The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014
Abstract
1. Introduction
2. Methods
2.1. Population
2.2. Data Analysis
| Response | Predictor |
| Model 1: Metabolic Syndrome (Yes/No) | Smooth(Sleep) |
| Model 2: Metabolic Syndrome (Yes/No) | Age, Sex, Race, Sitting, smooth(Sleep) |
| Model 3: Metabolic Syndrome (Yes/No) | Age, Race, Sitting, smooth(Sleep*Sex) |
| Model 4: Metabolic Syndrome Severity Score | Age, Sex, Race, Sitting, smooth(Sleep) |
| Model 5: Metabolic Syndrome Severity Score | Age, Race, Sitting, smooth(Sleep*Sex) |
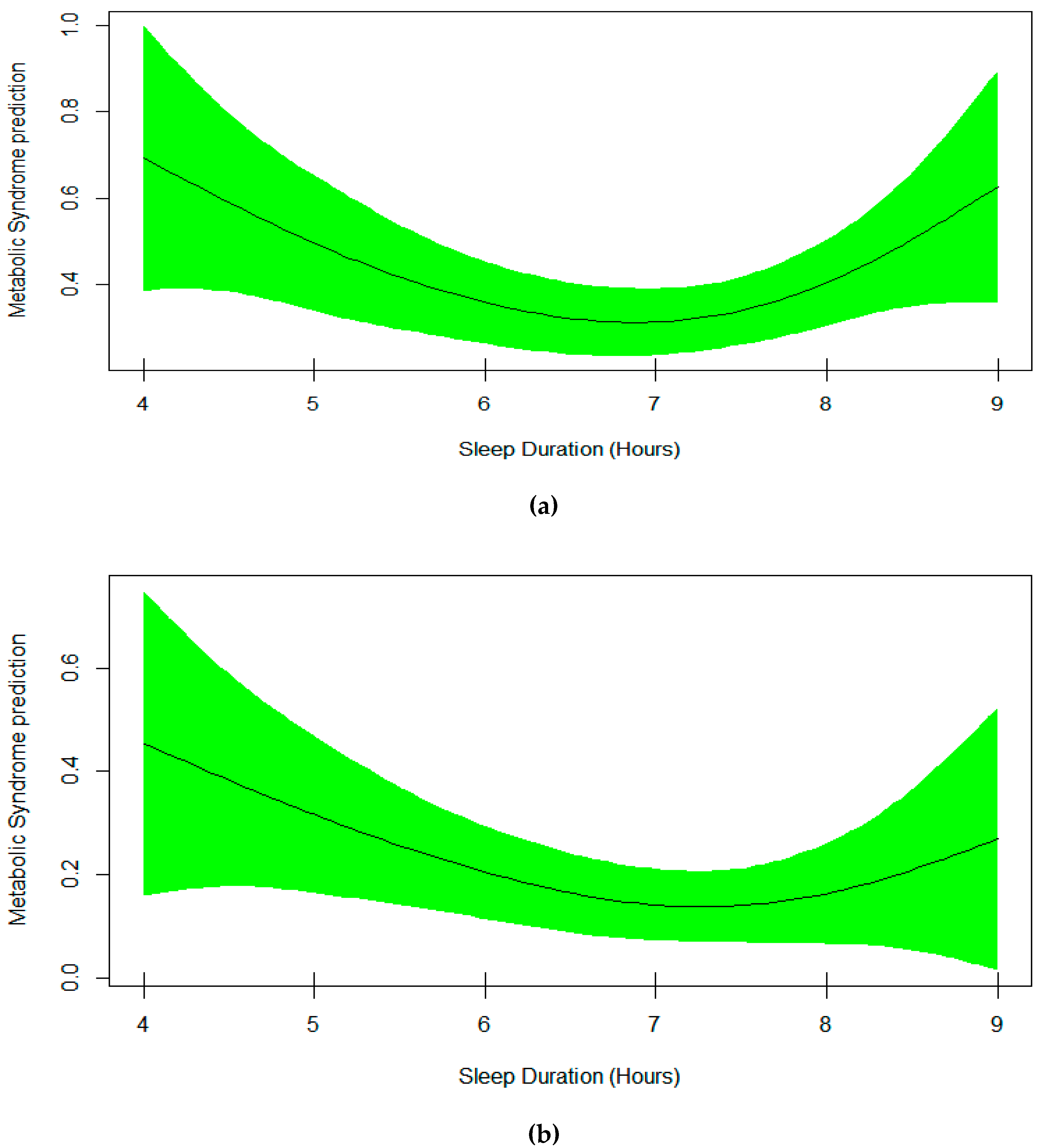
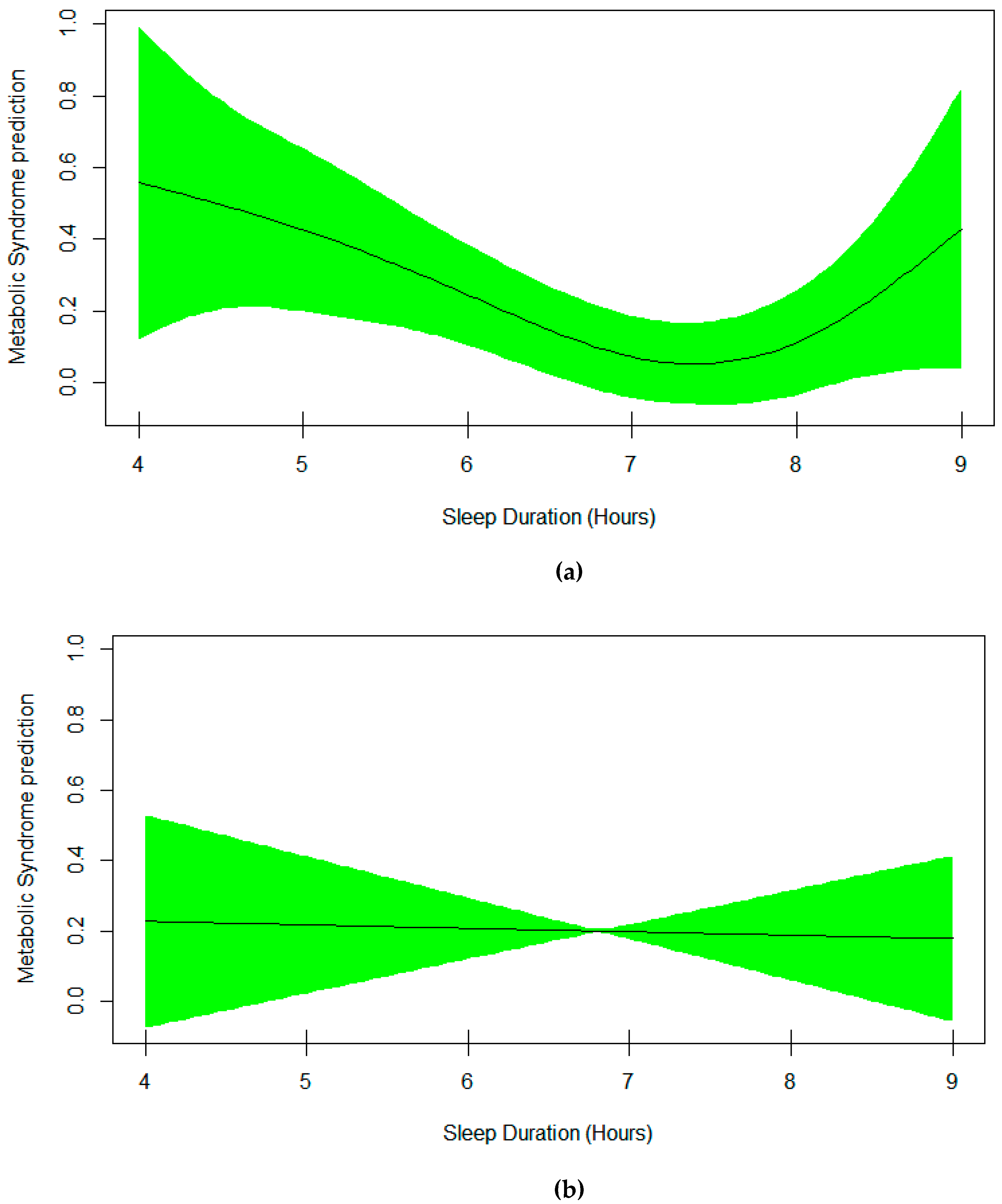
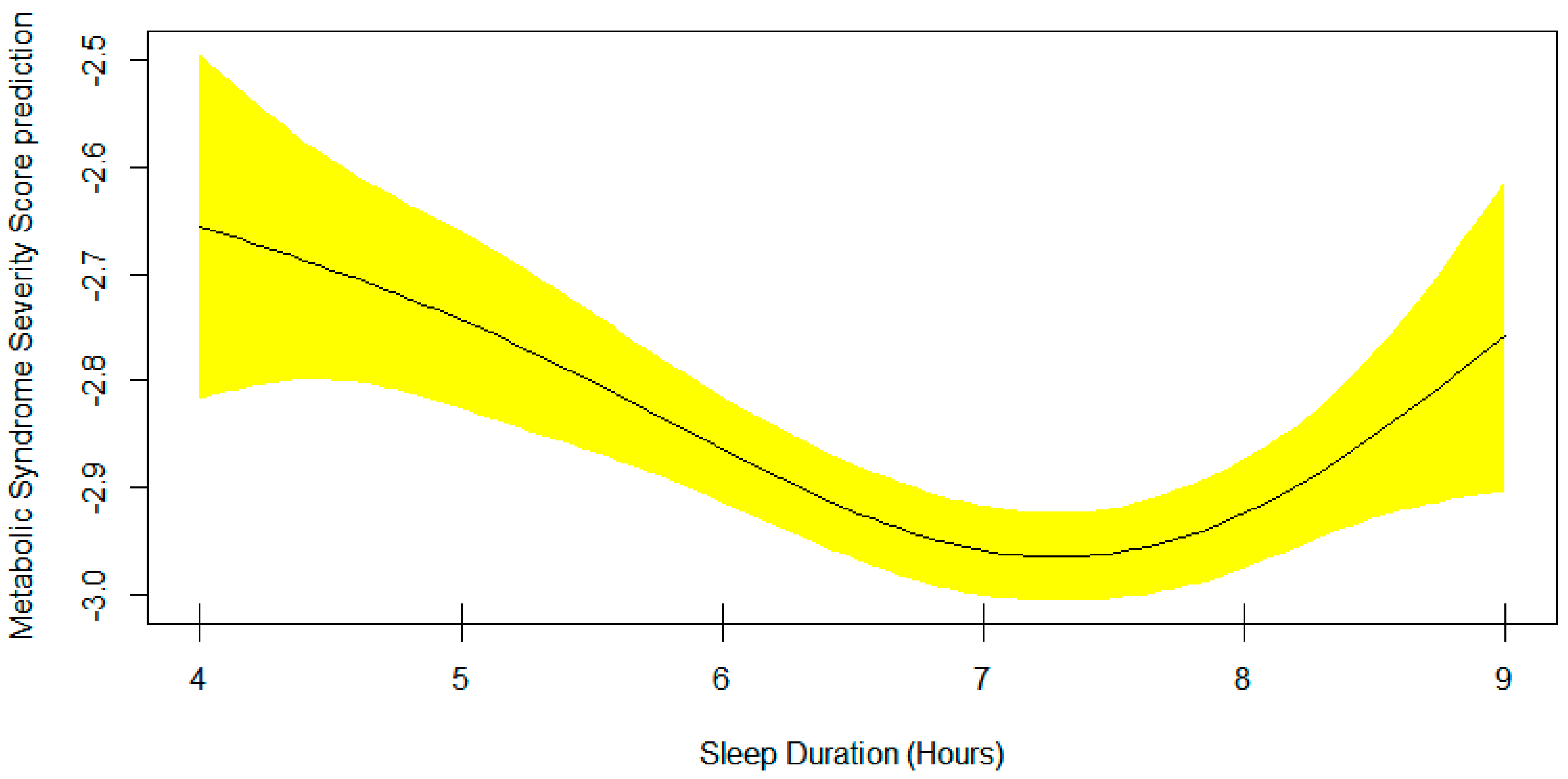
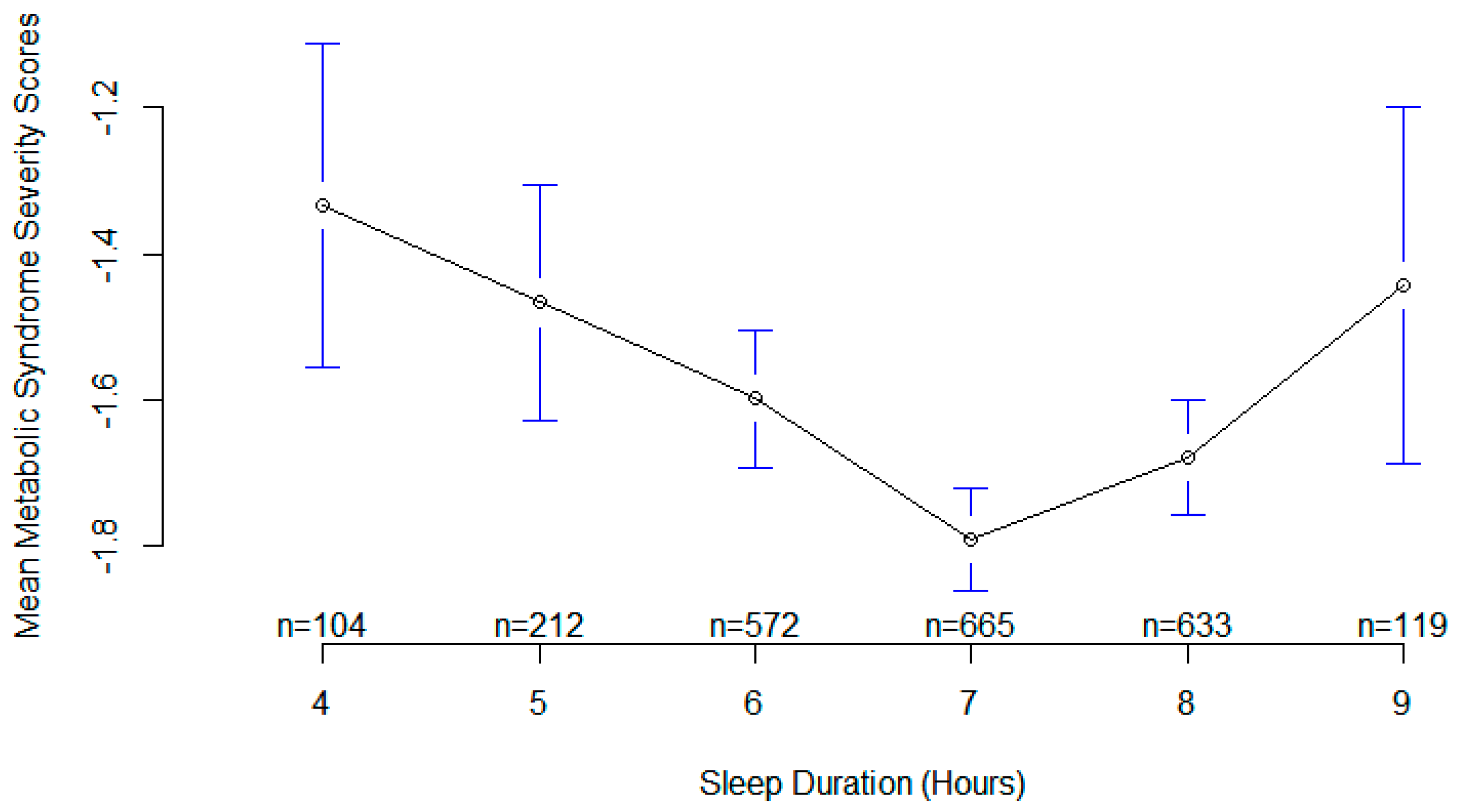
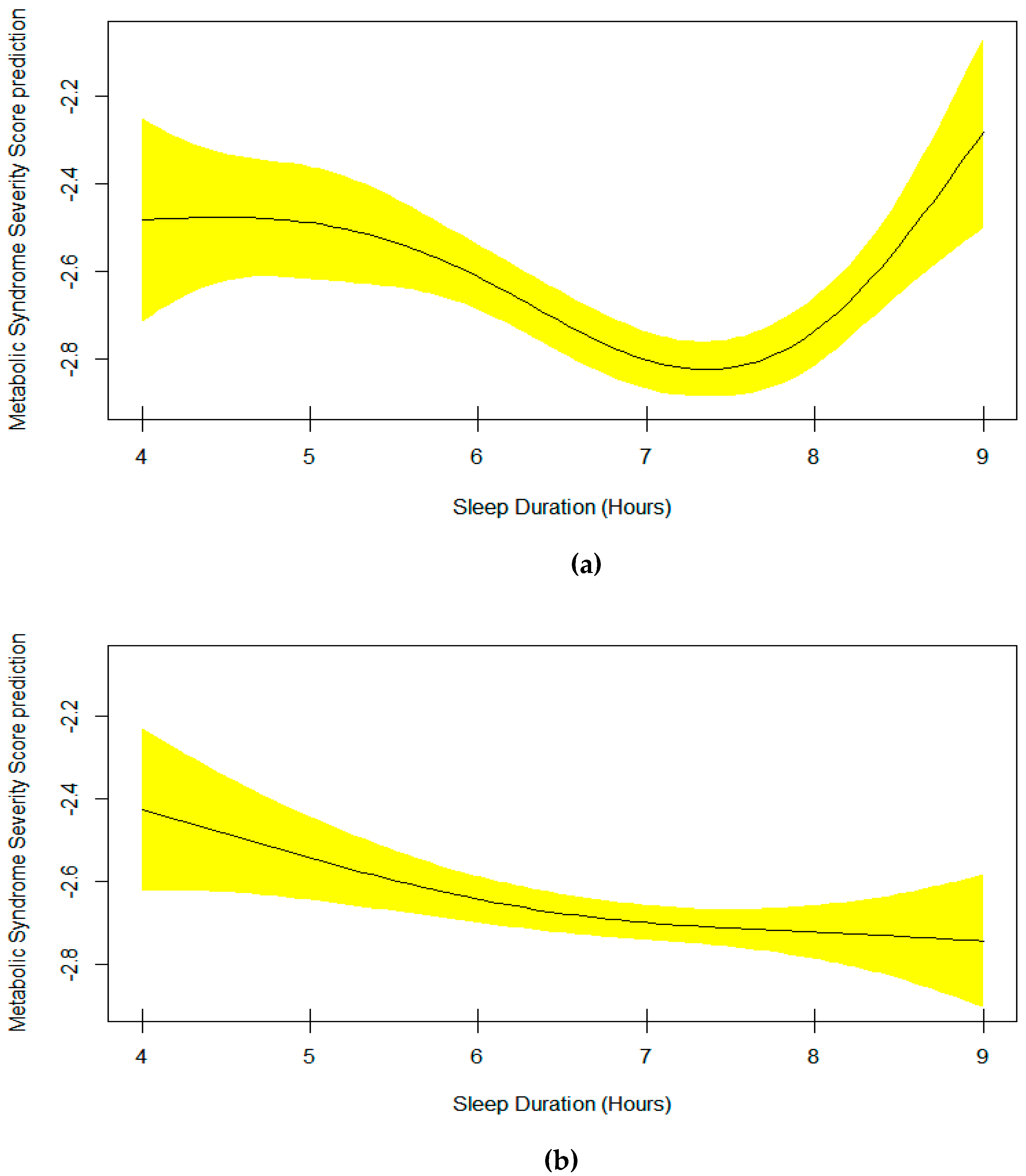
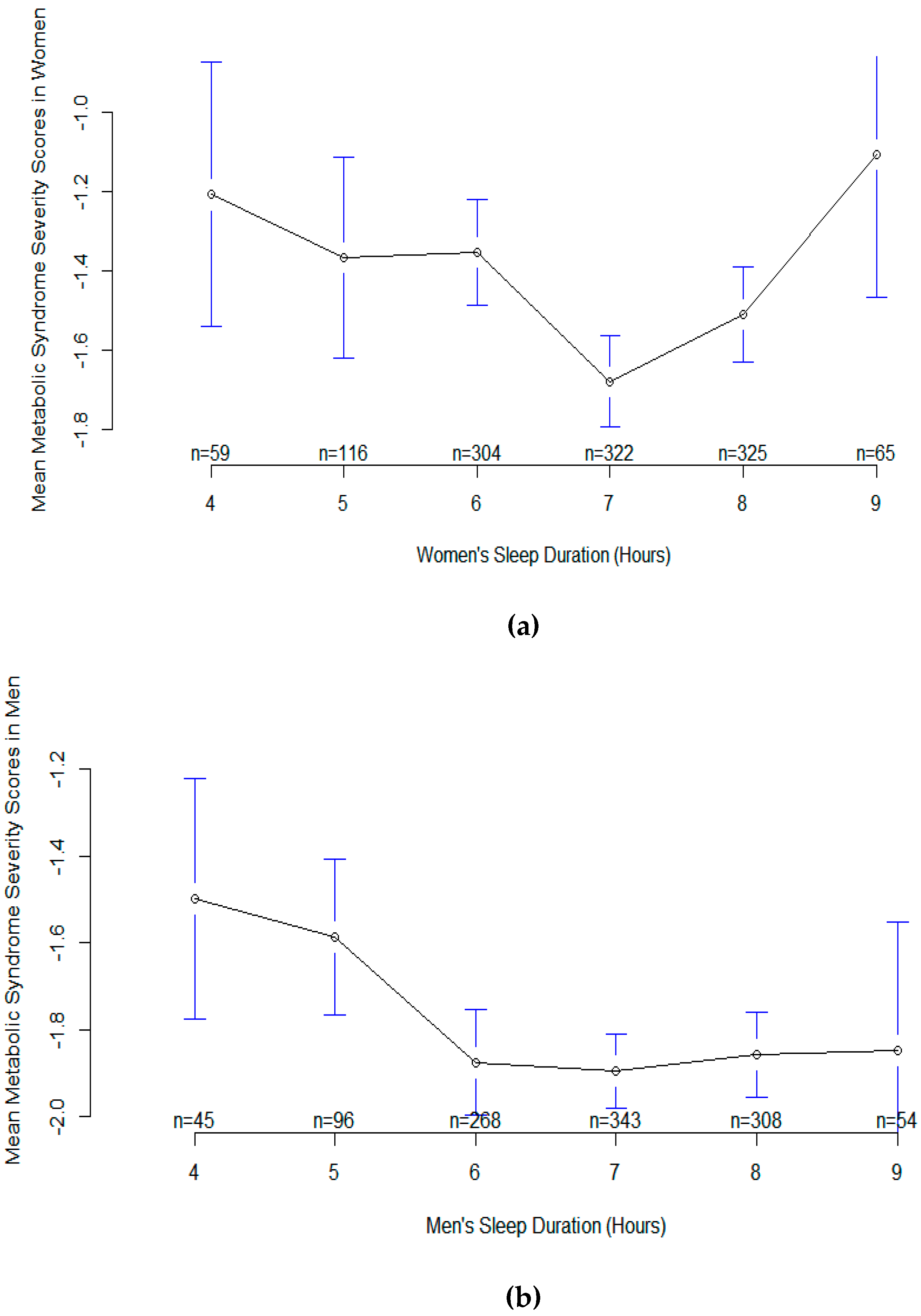
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Hillard, P.J.A.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Panel, C.C.; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Sleep 2015, 38, 1161–1183. [Google Scholar]
- Ostadrahimi, A.; Nikniaz, Z.; Faramarzi, E.; Mohammadpoorasl, A.; Ansarin, K.; Somi, M.H. Does long sleep duration increase risk of metabolic syndrome in Azar cohort study population? Health Promot. Perspect. 2018, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Hemati, Z.; Mozafarian, N.; Heshmat, R.; Ahadi, Z.; Motlagh, M.E.; Ziaodini, H.; Taheri, M.; Aminaee, T.; Qorbani, M.; Kelishadi, R. Association of sleep duration with metabolic syndrome and its components in children and adolescents; a propensity score-matched analysis: The CASPIAN-V study. Diabetol. Metab. Syndr. 2018, 10, 78. [Google Scholar] [CrossRef]
