The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurements
2.3. Statistical Analysis
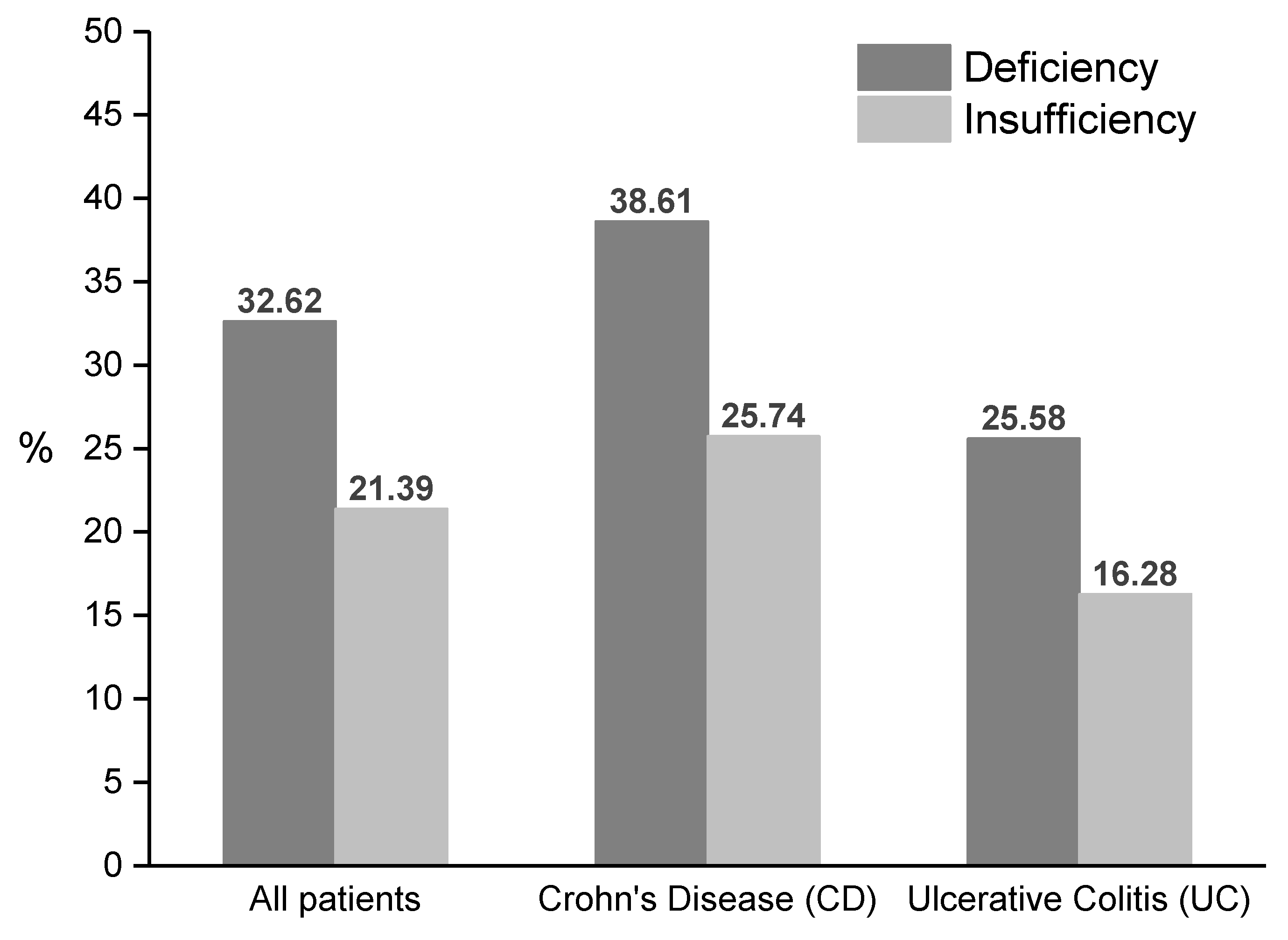
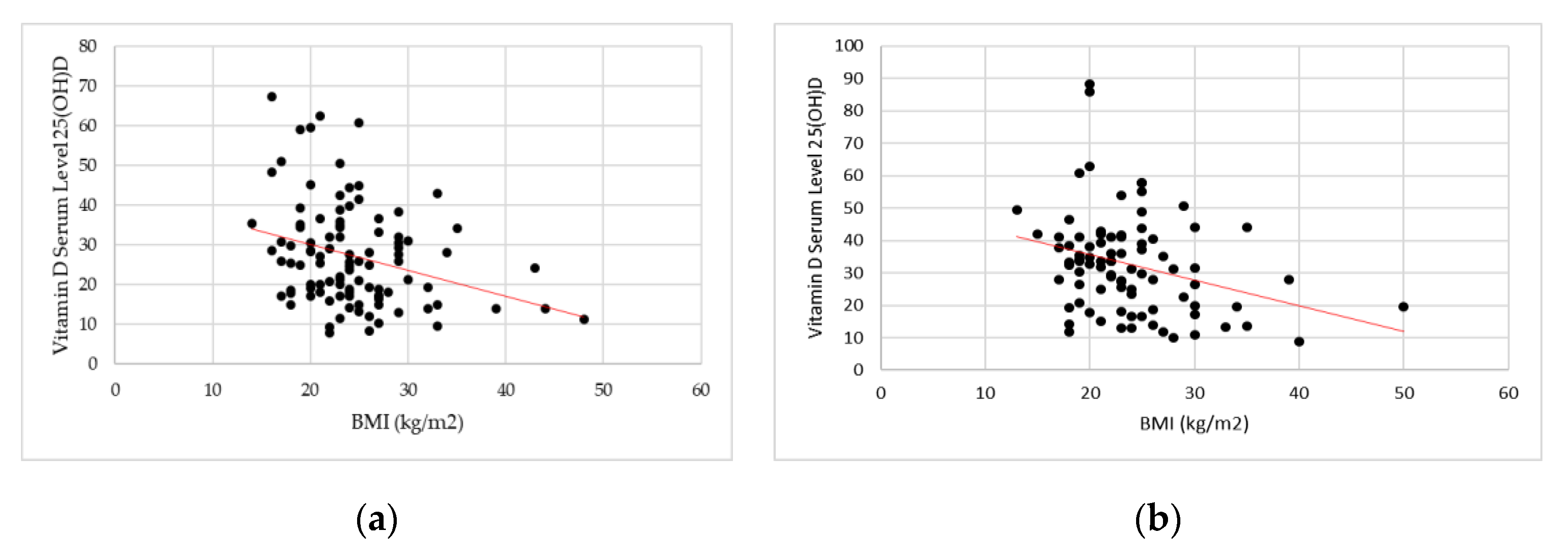
3. Results
4. Discussion
4.1. Direct Relationship: Vitamin D Deficiency as Risk Factor in the Onset of IBD or in the IBD Progression
4.2. Inverse Relationship: Onset Of Hypovitaminosis D In Patients with IBD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel disease with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Torki, M.; Gholamrezaei, A.; Mirbagher, L.; Danesh, M.; Kheiri, S.; Emami, M.H. Vitamin D Deficiency Associated with Disease Activity in Patients with Inflammatory Bowel Diseases. Dig. Dis. Sci. 2015, 60, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Hanauner, S.B. Inflammatory Bowel Disease: Epidemiology, Pathogenesis, and Therapeutic Opportunities. Inflamm. Bowel Dis. 2006, 12, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Series Gastroenterology 1 Inflammatory bowel disease: Cause and immunobiology. Apoptosis 2007, 369, 1627–1640. [Google Scholar]
- Raftery, T.; O’Sullivan, M. Optimal vitamin D levels in Crohn’s disease: A review. Proc. Nutr. Soc. 2015, 74, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Nedjat, S.; Vahedi, H.; Veghari, G.; Hosseinzadeh-Attar, M.J. Vitamin D Status and Its Relation to Inflammatory Markers in Patients with Mild to Moderate Ulcerative Colitis. Middle East J. Dig. Dis. 2018, 10, 84–89. [Google Scholar] [CrossRef]
- O’Sullivan, M. Vitamin D as a novel therapy in inflammatory bowel disease: New hope or false dawn? Proc. Nutr. Soc. 2015, 74, 5–12. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review article: Vitamin D and inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Kaplan, G.G. Environmental Risk Factors for Inflammatory Bowel Disease. Gastroenterol. Hepatol. (NY) 2010, 6, 339–346. [Google Scholar]
- Palmer, M.T.; Weaver, C.T. Linking vitamin D deficiency to inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Environmental risk factors for inflammatory bowel disease: A review. Dig. Dis. Sci. 2015, 60, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, S. Iron and vitamin D deficiency in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2016, 31, 27–28. [Google Scholar] [CrossRef] [Green Version]
- Ulitsky, A.; Ananthakrishnan, A.N.; Naik, A.; Skaros, S.; Zadvornova, Y.; Binion, D.G.; Issa, M. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. J. Parenter. Enter Nutr. 2011, 35, 308–316. [Google Scholar] [CrossRef]
- Yoon, S.M. Micronutrient deficiencies in inflammatory bowel disease: Trivial or crucial? Intest. Res. 2016, 14, 109. [Google Scholar] [CrossRef]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef]
