Intra-Abdominal Pressure as a Marker of Enteral Nutrition Intolerance in Critically Ill Patients. The PIANE Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Variables
2.2. Statistical Methods
Ethics Approval and Consent to Participate
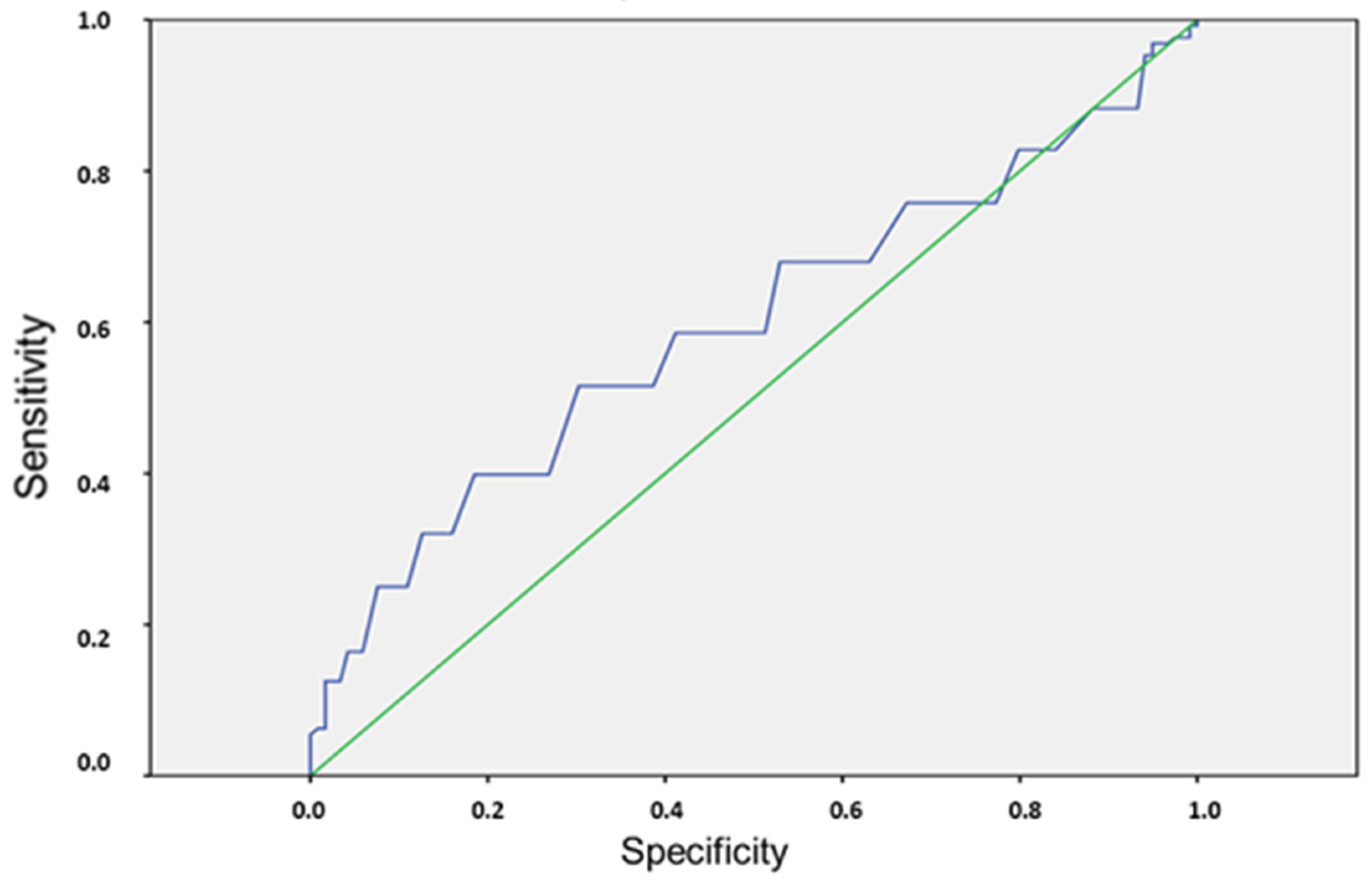
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Abdominal compartmental Syndrome |
| APACHE II | Acute Physiologic and chronic health evaluation II |
| ARDS | Acute Respiratory Distress Syndrome |
| EN | enteral nutrition |
| GI | gastrointestinal |
| GRV | gastric residual volume |
| IAP | intraabdominal pressure |
| ICU | Intensive Care Unit |
| SOFA | Sequential Organ Failure Assessment |
References
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, C.D.; Ioannidis, O.; Gkiomisi, A.I.; Botsios, D. Artificial nutrition and intestinal mucosal barrier functionality. Digestion 2013, 88, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wen, J.; Xu, L.; Zhou, S.; Gong, M.; Wen, P.; Xiao, X. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J. Surg. Res. 2013, 183, 592–597. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Martindale, R.G.; Rice, T.W.; Heyland, D.K. Feeding the critically ill patient. Crit. Care Med. 2014, 42, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Heighes, P.T.; Simpson, F.; Sweetman, E.A.; Davies, A.R. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: A meta-analysis of randomised controlled trials. Intensive Care Med. 2009, 35, 2018–2027. [Google Scholar] [CrossRef]
- Montejo, J.C. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Enteral nutrition-related gastrointestinal complications in critically ill patients: A multicenter study. Crit. Care Med. 1999, 27, 1447–1453. [Google Scholar] [CrossRef]
- Mentec, H.; Dupont, H.; Bocchetti, M.; Cani, P.; Ponche, F.; Bleichner, G. Upper digestive intolerance during enteral nutrition in critically ill patients: Frequency, risk factors, and complications. Crit. Care Med. 2001, 29, 1955–1961. [Google Scholar] [CrossRef]
- Montejo, J.C.; Miñambres, E.; Bordejé, L.; Mesejo, A.; Acosta, J.; Heras, A.; Ferré, M.; Fernández-Ortega, F.; Vaquerizo, C.I.; Manazanedo, R. Gastric residual volume during enteral nutrition in ICU patients: The REGANE study. Intensive Care Med. 2010, 36, 1386–1393. [Google Scholar] [CrossRef]
- Hurt, R.T.; McClave, S.A. Gastric residual volumes in critical illness: What do they really mean? Crit. Care Clin. 2010, 26, 481–490. [Google Scholar] [CrossRef]
- Reignier, J.; Mercier, E.; Le Gouge, A.; Boulin, T.; Desachy, A.; Bellec, F.; Clavel, M.; Frat, J.P.; Plantefeve, G.; Quenot, J.P.; et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: A randomized controlled trial. JAMA 2013, 309, 249–256. [Google Scholar] [CrossRef]
