Pleiotropic Effects of Modified Citrus Pectin
Abstract
:1. Introduction
2. Galectin-3
3. Cancer
4. Fibrotic Diseases
4.1. Aortic Stenosis
4.2. Additional Cardiovascular Effects
4.3. Kidney
4.4. Additional Fibrotic Diseases
5. Detoxification
6. Immune Function
7. Other Galectin-3 Inhibitors
8. Possible New Areas for MCP Research
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-cancer activities of pH-or heat-modified pectin. Front. Pharm. 2013, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Refai, L. Plant pectin: A potential source for cancer suppression. Am. J. Pharm. Toxicol. 2013, 8, 9–19. [Google Scholar] [CrossRef]
- Courts, F.L. Profiling of modified citrus pectin oligosaccharide transport across Caco-2 cell monolayers. PharmaNutrition 2013, 1, 22–31. [Google Scholar] [CrossRef]
- Ried, K.; Eng, P.; Sali, A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effectiveness: An observational study. Asian Pac. J. Cancer Prev. 2017, 18, 2275–2285. [Google Scholar] [CrossRef]
- Azémar, M.; Hildenbrand, B.; Haering, B.; Heim, M.E.; Unger, C. Clinical benefit in patients with advanced solid tumors treated with modified citrus pectin: A prospective pilot study. Clin. Med. Oncol. 2007, 1, 73–80. [Google Scholar] [CrossRef]
- Guess, B.; Scholz, M.; Strum, S.; Lam, R.; Johnson, H.; Jennrich, R. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: A phase II pilot study. Prostate Cancer Prostatic Dis. 2003, 6, 301–304. [Google Scholar] [CrossRef]
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, S.; Li, J.-C.; Feng, J.-X. Galectin 3 inhibition attenuates renal injury progression in cisplatin-induced nephrotoxicity. Biosci. Rep. 2018, 38, BSR20181803. [Google Scholar] [CrossRef]
- Wu, K.-L.; Kuo, C.-M.; Huang, E.-Y.; Pan, H.-M.; Huang, C.-C.; Chen, Y.-F.; Hsiao, C.-C.; Yang, K.D. Extracellular galectin-3 facilitates colon cancer cell migration and is related to the epidermal growth factor receptor. Am. J. Transl. Res. 2018, 10, 2402–2412. [Google Scholar]
- Conti, S.; Vexler, A.; Hagoel, L.; Kalich-Philosoph, L.; Corn, B.W.; Honig, N.; Shtraus, N.; Meir, Y.; Ron, I.; Eliaz, I. Modified citrus pectin as a potential sensitizer for radiotherapy in prostate cancer. Integr. Cancer 2018, 17, 1225–1234. [Google Scholar] [CrossRef]
- Fang, T.; Liu, D.-D.; Ning, H.-M.; Liu, D.; Sun, J.-Y.; Huang, X.-J.; Dong, Y.; Geng, M.-Y.; Yun, S.-F.; Yan, J. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharm. Sin. 2018, 39, 1885–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Li, P.; Lu, S.-M.; Ling, Z.-Q. Chemoprevention of low-molecular-weight citrus pectin (LCP) in gastrointestinal cancer cells. Int. J. Biol. Sci. 2016, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Mellem, J.; Naicker, T.; Reddy, L. Chemoprevention of azoxymethane-induced colonic carcinogenesis in Balb/c mice using a modified pectin alginate probiotic. Anticancer Res. 2015, 35, 4765–4775. [Google Scholar] [PubMed]
- Jiang, J.; Eliaz, I.; Sliva, D. Synergistic and additive effects of modified citrus pectin with two polybotanical compounds, in the suppression of invasive behavior of human breast and prostate cancer cells. Integr. Cancer 2013, 12, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hossein, G.; Keshavarz, M.; Ahmadi, S.; Naderi, N. Synergistic effects of PectaSol-C modified citrus pectin an inhibitor of Galectin-3 and paclitaxel on apoptosis of human SKOV-3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 7561–7568. [Google Scholar] [CrossRef]
- Tehranian, N.; Sepehri, H.; Mehdipour, P.; Biramijamal, F.; Hossein-Nezhad, A.; Sarrafnejad, A.; Hajizadeh, E. Combination effect of PectaSol and Doxorubicin on viability, cell cycle arrest and apoptosis in DU-145 and LNCaP prostate cancer cell lines. Cell Biol. Int. 2012, 36, 601–610. [Google Scholar] [CrossRef]
- Yan, J.; Katz, A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and-independent prostate cancer cells. Integr. Cancer 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; He, X.; Fan, L.; Yang, G.; Chen, X.; Lin, X.; Du, L.; Li, Z.; Ye, H. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int. J. Gynecol. Cancer 2008, 18, 652–659. [Google Scholar] [CrossRef]