- Nobile, S.; Tenace, M.A.; Pappa, H.M. The Role of Vitamin D in the Pathogenesis of Inflammatory Bowel Disease. Gastrointest. Disord. 2019, 1, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Limketkai, B.N.; Mullin, G.E.; Limsui, D.; Parian, A.M. Role of Vitamin D in inflammatory bowel disease. Nutr. Clin. Pract. 2017, 32, 337–345. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Rejnmark, L.; Moss, A.C. Role of vitamin D in the natural history of inflammatory bowel disease. J. Crohn’s Colitis 2018, 12, 742–752. [Google Scholar] [CrossRef]
- Caviezel, D.; Maissen, S.; Niess, J.H.; Kiss, C.; Hrux, P. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, H.; Schmidt, M.; Huth, A.; Reiner, J.; Glass, Ä.; Lamprecht, G. Clinical factors are associated with vitamin D levels in IBD patients: A retrospective analysis. J. Dig. Dis. 2018, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Lubel, J.S.; Sparrow, M.P.; Holt, S.G.; Gibson, P.R. Review article: Vitamin D and inflammatory bowel disease—Established concepts and future directions. Aliment. Pharmacol. Ther. 2012, 36, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Gominak, S.C. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmun. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Hanauner, S.B.; Li, Y.C. Mechanisms of disease: Vitamin D and inflammatory bowel disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2015, 2, 308–315. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Lubel, J.S.; Gibson, P.R. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2634–2643. [Google Scholar] [CrossRef]
- Alrefai, D.; Jones, J.; El-Matary, W.; Whiting, S.J.; Aljebreen, A.; Mirhosseini, N.; Vatanparast, H. The association of vitamin D status with disease activity in a cohort of crohn′s disease patients in Canada. Nutrients 2017, 9, 1112. [Google Scholar] [CrossRef]
- Ham, M.; Longhi, M.S.; Lahiff, C.; Cheifetz, A.; Robson, S.; Moss, A.C. Vitamin D levels in adults with Crohn’s disease are responsive to disease activity and treatment. Inflamm. Bowel Dis. 2014, 20, 856–860. [Google Scholar] [CrossRef]
- Reich, K.; Fedorak, R.N.; Madsen, K.; Kroeker, K.I. Vitamin D improves inflammatory bowel disease outcome: Basic science and clinical review. World J. Gastroenterol. 2014, 20, 4934–4947. [Google Scholar] [CrossRef]
- Kabbani, T.; Koutroubakis, I.; Schoen, R.; Ramos-Rivers, C.; Shah, N.; Swoger, J.; Regueiro, M.; Barrie, A.; Schwartz, M.; Hashash, J.; et al. Association of vitamin D level with clinical status in inflammatory bowel disease: A 5-year longitudinal study. Am. J. Gastroenterol. 2016, 111, 712–719. [Google Scholar] [CrossRef]