- Kim, C.E.; Shin, S.; Lee, H.-W.; Lim, J.; Lee, J.-K.; Shin, A.; Kang, D. Association between sleep duration and metabolic syndrome: A cross-sectional study. BMC Public Health 2018, 18, 720. [Google Scholar] [CrossRef]
- Itani, O.; Kaneita, Y.; Tokiya, M.; Jike, M.; Murata, A.; Nakagome, S.; Otsuka, Y.; Ohida, T. Short sleep duration, shift work, and actual days taken off work are predictive life-style risk factors for new-onset metabolic syndrome: A seven-year cohort study of 40,000 male workers. Sleep Med. 2017, 39, 87–94. [Google Scholar] [CrossRef]
- Deng, H.-B.; Tam, T.; Zee, B.C.-Y.; Chung, R.Y.-N.; Su, X.; Jin, L.; Chan, T.-C.; Chang, L.-Y.; Yeoh, E.-K.; Lao, X.Q. Short sleep duration increases metabolic impact in healthy adults: A population-based cohort study. Sleep 2017, 40, zsx130. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; He, F.; LaGrotte, C.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Impact of the metabolic syndrome on mortality is modified by objective short sleep duration. J. Am. Heart Assoc. 2017, 6, e005479. [Google Scholar] [CrossRef]
- Chaput, J.-P.; McNeil, J.; Després, J.-P.; Bouchard, C.; Tremblay, A. Short sleep duration as a risk factor for the development of the metabolic syndrome in adults. Prev. Med. 2013, 57, 872–877. [Google Scholar] [CrossRef]