- Ibarrola, J.; Matilla, L.; Martínez-Martínez, E.; Gueret, A.; Fernández-Celis, A.; Henry, J.-P.; Nicol, L.; Jaisser, F.; Mulder, P.; Ouvrard-Pascaud, A. Myocardial Injury After Ischemia/Reperfusion Is Attenuated By Pharmacological Galectin-3 Inhibition. Sci. Rep. 2019, 9, 9607. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Hao, X.; Zhang, Y.; Deng, W. Perindopril and a galectin-3 inhibitor improve ischemic heart failure in rabbits by reducing Gal-3 expression and myocardial fibrosis. Front. Physiol. 2019, 10, 267. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation: Mechanistic Insights and Clinical Implications. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Liu, L.; Nakano, F.; Kawakita, F.; Kanamaru, H.; Nakatsuka, Y.; Okada, T.; Suzuki, H. Modified citrus pectin prevents blood-brain barrier disruption in mouse subarachnoid hemorrhage by inhibiting galectin-3. Stroke 2018, 49, 2743–2751. [Google Scholar] [CrossRef]
- Ibarrola, J.; Arrieta, V.; Sádaba, R.; Martinez-Martinez, E.; Garcia-Peña, A.; Alvarez, V.; Fernández-Celis, A.; Gainza, A.; Santamaría, E.; Fernández-Irigoyen, J. Galectin-3 down-regulates antioxidant peroxiredoxin-4 in human cardiac fibroblasts: A new pathway to induce cardiac damage. Clin. Sci. 2018, 132, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Marín-Royo, G.; Gallardo, I.; Martínez-Martínez, E.; Gutiérrez, B.; Jurado-López, R.; López-Andrés, N.; Gutiérrez-Tenorio, J.; Rial, E.; Bartolomé, M.a.V.; Nieto, M.L. Inhibition of galectin-3 ameliorates the consequences of cardiac lipotoxicity in a rat model of diet-induced obesity. Dis. Model. Mech. 2018, 11, dmm032086. [Google Scholar] [CrossRef]
- Fernandez-García, C.-E.; Tarin, C.; Roldan-Montero, R.; Martinez-Lopez, D.; Torres-Fonseca, M.; Lindhot, J.S.; de Ceniga, M.V.; Egido, J.; Lopez-Andres, N.; Blanco-Colio, L.-M. Increased galectin-3 levels are associated with abdominal aortic aneurysm progression and inhibition of galectin-3 decreases elastase-induced AAA development. Clin. Sci. 2017, 131, 2707–2719. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, M.; Zhao, P.; Jia, M.; Liu, B.; Jia, Q.; Guo, J.; Dou, L.; Li, J. Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol. Med. Rep. 2017, 16, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, V.; Martinez-Martinez, E.; Ibarrola, J.; Alvarez, V.; Sádaba, R.; Garcia-Peña, A.; Fernández-Celis, A.; Cachofeiro, V.; Rossignol, P.; López-Andrés, N. A role for galectin-3 in the development of early molecular alterations in short-term aortic stenosis. Clin. Sci. 2017, 131, 935–949. [Google Scholar] [CrossRef]
- Ibarrola, J.; Martínez-Martínez, E.; Sádaba, J.; Arrieta, V.; García-Peña, A.; Álvarez, V.; Fernández-Celis, A.; Gainza, A.; Rossignol, P.; Cachofeiro Ramos, V. Beneficial effects of galectin-3 blockade in vascular and aortic valve alterations in an experimental pressure overload model. Int. J. Mol. Sci. 2017, 18, 1664. [Google Scholar] [CrossRef]
- Sádaba, J.R.; Martínez-Martínez, E.; Arrieta, V.; Álvarez, V.; Fernández-Celis, A.; Ibarrola, J.; Melero, A.; Rossignol, P.; Cachofeiro, V.; López-Andrés, N. Role for galectin-3 in calcific aortic valve stenosis. J. Am. Heart Assoc. 2016, 5, e004360. [Google Scholar] [CrossRef]
- Vergaro, G.; Prud’homme, M.; Fazal, L.; Merval, R.; Passino, C.; Emdin, M.; Samuel, J.-L.; Cohen Solal, A.; Delcayre, C. Inhibition of galectin-3 pathway prevents isoproterenol-induced left ventricular dysfunction and fibrosis in mice. Hypertension 2016, 67, 606–612. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; López-Ándres, N.; Jurado-López, R.; Rousseau, E.; Bartolomé, M.V.; Fernández-Celis, A.; Rossignol, P.; Islas, F.; Antequera, A.; Prieto, S. Galectin-3 participates in cardiovascular remodeling associated with obesity. Hypertension 2015, 66, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arter. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Ibarrola, J.; Fernández-Celis, A.; Calvier, L.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; López-Andrés, N. Galectin-3 pharmacological inhibition attenuates early renal damage in spontaneously hypertensive rats. J. Hypertens. 2018, 36, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef]
- Kolatsi-Joannou, M.; Price, K.L.; Winyard, P.J.; Long, D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 2011, 6, e18683. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Calvier, L.; Rossignol, P.; Rousseau, E.; Fernandez-Celis, A.; Jurado-Lopez, R.; Laville, M.; Cachofeiro, V.; Lopez-Andres, N. Galectin-3 inhibition prevents adipose tissue remodelling in obesity. Int. J. Obes. (Lond.) 2016, 40, 1034–1038. [Google Scholar] [CrossRef]