- Malbrain, M.L. Different techniques to measure intra-abdominal pressure (IAP): Time for a critical re-appraisal. Intensive Care Med. 2004, 30, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Cheatham, M.L.; Kirkpatrick, A.; Sugrue, M.; Parr, M.; De Waele, J.; Leppäniemi, A.; Olvera, C.; Ivatury, R.; D’Amours, S.; et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006, 32, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, M. Intra-abdominal pressure: Time for clinical practice guidelines? Intensive Care Med. 2002, 28, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Pal, K.M.; Jajja, M.R.; Nawaz, Z.; Koondhar, R.; Nasim, S. Intra abdominal hypertension; incidence, prevalence and outcomes in a mixed intensive care unit: Prospective cohort study. Int. J. Surg. 2015, 19, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.J.; Lumsdaine, W.; Moore, E.E.; Moore, F.A. Postinjury abdominal compartment syndrome: From recognition to prevention. Lancet 2014, 384, 1466–1475. [Google Scholar] [CrossRef]
- Rastogi, P.; Iyer, D.; Aneman, A.; D’Amours, S. Intra-abdominal hypertension and abdominal compartment syndrome: Pathophysiological and non-operative management. Minerva Anestesiol. 2014, 80, 922–932. [Google Scholar]
- Sánchez-Miralles, A.; Castellanos, G.; Badenes, R.; Conejero, R. Abdominal compartment syndrome and acute intestinal distress syndrome. Med. Intensiva 2011, 37, 99–109. [Google Scholar] [CrossRef]
- Schein, M.; Wittmann, D.H.; Aprahamian, C.C.; Condon, R.E. The abdominal compartment syndrome: The physiological and clinical consequences of elevated intra-abdominal pressure. J. Am. Coll. Surg. 1996, 180, 745–753. [Google Scholar]
- Sugrue, M. Abdominal compartment syndrome. Curr. Opin. Crit. Care 2005, 11, 333–338. [Google Scholar] [CrossRef]
- Ivatury, R.; Diebel, L. Intra-Abdominal Hypertension and the Splanchnic Bed; Ivatury, R., Cheatham, M.L., Malbrain, M.L., Sugrue, M., Eds.; Abdominal Compartment Syndrome; Landes Bioscience: Austin, TX, USA, 2006; pp. 129–137. [Google Scholar]
- Raeburn, C.D.; Moore, E.E. Abdominal Compartment Syndrome Provokes Multiple Organ Failure: Animal and Human Supporting Evidence; Ivatury, R., Cheatham, M.L., Malbrain, M.L., Sugrue, M., Eds.; Abdominal Compartment Syndrome; Landes Bioscience: Austin, TX, USA, 2006; pp. 157–169. [Google Scholar]
- Diebel, L.; Dulchavsky, S.; Wilson, R.F. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J. Trauma 1992, 33, 45–48. [Google Scholar] [CrossRef]
- Reintam, A.; Parm, P.; Kitus, R.; Starkopf, J.; Kern, H. Gastrointestinal failure score in critically ill patients: A prospective observational study. Crit. Care 2008, 12, R90. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Oddo, M.; Lavanchy, J.; Longchamp, C.; Delodder, F.; Schaller, M.D. Gastrointestinal failure score in critically ill patients. Crit. Care 2008, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Bonet Saris, A.; Márquez-Vácaro, J.A.; Serón, C.; Metabolism and Nutrition Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Guidelines for specialized nutritional and metabolic support in the critically-ill patient: Update. Consensus SEMICYUC-SENPE: Macronutrient and micronutrient requirements. Nutr. Hosp. 2011, 26 (Suppl. 2), 16–20. [Google Scholar]