- Gubatan, J.; Mitsuhashi, S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2017, 15, 240.e1–246.e1. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, G.; Mihai, C.; Dranga, M.; Prelipcean, C.C. Serum 25-hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J. Gastroenterol. 2014, 20, 2393–2396. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Kim, Y.S.; Lee, B.K.; Choi, J.H.; Kim, J.Y.; Moon, J.S. Vitamin D deficiency is associated with disease activity in patients with Crohn’s disease. Intest. Res. 2019, 17, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.P.; Hvas, C.L.; Agnholt, J.; Christensen, L.A.; Heickendorff, L.; Dahlerup, J.F. Active Crohn’s disease is associated with low vitamin D levels. J. Crohn’s Colitis 2013, 7, e407–e413. [Google Scholar]
- Frigstad, S.O.; Høivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Torp, R.; et al. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Blanck, S.; Aberra, F. Vitamin D deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013, 58, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, Z.; Porojnicu, A.C.; Lindberg, F.; Hexeberg, S.; Moan, J. The Dependency of Vitamin D Status on Body Mass Index, Gender, Age and Season. Anticancer Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Nishiyama, V.K.G.; Albertini, S.M.; de Moraes, C.M.Z.G.; de Godoy, M.F.; Netinho, J.G. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq. Gastroenterol. 2018, 55, 397–402. [Google Scholar] [CrossRef]
- Pallav, K.; Riche, D.; May, W.L.; Sanchez, P.; Gupta, N.K. Predictors of vitamin D deficiency in inflammatory bowel disease and health: A Mississippi perspective. World J. Gastroenterol. 2017, 23, 638–645. [Google Scholar] [CrossRef]
- Gilman, J.; Shanahan, F.; Cashman, K.D. Determinants of vitamin D status in adult Crohn’s disease patients, with particular emphasis on supplemental vitamin D use. Eur. J. Clin. Nutr. 2006, 60, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Chesworth, B.; Madill, J. Is phase angle an appropriate indicator of malnutrition in different disease states? A systematic review. Clin. Nutr. ESPEN 2019, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, C.R.; Park, K.H.; Hwang, J.H.; Kim, S.H. Predicting clinical outcomes using phase angle as assessed by bioelectrical impedance analysis in maintenance hemodialysis patients. Nutrition 2017, 41, 7–13. [Google Scholar] [CrossRef] [PubMed]
- do Amaral Paes, T.C.; de Oliveira, K.C.C.; de Carvalho Padilha, P.; Peres, W.A.F. Phase angle assessment in critically ill cancer patients: Relationship with the nutritional status, prognostic factors and death. J. Crit. Care 2018, 44, 430–435. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care. 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Peres, W.a.F.; Lento, D.F.; Baluz, K.; Ramalho, A. Phase angle as a nutritional evaluation tool in all stages of chronic liver disease. Nutr Hosp. 2012, 27, 2072–2078. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation, 2000, Series 894. Available online: https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 11 May 2019).