- Abu-Elsaad, N.M.; Elkashef, W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharm. 2016, 94, 554–562. [Google Scholar] [CrossRef]
- Eliaz, I.; Weil, E.; Schwarzbach, J.; Wilk, B. Modified Citrus Pectin/Alginate Dietary Supplement Increased Fecal Excretion of Uranium: A Family. Altern. Health Med. 2019, 25, 20–24. [Google Scholar]
- Zhao, Z.Y.; Liang, L.; Fan, X.; Yu, Z.; Hotchkiss, A.T.; Wilk, B.J.; Eliaz, I. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern. Health Med. 2008, 14, 34–38. [Google Scholar]
- Eliaz, I.; Weil, E.; Wilk, B. Integrative medicine and the role of modified citrus pectin/alginates in heavy metal chelation and detoxification-five case reports. Komplementmed 2007, 14, 358–364. [Google Scholar]
- Eliaz, I.; Hotchkiss, A.T.; Fishman, M.L.; Rode, D. The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother. Res. 2006, 20, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Merheb, R.; Abdel-Massih, R.M.; Karam, M.C. Immunomodulatory effect of natural and modified Citrus pectin on cytokine levels in the spleen of BALB/c mice. Int. J. Biol. Macromol. 2019, 121, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Mellem, J.; Reddy, L. The effect of modified citrus pectin-probiotic on faecal lactobacilli in Balb/c mice. Food Sci. Technol. 2017, 37, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157: H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Ramachandran, C.; Wilk, B.; Melnick, S.J.; Eliaz, I. Synergistic antioxidant and anti-inflammatory effects between modified citrus pectin and honokiol. Evid. Based Complement. Altern. Med. 2017, 2017, 8379843. [Google Scholar] [CrossRef]
- Dahdouh, E.; El-Khatib, S.; Baydoun, E.; Abdel-Massih, R.M. Additive Effect of MCP in Combination with Cefotaxime Against Staphylococcus aureus. Med. Chem. 2017, 13, 682–688. [Google Scholar] [CrossRef]
- Ramachandran, C.; Wilk, B.J.; Hotchkiss, A.; Chau, H.; Eliaz, I.; Melnick, S.J. Activation of human T-helper/inducer cell, T-cytotoxic cell, B-cell, and natural killer (NK)-cells and induction of natural killer cell activity against K562 chronic myeloid leukemia cells with modified citrus pectin. BMC Complement. Altern. Med. 2011, 11, 59. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Harazono, Y.; Nakajima, K.; Raz, A. Why anti-Bcl-2 clinical trials fail: A solution. Cancer Metastasis Rev. 2014, 33, 285–294. [Google Scholar] [CrossRef]
- Clementy, N.; Piver, E.; Bisson, A.; Andre, C.; Bernard, A.; Pierre, B.; Fauchier, L.; Babuty, D. Galectin-3 in atrial fibrillation: Mechanisms and therapeutic implications. Int. J. Mol. Sci. 2018, 19, 976. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy. Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Zhang, T.; Xue, H.; Wang, X.; Foday, A.D.; Tai, G.; Zhou, Y. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj. J. 2012, 29, 159–165. [Google Scholar] [CrossRef]
- Kim, H.-R.C.; Lin, H.-M.; Biliran, H.; Raz, A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999, 59, 4148–4154. [Google Scholar]
- Hayashi, A.; Gillen, A.C.; Lott, J.R. Effects of daily oral administration of quercetin chalcone and modified citrus pectin on implanted colon-25 tumor growth in Balb-c mice. Altern. Med. Rev. 2000, 5, 546–552. [Google Scholar]
- Hsieh, T.; Wu, J.M. Changes in cell growth, cyclin/kinase, endogenous phosphoproteins and nm23 gene expression in human prostatic JCA-1 cells treated with modified citrus pectin. Biochem. Mol. Biol. Int. 1995, 37, 833–841. [Google Scholar]
- Lehr, J.E.; Pienta, K.J. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J. Natl. Cancer Inst. 1998, 90, 118–123. [Google Scholar] [CrossRef]
- Glinsky, V.V.; Glinsky, G.V.; Rittenhouse-Olson, K.; Huflejt, M.E.; Glinskii, O.V.; Deutscher, S.L.; Quinn, T.P. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001, 61, 4851–4857. [Google Scholar]
- Khaldoyanidi, S.K.; Glinsky, V.V.; Sikora, L.; Glinskii, A.B.; Mossine, V.V.; Quinn, T.P.; Glinsky, G.V.; Sriramarao, P. MDA-MB-435 human breast carcinoma cell homo-and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen-galectin-3 interactions. J. Biol. Chem. 2003, 278, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Glinsky, G.V.; Glinskii, O.V.; Huxley, V.H.; Turk, J.R.; Mossine, V.V.; Deutscher, S.L.; Pienta, K.J.; Quinn, T.P. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003, 63, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Glinskii, O.V.; Turk, J.R.; Pienta, K.J.; Huxley, V.H.; Glinsky, V.V. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumour cells. J. Physiol. 2004, 554, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Platt, D.; Raz, A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J. Natl. Cancer Inst. 1992, 84, 438–442. [Google Scholar] [CrossRef]