- Stefani, K.M.; Kim, H.C.; Kim, J.; Oh, K.; Suh, I. The influence of sex and age on the relationship between sleep duration and metabolic syndrome in Korean adults. Diabetes Res. Clin. Pract. 2013, 102, 250–259. [Google Scholar] [CrossRef]
- Sung, V.; Beebe, D.W.; VanDyke, R.; Fenchel, M.C.; Crimmins, N.A.; Kirk, S.; Hiscock, H.; Amin, R.; Wake, M. Does sleep duration predict metabolic risk in obese adolescents attending tertiary services? A cross-sectional study. Sleep 2011, 34, 891–898. [Google Scholar] [PubMed]
- Wu, J.; Xu, G.; Shen, L.; Zhang, Y.; Song, L.; Yang, S.; Yang, H.; Liang, Y.; Wu, T.; Wang, Y. Daily sleep duration and risk of metabolic syndrome among middle-aged and older Chinese adults: Cross-sectional evidence from the Dongfeng–Tongji cohort study. BMC Public Health 2015, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; He, D.; Zhang, M.; Xue, J.; Zhou, D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med. Rev. 2014, 18, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. Harrison’s Manual of Medicine; McGraw-Hill Medical: New York, NY, USA, 2013. [Google Scholar]
- Lee, A.M.; Gurka, M.J.; DeBoer, M.D. A MetS severity score to estimate risk in adolescents and adults: Current evidence and future potential. Expert Rev. Cardiovasc. Ther. 2016, 14, 411–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruppert, D.; Wand, M.P.; Carroll, R.J. Semiparametric regression during 2003–2007. Electron. J. Stat. 2009, 3, 1193. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Smiley, A.; Wolter, S.; Nissan, D. Mechanisms of Association of Sleep and Metabolic Syndrome. J. Med. Clin. Res. Rev. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Benedetti, A.; Abrahamowicz, M. Using generalized additive models to reduce residual confounding. Stat. Med. 2004, 23, 3781–3801. [Google Scholar] [CrossRef]
- Moore, L.; Hanley, J.A.; Turgeon, A.F.; Lavoie, A. A comparison of generalized additive models to other common modeling strategies for continuous covariates: Implications for risk adjustment. J. Biomet. Biostat. 2011, 2, 109. [Google Scholar] [CrossRef]
- Austin, P.C.; Brunner, L.J. Inflation of the type I error rate when a continuous confounding variable is categorized in logistic regression analyses. Stat. Med. 2004, 23, 1159–1178. [Google Scholar] [CrossRef]
- Boucher, K.M.; Slattery, M.L.; Berry, T.D.; Quesenberry, C.; Anderson, K. Statistical methods in epidemiology: A comparison of statistical methods to analyze dose–response and trend analysis in epidemiologic studies. J. Clin. Epidemiol. 1998, 51, 1223–1233. [Google Scholar] [CrossRef]
- Brenner, H.; Blettner, M. Controlling for continuous confounders in epidemiologic research. Epidemiology 1997, 8, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.; Brady, M.J. The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics 1968, 24, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Gurka, M.J.; Golden, S.H.; Musani, S.K.; Sims, M.; Vishnu, A.; Guo, Y.; Cardel, M.; Pearson, T.A.; DeBoer, M.D. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: The Atherosclerosis Risk in Communities Study and Jackson Heart Study. Diabetologia 2017, 60, 1261–1270. [Google Scholar] [CrossRef]
- Dimitrov, B.D.; Bahchevanov, K.M.; Atanassova, P.A.; Mitkov, M.D.; Massaldjieva, R.I.; Chompalov, K.A.; Hadzhipetrov, G.K. Metabolic syndrome severity score: Range and associations with cardiovascular risk factors. Arch. Med. Sci. Atheroscler. Dis. 2016, 1, e90. [Google Scholar] [CrossRef]
- Guo, Y.; Musani, S.K.; Sims, M.; Pearson, T.A.; DeBoer, M.D.; Gurka, M.J. Assessing the added predictive ability of a metabolic syndrome severity score in predicting incident cardiovascular disease and type 2 diabetes: The Atherosclerosis Risk in Communities Study and Jackson Heart Study. Diabetol. Metab. Syndr. 2018, 10, 42. [Google Scholar] [CrossRef]
- Gurka, M.J.; Filipp, S.L.; Musani, S.K.; Sims, M.; DeBoer, M.D. Use of BMI as the marker of adiposity in a metabolic syndrome severity score: Derivation and validation in predicting long-term disease outcomes. Metabolism 2018, 83, 68–74. [Google Scholar] [CrossRef]
- Huh, J.H.; Lee, J.H.; Moon, J.S.; Sung, K.C.; Kim, J.Y.; Kang, D.R. Metabolic Syndrome Severity Score in Korean Adults: Analysis of the 2010–2015 Korea National Health and Nutrition Examination Survey. J. Korean Med. Sci. 2019, 34, e48. [Google Scholar] [CrossRef]