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Series Gastroenterology 2 Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Atef, S.H. Vitamin D assays in clinical laboratory: Past, present and future challenges. J. Steroid. Biochem. Mol. Biol. 2018, 175, 136–137. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Shivananda, S.; Lennard-Jones, J.; Logan, R.; Fear, N.; Price, A.; Carpenter, L.; Van Blankenstein, M. Incidence of inflammatory bowel disease across Europe: Is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut 1996, 39, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Nerich, V.; Monnet, E.; Etienne, A.; Louafi, S.; Ramée, C.; Rican, S.; Weill, A.; Vallier, N.; Vanbockstael, V.; Auleley, G.-R.; et al. Geographical variations of inflammatory bowel disease in France: A study based on national health insurance data. Inflamm. Bowel Dis. 2006, 13, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Scotti, G.B.; Afferria, M.T.; de Carolisa, A.; Vaiarelloa, V.; Fassinob, V.; Ferroneb, F.; Minisolab, S.; Niedduc, L.; Verniaa, P. Factors affecting vitamin D deficiency in active inflammatory bowel diseases. Dig. Liver Dis. 2018, 6–11. [Google Scholar] [CrossRef]
- Lippi, G.; Nouvenne, A.; Ticinesi, A.; Bonelli, P.; Salvagno, G.L.; Cervellin, G. The burden of vitamin D deficiency in a mediterranean country without a policy of food fortification. Acta Biomed. 2015, 86, 59–62. [Google Scholar] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Saneei, P.; Siassi, F.; Esmaillzadeh, A. Vitamin D status in relation to Crohn’s disease: Meta-analysis of observational studies. Nutrition 2016, 32, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.J.; George, B.; Pulimood, A.B.; Seshadri, M.S.; Chacko, A. 25 (OH) vitamin D level in Crohn’s disease: Association with sun exposure & disease activity. Indian J. Med. Res. 2009, 130, 133–137. [Google Scholar] [PubMed]
- Hassan, V.; Hassan, S.; Seyed-Javad, P.; Ahmad, K.; Asieh, H.; Maryam, S.; Farid, F.; Siavash, A. Association between serum 25 (OH) vitamin D concentrations and inflammatory bowel diseases (IBDS) activity. Med. J. Malay. 2013, 68, 34–38. [Google Scholar]
- Armitage, E.L.; Aldhous, M.C.; Anderson, N.; Drummond, H.E.; Riemersma, R.A.; Ghosh, S.; Satsangi, J. Incidence of juvenile-onset Crohn’s disease in Scotland: Association with northern latitude and affluence. Gastroenterology 2004, 127, 1051–1057. [Google Scholar] [CrossRef]
- Farraye, F.A.; Nimitphong, H.; Stucchi, A.; Dendrinos, K.; Boulanger, A.B.; Vijjeswarapu, A.; Tanennbaum, A.; Biancuzzo, R.; Chen, T.C.; Holick, M.F. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 2116–2121. [Google Scholar] [CrossRef]