- Pienta, K.J.; Nailk, H.; Akhtar, A.; Yamazaki, K.; Replogle, T.S.; Lehr, J.; Donat, T.L.; Tait, L.; Hogan, V.; Raz, A. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J. Natl. Cancer Inst. 1995, 87, 348–353. [Google Scholar] [CrossRef]
- Inohara, H.; Raz, A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj. J. 1994, 11, 527–532. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Huxley, V.H.; Glinsky, G.V.; Pienta, K.J.; Raz, A.; Glinsky, V.V. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia 2005, 7, 522–527. [Google Scholar] [CrossRef]
- Al-Mehdi, A.; Tozawa, K.; Fisher, A.; Shientag, L.; Lee, A.; Muschel, R. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102. [Google Scholar] [CrossRef]
- Sathisha, U.; Jayaram, S.; Nayaka, M.H.; Dharmesh, S.M. Inhibition of galectin-3 mediated cellular interactions by pectic polysaccharides from dietary sources. Glycoconj. J. 2007, 24, 497–507. [Google Scholar] [CrossRef]
- Chambers, A.; Groom, A.; MacDonald, I. Dissemination and Growth of Cancer Cells in Metastatic Sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Nakahara, S.; Oka, N.; Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Liu, F.-T. Galectins in cell growth and apoptosis. Cell Mol. Life Sci. 2003, 60, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Nakahara, S.; Hogan, V.; Raz, A. Galectin-3 in apoptosis, a novel therapeutic target. J. Bioenerg. Biomembr. 2007, 39, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.D.; Glinskii, O.V.; Mossine, V.V.; Turk, J.R.; Mawhinney, T.P.; Anthony, D.C.; Henry, C.J.; Huxley, V.H.; Glinsky, G.V.; Pienta, K.J. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia 2007, 9, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Honjo, Y.; Sarvis, R.; Akahani, S.; Hogan, V.; Pienta, K.J.; Raz, A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 2000, 156, 899–909. [Google Scholar] [CrossRef]
- Matarrese, P.; Tinari, N.; Semeraro, M.L.; Natoli, C.; Iacobelli, S.; Malorni, W. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 2000, 473, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.-R.C.; Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar]
- Yu, F.; Finley, R.L.; Raz, A.; Kim, H.-R.C. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria A role for synexin in galectin-3 translocation. J. Biol. Chem. 2002, 277, 15819–15827. [Google Scholar] [CrossRef]
- Oka, N.; Nakahara, S.; Takenaka, Y.; Fukumori, T.; Hogan, V.; Kanayama, H.-O.; Yanagawa, T.; Raz, A. Galectin-3 inhibits tumor necrosis factor–related apoptosis-inducing ligand–induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005, 65, 7546–7553. [Google Scholar] [CrossRef]
- Fukumori, T.; Oka, N.; Takenaka, Y.; Nangia-Makker, P.; Elsamman, E.; Kasai, T.; Shono, M.; Kanayama, H.-O.; Ellerhorst, J.; Lotan, R. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 2006, 66, 3114–3119. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Itamochi, H.; Kigawa, J.; Kanamori, Y.; Shimada, M.; Takahashi, M.; Shimogai, R.; Kawaguchi, W.; Sato, S.; Terakawa, N. Galectin-3 may contribute to Cisplatin resistance in clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 2007, 17, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Li, G.; Podar, K.; Hideshima, T.; Neri, P.; He, D.; Mitsiades, N.; Richardson, P.; Chang, Y.; Schindler, J. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 2005, 65, 8350–8358. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Huang, Z.-L.; Yang, G.-H.; Lu, W.-Q.; Yu, N.-R. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef]
- Do Prado, S.B.R.; Shiga, T.M.; Harazono, Y.; Hogan, V.A.; Raz, A.; Carpita, N.C.; Fabi, J.P. Migration and proliferation of cancer cells in culture are differentially affected by molecular size of modified citrus pectin. Carbohydr. Polym. 2019, 211, 141–151. [Google Scholar] [CrossRef]
- Strum, S.; Scholz, M.; McDermed, J.; McCulloch, M.; Eliaz, I. Modified citrus pectin slows PSA doubling time: A pilot clinical trial. In Proceedings of the International Conference on Diet and Prevention of Cancer, Tampere, Finland, 28 May–1 June 1999. [Google Scholar]
