2 ± 1.0 | <0.0001 | <0.0001 |
| Cholesterol (mg/1000 Kcal) | 191 ± 54 | 183 ± 54 | 180 ± 50 | 172 ± 49 | <0.0001 | <0.0001 |
| Carbohydrates (% of TE) | 43.1 ± 7.7 | 44.6 ± 7.5 | 45.4 ± 7.0 | 46.9 ± 6.8 | <0.0001 | <0.0001 |
| Added Sugar (% of TE) | 2.7 ± 3.7 | 2.4 ± 3.3 | 2.3 ± 3.2 | 1.9 ± 2.5 | <0.0001 | <0.0001 |
| Fiber (g/1000 Kcal) | 11.5 ± 3.0 | 11.0 ± 2.9 | 10.5 ± 2.6 | 10.1 ± 2.4 | <0.0001 | <0.0001 |
| Glycemic index (%) | 53.0 ± 3.6 | 52.4 ± 3.2 | 51.5 ± 2.9 | 49.7 ± 2.9 | <0.0001 | <0.0001 |
| Glycemic load | 111 ± 64 | 118 ± 65 | 119 ± 50 | 111 ± 46 | 0.078 | 0.341 |
| Alcohol (g/day) | 10.7 ± 15.9 | 12.2 ± 18.4 | 11.2 ± 14.6 | 9.1 ± 13.8 | 0.046 | 0.023 |
| Quartile 1 (n = 640) | Quartile 2 (n = 641) | Quartile 3 (n = 641) | Quartile 4 (n = 640) | p for Anova or χ2 | p for Trend | |
|---|---|---|---|---|---|---|
| Pasta (g/1000 Kcal) | 7.5 ± 4.4 (0.0–15.8) | 19.4 ± 4.2 (15.9–27.4) | 30.8 ± 5.0 (27.5–41.1) | 52.4 ± 14.0 (41.2–120.0) | ||
| BMI (Kg/m2) | 30.1 ± 4.4 | 30.5 ± 4.5 | 30.2 ± 4.5 | 30.4 ± 4.4 | 0.362 | 0.569 |
| BMI ≥30 Kg/m2 (%) | 48.6 | 49.5 | 48.0 | 49.7 | 0.926 | |
| Waist Circumference (cm) | 103.9 ± 11.2 | 104.8 ± 11.1 | 104.7 ± 11.2 | 104.4 ± 11.3 | 0.497 | 0.524 |
| Waist-to-hip ratio | 0.98 ± 0.07 | 0.98 ± 0.06 | 0.98 ± 0.07 | 0.98 ± 0.07 | 0.545 | 0.154 |
| With Abdominal obesity * (%) | 71.9 | 74.6 | 75.0 | 74.1 | 0.589 | |
| HbA1c (%) | 7.67 ± 0.51 | 7.70 ± 0.50 | 7.67 ± 0.51 | 7.68 ± 0.51 | 0.707 | 0.915 |
| Proportion with HbA1c <7.5% | 26.0 | 23.7 | 25.8 | 24.4 | 0.445 | |
| LDL-cholesterol (mg/dl) | 104.7 ± 32.3 | 102.3 ± 31.5 | 102.2 ± 31.6 | 102.0 ± 30.3 | 0.364 | 0.142 |
| HDL-cholesterol (mg/dl) | 47.8 ± 12.5 | 45.5 ± 11.8 | 46.1 ± 11.9 | 44.9 ± 11.3 | <0.0001 | <0.0001 |
| LDL/HDL Ratio | 2.32 ± 0.90 | 2.36 ± 0.90 | 2.33 ± 0.90 | 2.39 ± 0.92 | 0.571 | 0.271 |
| Triglycerides (mg/dl) | 145.7 ± 71.5 | 153.4 ± 80.2 | 148.7 ± 72.6 | 155.2 ± 75.4 | 0.095 | 0.072 |
| Non-HDL Cholesterol (mg/dl) | 133.8 ± 36.0 | 133.3 ± 36.3 | 132.9 ± 37.9 | 133.2 ± 35.0 | 0.980 | 0.963 |
| Systolic blood pressure (mm/Hg) | 132.5 ± 14.3 | 133.7 ± 13.9 | 135.2 ± 15.2 | 133.8 ± 15.0 | 0.034 | 0.012 |
| Diastolic blood pressure (mm/Hg) | 78.9 ± 8.8 | 79.8 ± 8.6 | 80.1 ± 8.3 | 79.4 ± 8.4 | 0.103 | 0.300 |
| On lipid lowering medications (%) | 59.7 | 66.1 | 62.8 | 59.9 | 0.080 | |
| On antihypertensive medications (%) | 93.6 | 90.3 | 94.1 | 92.0 | 0.104 |
| Standardized ß-Coefficient | Standard Error | p-Value | |
|---|---|---|---|
| Dependent variable HDL-cholesterol | |||