- Horan, T.C.; Andrus, M.; Dudeck, MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Reintam, A.; Parm, P.; Kitus, R.; Starkopf, J. Intra-Abdominal Hypertension and Gastrointestinal Symptoms in Mechanically Ventilated Patients. Crit. Care Res. Pract. 2011, 2011, 982507. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.W.; Roberts, D.J.; De Waele, J.; Jaeschke, R.; Malbrain, M.L.; De Keulenaer, B.; Duchesne, J.; Bjorck, M.; Leppaniemi, A.; Ejicke, J.C.; et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013, 39, 1190–1206. [Google Scholar] [CrossRef]
- Reintam, A.; Malbrain, M.L.; Starkopf, J.; Fruhwald, J.; Jakob, S.M.; De Waele, J.; Braun, J.P.; Poeze, M.; Spies, C. Gastrointestinal function in intensive care patients: Terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012, 38, 384–394. [Google Scholar] [CrossRef]
- Bejarano, N.; Navarro, S.; Rebasa, P.; García-Esquirol, O.; Hermoso, J. Intra-abdominal pressure as a prognostic factor for tolerance of enteral nutrition in critical patients. JPEN J. Parenter. Enteral Nutr. 2013, 37, 352–360. [Google Scholar] [CrossRef]
- Hill, L.T.; Hill, B.; Miller, M.; Michell, W.L. The effect of intra-abdominal hypertension on gastro-intestinal function. S. Afr. J. Crit. Care 2011, 27, 12–19. [Google Scholar]
- Vidal, M.G.; Ruiz, J.; Gonzalez, F.; Toro, M.A.; Loudet, C.; Balasini, C.; Canales, H.; Reina, R.; Estenssoro, E. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit. Care Med. 2008, 36, 1823–1831. [Google Scholar] [CrossRef]
- Holodinsky, J.K.; Roberts, D.J.; Ball, C.G.; Blaser, A.R.; Starkopf, J.; Zygun, D.A.; Stelfox, H.T.; Malbrain, M.L.; Jaeschke, R.C.; Kirkpatrick, A.W. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: A systematic review and meta-analysis. Crit. Care 2013, 17, R249. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Chiumello, D.; Cesana, B.M.; Blaser, A.R.; Starkopf, J.; Sugrue, M.; Pelosi, P.; Severgnini, P.; Hernandez, G.; Brienza, N.; et al. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: The wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol. 2014, 80, 293–306. [Google Scholar] [PubMed]
- Reintam, A.; Poeze, M.; Malbrain, M.; Björck, M.; Oudermans-van Straaten, H.; Starkopf, J. Gastro-intestinal Failure Trial Group. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: A prospective multicentre study. Intensive Care Med. 2013, 39, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Van Stappen, J.; Pigozzi, C.; Tepaske, R.; Van Regenmortel, N.; De Laet, I.; Schoonheyydt, K.; Dits, H.; Severdnini, P.; Roberts, D.J.; Malbrain, M.L. Validation of a novel method for measuring intra-abdominal pressure and gastric residual volume in critically ill patients. Anaesthesiol. Intensive Ther. 2014, 46, 245–254. [Google Scholar] [CrossRef] [Green Version]