- Keizman, D.; Frenkel, M.A.; Peer, A.; Rosenbaum, E.; Margel, D.; Sarid, D.L.; Neiman, V.; Gottfried, M.; Maimon, N.; Leibovitch, I. Effect of pectasol-c modified citrus pectin (P-MCP) treatment (tx) on PSA dynamics in non-metastatic biochemically relapsed prostate cancer (BRPC) patients (pts): Results of a prospective phase II study. J. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Arrieta, V.; Sádaba, J.; Álvarez, V.; Rodríguez, J.; López-Andrés, N. Galectin-3 as a novel biotarget in cardiovascular alterations associated to development of severe aortic stenosis. Sist. Sanit. Navar. 2019, 72347. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; André, S.; Crijns, H.J.; Gabius, H.-J.; Maessen, J. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef]
- Ho, M.-K.; Springer, T. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J. Immunol. 1982, 128, 1221–1228. [Google Scholar]
- De Boer, R.A.; Van Der Velde, A.R.; Mueller, C.; Van Veldhuisen, D.J.; Anker, S.D.; Peacock, W.F.; Adams, K.F.; Maisel, A. Galectin-3: A modifiable risk factor in heart failure. Cardiovasc. Drugs 2014, 28, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridonos, M.; McNeill, E.; de Bono, J.P.; Smith, A.; Burnand, K.G.; Channon, K.M.; Greaves, D.R. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, R.; Zhang, Q.; Xu, Z.; Xu, F.; Li, D.; Li, Y. Microtia patients: Auricular chondrocyte ECM is promoted by CGF through IGF-1 activation of the IGF-1R/PI3K/AKT pathway. J. Cell. Physiol. 2019, 234, 21817–21824. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.I.; Jefferies, K.C.; Panjwani, N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J. Biol. Chem. 2011, 286, 29913–29921. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Calvier, L.; Fernández-Celis, A.; Rousseau, E.; Jurado-López, R.; Rossoni, L.V.; Jaisser, F.; Zannad, F.; Rossignol, P.; Cachofeiro, V. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension 2015, 66, 767–775. [Google Scholar] [CrossRef]
- Leclere, L.; Fransolet, M.; Cote, F.; Cambier, P.; Arnould, T.; Van Cutsem, P.; Michiels, C. Heat-modified citrus pectin induces apoptosis-like cell death and autophagy in HepG2 and A549 cancer cells. PLoS ONE 2015, 10, e0115831. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Liu, X.; Hadoke, P.W.; Miller, M.R.; Newby, D.E.; Sethi, T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology 2013, 23, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Gu, X.; Gong, M.; Guo, G.; Han, K.; An, R. Galectin-3 inhibition sensitizes human renal cell carcinoma cells to arsenic trioxide treatment. Cancer Biol. 2013, 14, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Menachem, A.; Bodner, O.; Pastor, J.; Raz, A.; Kloog, Y. Inhibition of malignant thyroid carcinoma cell proliferation by Ras and galectin-3 inhibitors. Cell Death Discov. 2015, 1, 15047. [Google Scholar] [CrossRef] [Green Version]
- Ruvolo, P.P.; Ruvolo, V.R.; Benton, C.B.; AlRawi, A.; Burks, J.K.; Schober, W.; Rolke, J.; Tidmarsh, G.; Hail, N., Jr.; Davis, R.E. Combination of galectin inhibitor GCS-100 and BH3 mimetics eliminates both p53 wild type and p53 null AML cells. Biochim. Biophys. Acta 2016, 1863, 562–571. [Google Scholar] [CrossRef]
- Clark, M.C.; Pang, M.; Hsu, D.K.; Liu, F.-T.; De Vos, S.; Gascoyne, R.D.; Said, J.; Baum, L.G. Galectin-3 binds to CD45 on diffuse large B-cell lymphoma cells to regulate susceptibility to cell death. Blood 2012, 120, 4635–4644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demotte, N.; Wieërs, G.; Van Der Smissen, P.; Moser, M.; Schmidt, C.; Thielemans, K.; Squifflet, J.-L.; Weynand, B.; Carrasco, J.; Lurquin, C. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010, 70, 7476–7488. [Google Scholar] [CrossRef] [PubMed]
- Streetly, M.J.; Maharaj, L.; Joel, S.; Schey, S.A.; Gribben, J.G.; Cotter, F.E. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood 2010, 115, 3939–3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Nangia-Makker, P.; Balan, V.; Hogan, V.; Raz, A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010, 1, e101. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Bojic, S.; Stojanovic, M.; Nilsson, U.; Leffler, H.; Besra, G.S.; Arsenijevic, N.; Paunovic, V.; Trajkovic, V. Gal-3 regulates the capacity of dendritic cells to promote NKT-cell-induced liver injury. Eur. J. Immunol. 2015, 45, 531–543. [Google Scholar] [CrossRef]