| Intake of pasta (g/1000 Kcal) | −0.057 | 0.007 | 0.003 |
| Gender (female/male) | 0.330 | 0.535 | 0.000 |
| BMI (Kg/m2) | −0.170 | 0.050 | 0.000 |
| Age (years) | 0.093 | 0.034 | 0.000 |
| Fiber intake (g/1000 Kcal) | 0.004 | 0.032 | 0.836 |
| Alcohol (g/day) | 0.171 | 0.015 | 0.000 |
| Smoking (yes/no) | −0.018 | 0.266 | 0.370 |
| Dependent variable Systolic Blood Pressure | |||
| Intake of pasta (g/1000 Kcal) | 0.031 | 0.010 | 0.133 |
| Gender (female/ male) | −0.026 | 0.833 | 0.261 |
| BMI (Kg/m2) | 0.108 | 0.078 | 0.000 |
| Age (years) | 0.126 | 0.054 | 0.000 |
| Fiber intake (g/1000 Kcal) | −0.045 | 0.049 | 0.024 |
| Alcohol (g/day) | 0.001 | 0.023 | 0.970 |
| Smoking (yes/no) | 0.044 | 0.415 | 0.040 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, M.; Masulli, M.; Rivellese, A.A.; Bonora, E.; Babini, A.C.; Sartore, G.; Corsi, L.; Buzzetti, R.; Citro, G.; Baldassarre, M.P.A.; et al. Pasta Consumption and Connected Dietary Habits: Associations with Glucose Control, Adiposity Measures, and Cardiovascular Risk Factors in People with Type 2 Diabetes—TOSCA.IT Study. Nutrients 2020, 12, 101. https://doi.org/10.3390/nu12010101
Vitale M, Masulli M, Rivellese AA, Bonora E, Babini AC, Sartore G, Corsi L, Buzzetti R, Citro G, Baldassarre MPA, et al. Pasta Consumption and Connected Dietary Habits: Associations with Glucose Control, Adiposity Measures, and Cardiovascular Risk Factors in People with Type 2 Diabetes—TOSCA.IT Study. Nutrients. 2020; 12(1):101. https://doi.org/10.3390/nu12010101
Chicago/Turabian StyleVitale, Marilena, Maria Masulli, Angela Albarosa Rivellese, Enzo Bonora, Anna Carla Babini, Giovanni Sartore, Laura Corsi, Raffaella Buzzetti, Giuseppe Citro, Maria Pompea Antonia Baldassarre, and et al. 2020. "Pasta Consumption and Connected Dietary Habits: Associations with Glucose Control, Adiposity Measures, and Cardiovascular Risk Factors in People with Type 2 Diabetes—TOSCA.IT Study" Nutrients 12, no. 1: 101. https://doi.org/10.3390/nu12010101
APA StyleVitale, M., Masulli, M., Rivellese, A. A., Bonora, E., Babini, A. C., Sartore, G., Corsi, L., Buzzetti, R., Citro, G., Baldassarre, M. P. A., Bossi, A. C., Giordano, C., Auciello, S., Dall’Aglio, E., Iannarelli, R., Tonutti, L., Sacco, M., Di Cianni, G., Clemente, G., ... Vaccaro, O. (2020). Pasta Consumption and Connected Dietary Habits: Associations with Glucose Control, Adiposity Measures, and Cardiovascular Risk Factors in People with Type 2 Diabetes—TOSCA.IT Study. Nutrients, 12(1), 101. https://doi.org/10.3390/nu12010101