- Lee, K. Moderation Effect of Handgrip Strength on the Associations of Obesity and Metabolic Syndrome With Fatty Liver in Adolescents. J. Clin. Densitom. 2019. [Google Scholar] [CrossRef]
- Masson, W.; Epstein, T.; Huerín, M.; Lobo, L.M.; Molinero, G.; Angel, A.; Masson, G.; Millán, D.; De Francesca, S.; Vitagliano, L.; et al. Cardiovascular risk stratification in patients with metabolic syndrome Without diabetes or cardiovascular disease: Usefulness of metabolic syndrome severity score. High Blood Press. Cardiovasc. Prev. 2017, 24, 297–303. [Google Scholar] [CrossRef]
- Smiley, A.; King, D.; Harezlak, J.; Dinh, P.; Bidulescu, A. The association between sleep duration and lipid profiles: The NHANES 2013–2014. J. Diabetes Metab. Disord. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Smiley, A. Association of Sleep and Metabolic Syndrome/Metabolic Syndrome Components among Participants in Jackson Heart Study (JHS) and the Reasons for Geographic and Racial Differences in Stroke (REGARDS). Ph.D. Thesis, Indiana University, Bloomington, IN, USA, June 2019. [Google Scholar]
| Participants’ Characteristics | Metabolic Syndrome | |||
|---|---|---|---|---|
| No, n = 1735 | Yes, n = 793 | p | ||
| Age, Years, Mean (SD) | 48.5 (18.6) | 46.5 (17.6) | 0.02 | |
| Sex, n (%) | Male | 836 (33.1%) | 376 (14.9%) | 0.0001 |