- Volarevic, V.; Milovanovic, M.; Ljujic, B.; Pejnovic, N.; Arsenijevic, N.; Nilsson, U.; Leffler, H.; Lukic, M.L. Galectin-3 deficiency prevents concanavalin A–induced hepatitis in mice. Hepatology 2012, 55, 1954–1964. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J. Regulation of transforming growth factor-β1–driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.-I.; Friedman, S.L. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef]
- Traber, P.G.; Zomer, E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS ONE 2013, 8, e83481. [Google Scholar] [CrossRef]
- Iacobini, C.; Fantauzzi, C.B.; Pugliese, G.; Menini, S. Role of galectin-3 in bone cell differentiation, bone pathophysiology and vascular osteogenesis. Int. J. Mol. Sci. 2017, 18, 2481. [Google Scholar] [CrossRef]
- Colnot, C.; Sidhu, S.; Poirier, F.; Balmain, N. Cellular and subcellular distribution of galectin-3 in the epiphyseal cartilage and bone of fetal and neonatal mice. Cell. Mol. Biol. (Noisy-Le-Grandfrance) 1999, 45, 1191–1202. [Google Scholar]
- Stock, M.; Schäfer, H.; Stricker, S.; Gross, G.; Mundlos, S.; Otto, F. Expression of galectin-3 in skeletal tissues is controlled by Runx2. J. Biol. Chem. 2003, 278, 17360–17367. [Google Scholar] [CrossRef] [PubMed]
- Weilner, S.; Keider, V.; Winter, M.; Harreither, E.; Salzer, B.; Weiss, F.; Schraml, E.; Messner, P.; Pietschmann, P.; Hildner, F. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging (Albany N. Y.) 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Niida, S.; Amizuka, N.; Hara, F.; Ozawa, H.; Kodama, H. Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. J. Bone Miner. Res. 1994, 9, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Jarolim, E.; Gscheidlinger, R.; Oberhuber, G.; Neuchrist, C.; Lucas, T.; Bises, G.; Radauer, C.; Willheim, M.; Scheiner, O.; Liu, F.-T. The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn’s disease patients, and tumour necrosis factor alpha decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur. J. Gastroenterol. Hepatol. 2002, 14, 145–152. [Google Scholar] [CrossRef]
- Lippert, E.; Falk, W.; Bataille, F.; Kähne, T.; Naumann, M.; Goeke, M.; Herfarth, H.; Schoelmerich, J.; Rogler, G. Soluble galectin-3 is a strong, colonic epithelial-cell-derived, lamina propria fibroblast-stimulating factor. Gut 2007, 56, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Puthenedam, M.; Wu, F.; Shetye, A.; Michaels, A.; Rhee, K.-J.; Kwon, J.H. Matrilysin-1 (MMP7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm. Bowel Dis. 2010, 17, 260–267. [Google Scholar] [CrossRef]
- Lippert, E.; Stieber-Gunckel, M.; Dunger, N.; Falk, W.; Obermeier, F.; Kunst, C. Galectin-3 modulates experimental colitis. Digestion 2015, 92, 45–53. [Google Scholar] [CrossRef]
- Tsai, H.-F.; Wu, C.-S.; Chen, Y.-L.; Liao, H.-J.; Chyuan, I.-T.; Hsu, P.-N. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J. Mol. Med. 2016, 94, 545–556. [Google Scholar] [CrossRef]
- Simovic Markovic, B.; Nikolic, A.; Gazdic, M.; Bojic, S.; Vucicevic, L.; Kosic, M.; Mitrovic, S.; Milosavljevic, M.; Besra, G.; Trajkovic, V. Galectin-3 plays an important pro-inflammatory role in the induction phase of acute colitis by promoting activation of NLRP3 inflammasome and production of IL-1β in macrophages. J. Crohn’s Colitis 2016, 10, 593–606. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.; Sears, D.; Shen, Z.; Cui, B. Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell 2016, 167, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, S.J.; Kang, H.G.; Lee, H.W.; Kim, J.H.; Hwang, K.A.; Song, J.; Chun, K.H. Galectin-3 activates PPARγ and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology 2015, 156, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; de Lemos, J.A.; Ayers, C.; Grodin, J.L.; Lingvay, I. Association of Galectin-3 with Diabetes Mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019, 104, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
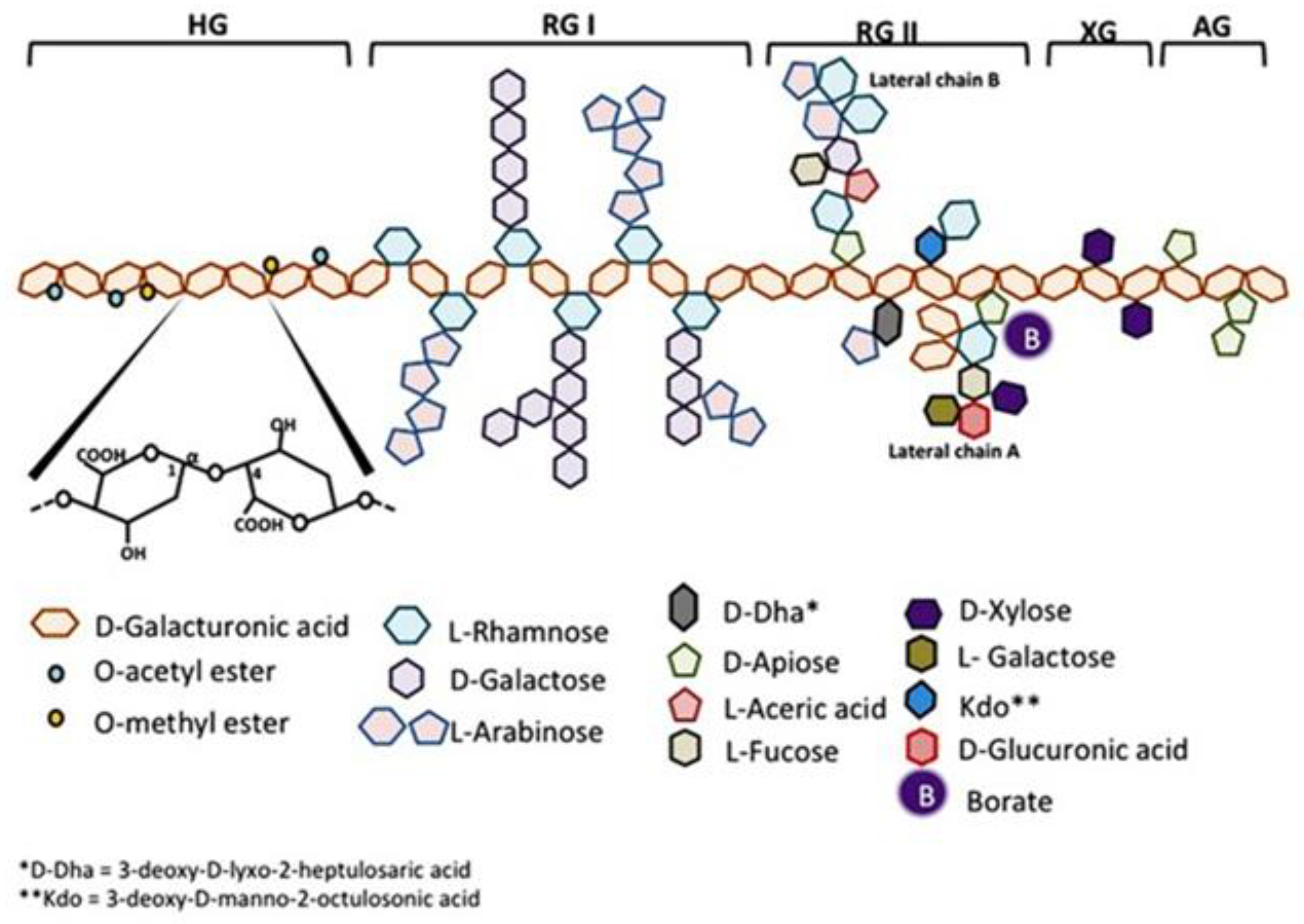
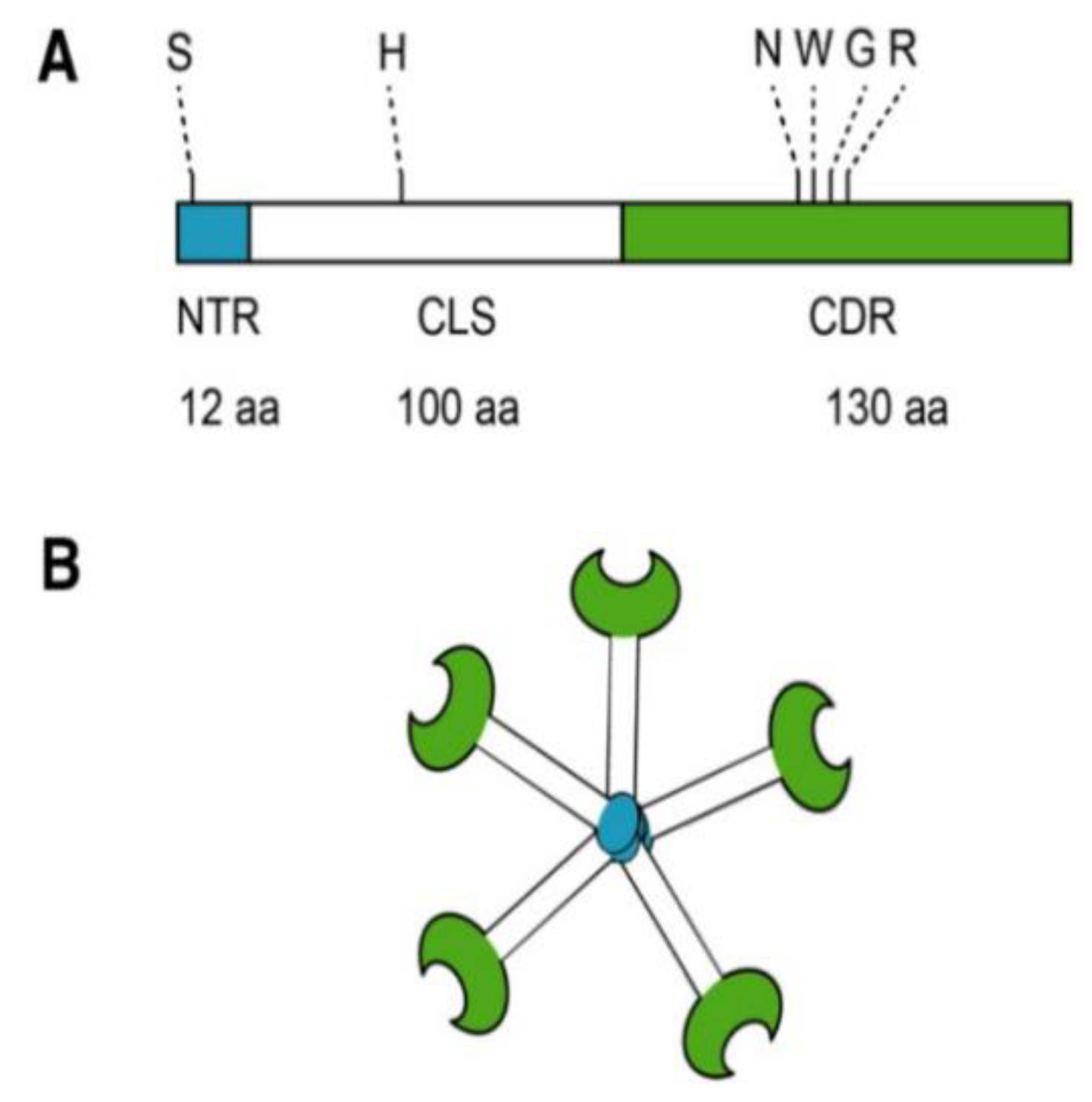
| Main Indication | Study Type | Disease Model | Species Studied | Reference | Summary of Results |
|---|---|---|---|---|---|
| Cancer | Clinical trial | Circulating tumor cells | Human | [4] | Nutrients with anti-carcinogenic properties could reduce circulating tumor cell count, and included curcumin, garlic, green tea, grape seed, MCP, and medicinal mushroom extract |