| Overall | GROUP A (n = 119) | GROUP B (n = 128) | p | |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 62.0 ± 14.7 | 62.5 ± 15.6 | 61.6 ± 13.9 | 0.64 |
| Sex distribution (%): | 0.37 | |||
| Males | 63.6% | 66.4% | 60.9% | |
| Females | 36.4% | 33.6% | 39.1% | |
| Admission diagnosis (% of patients): | 0.42 | |||
| Medical | 79.8% | 83.1% | 76.5% | |
| Surgical | 5.7% | 5.0% | 6.3% | |
| Trauma | 8.9% | 5.9% | 11.8% | |
| APACHE II (first 24 h) (mean ± SD) | 21.4 ± 7.8 | 22.1 ± 8.6 | 20.8 ± 6.8 | 0.187 |
| SOFA on admission (mean ± SD) | 7.5 ± 3.2 | 7.6 ± 3.0 | 7.5 ± 3.5 | 0.78 |
| Admission to EN (hours) (mean ± SD) | 30.6 ± 23.5 | 30.2 ± 23.0 | 30.9 ± 24.1 | 0.82 |
| median (P25; 75) | 24 (3; 99) | 23 (3; 96) | 24 (4; 99) | |
| EN volume administered (mL/day) | ||||
| (mean ± SD) | 1107.6 ± 396.1 | 1062.4 ± 375.8 | 1149.6 ± 411.1 | 0.08 |
| EN volume ratio * (mean ± SD) | 86.9 ± 22.2% | 88.6 ± 20.6% | 86.1 ± 22.8% | 0.009 |
| Transition to oral diet (% of patients) | 42.5% | 52.9% | 32.8% | <0.002 |
| EN days (mean ± SD) | 13.3 ± 12.5 | 8.1 ± 8.4 | 18.1 ± 13.7 | <0.001 |
| Mechanical ventilation days | ||||
| (mean ± SD) | 13.8 ± 13.2 | 8.0 ± 7.7 | 19.3 ± 14.9 | <0.001 |
| ICU days (mean ± SD) | 18.8 ± 16.1 | 12.3 ± 11.4 | 24.8 ± 17.5 | <0.001 |
| ICU death | 52 (21.1%) | 24 (20.2%) | 28 (22.0%) | 0.757 |
| Complication (%) | n | All Patients (n = 247) | GROUP B (n = 128) |
|---|---|---|---|
| Diarrhea | 47 | 19.0% | 36.7% |
| Constipation | 43 | 17.4% | 33.6% |
| High gastric residual volume | 40 | 16.2% | 31.25% |
| Abdominal distension | 28 | 11.3% | 21.8% |
| Vomiting | 24 | 9.7% | 18.7% |
| Diet regurgitation | 16 | 6.5% | 12.5% |
| Aspiration | 2 | 0.8% | 1.5% |
| Overall | GROUP A (n = 119) | GROUP B (n = 128) | P | ||
|---|---|---|---|---|---|
| Daily IAP | Mean ± SD | 14.8 ± 4 | 14.8 ± 3.7 | 14.8 ± 4.1 | 0.801 |
| Maximum daily IAP | Mean ± SD | 18.1 ± 4.6 | 16.8 ± 4 | 19.4 ± 4.8 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordejé, M.L.; Montejo, J.C.; Mateu, M.L.; Solera, M.; Acosta, J.A.; Juan, M.; García-Córdoba, F.; García-Martínez, M.A.; Gastaldo, R.; PIANE STUDY GROUP SPAIN. Intra-Abdominal Pressure as a Marker of Enteral Nutrition Intolerance in Critically Ill Patients. The PIANE Study. Nutrients 2019, 11, 2616. https://doi.org/10.3390/nu11112616
Bordejé ML, Montejo JC, Mateu ML, Solera M, Acosta JA, Juan M, García-Córdoba F, García-Martínez MA, Gastaldo R, PIANE STUDY GROUP SPAIN. Intra-Abdominal Pressure as a Marker of Enteral Nutrition Intolerance in Critically Ill Patients. The PIANE Study. Nutrients. 2019; 11(11):2616. https://doi.org/10.3390/nu11112616
Chicago/Turabian StyleBordejé, M Luisa, Juan C. Montejo, M Lidón Mateu, Manuel Solera, Jose A. Acosta, Mar Juan, Francisco García-Córdoba, Miguel A. García-Martínez, Rosa Gastaldo, and PIANE STUDY GROUP SPAIN. 2019. "Intra-Abdominal Pressure as a Marker of Enteral Nutrition Intolerance in Critically Ill Patients. The PIANE Study" Nutrients 11, no. 11: 2616. https://doi.org/10.3390/nu11112616