| Female | 899 (35.6%) | 417 (16.5%) | ||
| Ethnicity, n (%) | Hispanic | 364 (14.4%) | 186 (6.9%) | 0.0001 |
| White | 729 (28.8%) | 388 (15.3%) | ||
| Black | 345 (13.6%) | 142 (5.6%) | ||
| Asian | 250 (9.9%) | 55 (2.2%) | ||
| Multiracial | 47 (1.9%) | 16 (0.6%) | ||
| Marital Status, n (%) | Married or Living with partner | 972 (40.7%) | 495 (20.7%) | 0.0001 |
| Widowed | 104 (4.3%) | 69 (2.9%) | ||
| Divorced or Separated | 191 (8.0%) | 130 (5.4%) | ||
| Never married | 345 (14.4%) | 85 (3.6%) | ||
| Education, n (%) | <9th grade | 104 (4.1%) | 79 (3.1%) | 0.0001 |
| 9–11th grade | 201 (8.0%) | 138 (5.5%) | ||
| High school | 334 (13.2%) | 173 (6.8%) | ||
| Some college | 481 (19.0%) | 247 (9.8%) | ||
| ≥College graduate | 490 (19.4%) | 141 (5.6%) | ||
| Sitting, Minutes/Day, Mean (SD) | 434 (527) | 470 (709) | 0.1 | |
| Sleep Duration, Hours, Mean (SD) | 6.82 (1.2) | 6.80 (1.3) | 0.6 | |
| Diastolic Blood Pressure, mmHg, Mean (SD) | 67.5 (11.2) | 71.5 (14.5) | 0.0001 | |
| Systolic Blood Pressure, mmHg, Mean (SD) | 117.5 (15.7) | 130.5 (17.8) | 0.0001 | |
| Fasting Blood Glucose, mg/dL, Mean (SD) | 98.2 (21) | 123.5 (46) | 0.0001 | |
| High Density Lipoprotein, mg/dL, Mean (SD) | 58.5 (15) | 44.0 (13) | 0.0001 | |
| Triglyceride, mg/dL, Mean (SD) | 93.2 (52) | 193.7 (129) | 0.0001 | |
| Waist Circumference, cm, Mean (SD) | 92.5 (14.4) | 110.1 (14.9) | 0.0001 | |
| R2 = 0.064 n = 2527 | Outcome: Metabolic Syndrome | |