| Cancer | Clinical trial | Advanced solid tumors | Human | [5] | Clinical benefits and life quality with far advanced solid tumors |
| Cancer | Clinical trial | Prostate cancer | Human | [6] | PSADT extended in 70% of patients |
| Cancer | Preclinical | Ovarian cancer | In vitro | [7] | MCP enhanced the PTX effect on ovarian cancer cells MCTS through the inhibition of STAT3 activity |
| Cancer | Preclinical | Cisplatin-induced nephrotoxicity | Mouse | [8] | MCP-treated mice demonstrated decreased renal fibrosis and apoptosis |
| Cancer | Preclinical | Colon cancer | In vitro, in vivo, and ex vivo | [9] | MCP inhibition of extracellular Gal-3 decreases colon cancer cell migration |
| Cancer | Preclinical | Prostate cancer and radiation therapy | In vitro | [10] | MCP reduced prostate cancer cell viability and synergistically enhanced cell sensitivity to ionizing radiation |
| Cancer | Preclinical | Bladder cancer | In vitro and mouse | [11] | Remarkable inhibitory effects of MCP on urinary bladder cancer cell proliferation and survival in vitro and in vivo mainly through Gal-3 |
| Cancer | Preclinical | Gastrointestinal cancer | Mouse | [12] | MCP effectively inhibits the growth and metastasis of gastrointestinal cancer cells, partly by down-regulating Bcl-xL and Cyclin B to promote apoptosis and suppress EMT |
| Cancer | Preclinical | Colonic carcinogenesis | Mouse | [13] | Modified L. acidophilus ATCC 4356 cell envelope improved the bioavailability and the anti-(colon) cancer effect of MCP |
| Cancer | Preclinical | Breast and prostate cancer | In vitro | [14] | Inhibits breast/prostate cancer cell migration and synergy with MCP |
| Cancer | Preclinical | Ovarian cancer | In vitro | [15] | MCP synergy with paclitaxel |
| Cancer | Preclinical | Prostate cancer | In vitro | [16] | MCP synergy with doxorubicin |
| Cancer | Preclinical | Prostate cancer | In vitro | [17] | MCP induced cell death and inhibition of the proliferation of prostate cancer |
| Cancer | Preclinical | Liver and colon cancer | Mouse | [18] | MCP inhibits liver metastasis of colon cancer |
| Cardiovascular | Preclinical | Myocardial infarction | Rat | [19] | MCP blockade of Gal-3 can prevent cardiac fibrosis, inflammation, and functional alterations |
| Cardiovascular | Preclinical | Ischemic heart failure | Rabbit | [20] | Perindopril and MCP comparably improve ischemic heart failure in rabbits by downregulating Gal-3 and reducing myocardial fibrosis |
| Cardiovascular | Preclinical | Myocardial fibrosis | Rat, mouse, and human | [21] | MCP -mediated Gal-3 inhibition in mice prevented the profibrotic and proinflammatory effects of cardiotrophin-1 |
| Cardiovascular | Preclinical | Blood-brain barrier disruption | Mouse | [22] | MCP prevents post-Subarachnoid Hemorrhage blood-brain barrier disruption possibly by inhibiting Gal-3, of which the mechanisms may include binding to TLR4 and activating ERK1/2, STAT3, and MMP-9 |
| Cardiovascular | Preclinical | Cardiovascular fibrosis | In vitro, in vivo, and ex vivo | [23] | The pharmacological inhibition of Gal-3 with MCP restored cardiac Prx-4 as well as prohibitin-2 levels and improved oxidative status in spontaneously hypertensive rats |
| Cardiovascular | Preclinical | Cardiac lipotoxicity | Rat | [24] | Gal-3 inhibition with MCP attenuates consequences of cardiac lipotoxicity induced by a high-fat diet, reducing total triglyceride and lysophosphatidylcholine levels |
| Cardiovascular | Preclinical | Abdominal aortic aneurysm | Mouse | [25] | Mice treated with MCP showed decreased aortic dilation, as well as elastin degradation, vascular smooth muscle cell loss, and macrophage content at day 14 post-elastase perfusion compared with control mice |
| Cardiovascular | Preclinical | Atherosclerotic lesions in apoE-deficiency | Mouse | [26] | MCP reduced the size of atherosclerotic lesions by inhibiting the adhesion of leukocytes to endothelial cells |
| Cardiovascular | Preclinical | Aortic stenosis | Rat | [27] | In short-term AS, the increase in myocardial Gal-3 expression associated with cardiac fibrosis and inflammation, alterations that were prevented by Gal-3 blockade with MCP |
| Cardiovascular | Preclinical | Cardiovascular fibrosis and aortic valve calcification | Rat | [28] | MCP treatment prevented the increase in Gal-3, media thickness, fibrosis, and inflammation in the aorta of pressure overload rats |