|---|---|---|
| B | p | |
| Age | 0.03 | 0.0001 |
| Sex | ||
| Male | Reference Group | |
| Female | 0.04 | 0.60 |
| Race | ||
| Hispanic | Reference Group | |
| White | −0.12 | 0.30 |
| Black | −0.32 | 0.01 |
| Asian | −0.88 | 0.0001 |
| Multiracial | −0.25 | 0.40 |
| Sitting | 0.00007 | 0.30 |
| Sleep | Smooth Curve, EDF = 2.03 | 0.20 |
| R2 = 0.19 n = 2305 | Outcome: Metabolic Syndrome | |
|---|---|---|
| B | p | |
| Age | 0.013 | 0.0001 |
| Sex | ||
| Male | Reference Group | |
| Female | 0.38 | 0.0001 |
| Race | ||
| Hispanic | Reference Group | |
| White | 0.35 | 0.0001 |
| Black | 0.95 | 0.0001 |
| Asian | 0.09 | 0.20 |
| Multiracial | 0.27 | 0.04 |
| Sitting | 0.00009 | 0.004 |
| Sleep | Smooth Curve, EDF = 2.94 | 0.0004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smiley, A.; King, D.; Bidulescu, A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients 2019, 11, 2582. https://doi.org/10.3390/nu11112582
Smiley A, King D, Bidulescu A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients. 2019; 11(11):2582. https://doi.org/10.3390/nu11112582
Chicago/Turabian StyleSmiley, Abbas, David King, and Aurelian Bidulescu. 2019. "The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014" Nutrients 11, no. 11: 2582. https://doi.org/10.3390/nu11112582
APA StyleSmiley, A., King, D., & Bidulescu, A. (2019). The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients, 11(11), 2582. https://doi.org/10.3390/nu11112582