| Cardiovascular | Preclinical | Aortic stenosis | Human and ex vivo | [29] | Gal-3 expression was blocked in VICs undergoing osteoblastic differentiation using MCP |
| Cardiovascular | Preclinical | Cardiovascular LV fibrosis | Mouse | [30] | MCP reversed induced LV dysfunction of HF with cardiac hyperaldosteronism |
| Cardiovascular | Preclinical | Cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension | Rat | [31] | MCP prevention of inflammation and fibrosis with hypertension |
| Cardiovascular | Preclinical | Heart fibrosis | Rat | [32] | MCP prevention of cardiac fibrosis |
| Cardiovascular | Preclinical | Vascular fibrosis | Rat | [33] | MCP reverses vascular hypertrophy and fibrosis |
| Kidney | Preclinical | Renal damage in spontaneous hypertension | Rat | [34] | The inflammatory mediators (monocyte chemoattractant protein-1, osteopontin, cd68, cd80, cd44, and cd45) were elevated in spontaneously hypertensive rats and attenuated by MCP |
| Kidney | Preclinical | Kidney fibrosis | Rat | [35] | In experimental models of mild kidney damage, the increase in renal Gal-3 expression paralleled with renal fibrosis and inflammation, while these alterations prevented with MCP |
| Kidney | Preclinical | Kidney fibrosis | Rat | [32] | MCP prevention of kidney fibrosis |
| Kidney | Preclinical | Acute kidney disease | In vitro | [36] | MCP inhibits renal fibrosis |
| Obesity | Preclinical | Adipose tissue remodeling | Rat | [37] | Despite no effect on body weight, adipose tissue weights or adiposity, MCP prevented adipose tissue fibrosis, inflammation and the increase in adipocyte differentiation markers in a model of diet-induced obesity |
| Obesity | Preclinical | Adipose tissue remodeling/fibrosis | Rat | [31] | MCP prevented an increase in pericellular collagen, adipose tissue inflammation and differentiation degree of the adipocytes |
| Liver | Preclinical | Liver fibrosis | Rat | [38] | MCP attenuates liver fibrosis through an antioxidant effect, the inhibition of Gal-3, and the induction of apoptosis |
| Detoxification | Clinical trial | Chronic low-level uranium exposure | Human | [39] | MCP, after a post-treatment period of 6 weeks, decreased in fecal excretion of uranium found in 5 of 6 participants |
| Detoxification | Clinical trial | Child lead toxicity | Human | [40] | Detoxification from lead toxicity in hospitalized children |
| Detoxification | Clinical trial | Lead and mercury toxicity | Human | [41] | MCP lowered body burden of lead and or mercury and chronic ailment improvements |
| Detoxification | Clinical trial | Toxic metals | Human | [42] | MCP detoxification of lead, cadmium, arsenic, and mercury |
| Immune | Preclinical | Immuno-modulation | Mouse | [43] | CP and mainly MCP have an immunomodulatory effect on the levels of cytokine secretion in the spleen of mice with a pro-inflammatory potential |
| Immune | Preclinical | Probiotic | Mouse | [44] | The number of fecal lactobacilli in the MCP alginate probiotic-treated mice significantly increased |
| Immune | Preclinical | Shiga toxin producing E. Coli | In vitro | [45] | MCP inhibits adhesion of shiga toxin, reduces shiga toxin cytotoxicity |
| Immune | Preclinical | Inflammation | In vitro | [46] | MCP: Honokiol (9:1) combination induced a synergistic effect on antioxidant activity suggesting that the mixture is significantly more efficient than individual compounds |
| Immune | Preclinical | Staphylococcus aureus | In vitro | [47] | MCP demonstrates in vitro antimicrobial activity alone and combination with cefotaxime against staphylococcus aureus. |
| Immune | Preclinical | Immune activation | Human blood and ex vivo | [48] | MCP significantly activated T-cells and natural killer cells |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliaz, I.; Raz, A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients 2019, 11, 2619. https://doi.org/10.3390/nu11112619
Eliaz I, Raz A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients. 2019; 11(11):2619. https://doi.org/10.3390/nu11112619
Chicago/Turabian StyleEliaz, Isaac, and Avraham Raz. 2019. "Pleiotropic Effects of Modified Citrus Pectin" Nutrients 11, no. 11: 2619. https://doi.org/10.3390/nu11112619
APA StyleEliaz, I., & Raz, A. (2019). Pleiotropic Effects of Modified Citrus Pectin. Nutrients, 11(11), 2619. https://doi.org/10.3390/nu11112619