- Tajika, M.; Matsuura, A.; Nakamura, T.; Suzuki, T.; Sawaki, A.; Kato, T.; Hara, K.; Ookubo, K.; Yamao, K.; Kato, M.; et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J. Gastroenterol. 2003, 39, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Somma, C.D.; Salzano, C.; Pugliese, G.; Alteriis, G.; Colao, A.; Savastano, S. Phase Angle: A Possible Biomarker to Quantify Inflammation in Subjects with Obesity and 25(OH)D Deficiency. Nutrients 2019, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Berry, D.J.; Lü, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef] [PubMed]

| Disease Activity | UC (Partial Mayo Score) | CD (Harvey-Bradshaw Index) |
|---|---|---|
| Remission | <2 | <5 |
| Mild Disease | 2–4 | 5–7 |
| Moderate Disease | 5–7 | 8–16 |
| Severe Disease | >7 | >16 |
| Parameter | Total (n = 206) | CD (n = 101) | UC (n = 105) | p |
|---|---|---|---|---|
| Gender (%) | ||||
| Female | 102 (49.51) | 52 (51.49) | 50 (47.62) | 0.579 |
| Male | 104 (50.49) | 49 (48.51) | 55 (52.38) | |
| Age | ||||
| At diagnosis, mean (SD | 36.78 (15.66) | 37.9 (16.64) | 35.7 (14.65) | 0.4606 |
| At the last follow-up, mean (SD) | 44.1 (15.77) | 43.96 (16.33) | 44.24 (15.28) | 0.8498 |
| Smoke (%) | ||||
| Non Smoker | 36 (52.94) | 13 (50.00) | 23 (54.76) | 0.354 |
| Smoker | 15 (22.06) | 8 (30.77) | 7 (16.67) | |
| Former Smoker | 17 (25.00) | 5 (19.23) | 12 (28.57) | |
| Body Mass Index (BMI, kg/m2), mean (SD) | 24.03 (5.68) | 24.43 (5.92) | 23.66 (5.44) | 0.3192 |
| Phase Angle, mean (SD) | 5.41 (1.00) | 5.40 (1.00) | 5.50 (1.00) | 0.615 |
| Glycemia (%) | ||||
| <65 mg/dL | 57 (30.65) | 27 (29.03) | 30 (32.26) | 0.868 |
| 65–110 mg/dL | 118 (63.44) | 60 (64.52) | 58 (62.37) | |
| >110 mg/dL | 11 (5.91) | 6 (6.45) | 5 (5.38) | |
| Hemoglobin (%) | ||||
| low | 23 (12.71) | 12 (13.04) | 11 (12.36) | 0.608 |
| normal | 154 (85.08) | 77 (83.70) | 77 (86.52) | |
| high | 4 (2.21) | 3 (3.26) | 1 (1.12) | |
| CRP (%) | ||||
| <5.0 mg/L | 133 (72.28) | 67 (72.04) | 66 (72.53) | 0.941 |
| >5.0 mg/L | 51 (27.73) | 26 (27.96) | 25 (27.47) | |
| Total Proteins (%) | ||||
| <65 g/L | 5 (2.87) | 3 (3.53) | 2 (2.25) | 0.573 |
| 65–85 g/L | 153 (87.93) | 76 (89.41) | 77 (86.52) | |
| >85 g/L | 16 (9.20) | 6 (7.06) | 10 (11.24) | |
| Albumin (%) | ||||
| <34 g/L | 10 (6.02) | 7 (8.54) | 3 (3.57) | 0.254 |
| 34–48 g/L | 155 (93.37) | 75 (91.46) | 80 (95.24) | |
| >48 g/L | 1 (0.60) | - | 1 (1.19) | |
| Folates (%) | ||||
| <4 ng/mL | 55 (32.16) | 34 (40.96) | 21 (23.86) | 0.017 |
| >4 ng/mL | 116 (67.84) | 49 (59.04) | 67 (76.14) | |
| Iron (%) | ||||
| low | 72 (43.11) | 49 (48.51) | 23 (34.85) | 0.031 |
| normal | 92 (55.09) | 52 (51.49) | 40 (60.61) | |
| high | 3 (1.80) | - | 3 (4.55) | |
| Vitamin B12 (%) | ||||
| low | 23 (12.11) | 22 (22.45) | 1 (1.09) | 0.000 |
| normal | 158 (83.16) | 72 (73.47) | 86 (93.48) | |
| high | 9 (4.74) | 4 (4.08) | 5 (5.43) | |
| Parameter | Total (n = 206) | CD (n = 101) | UC (n = 105) | p |
|---|---|---|---|---|
| Disease duration (years), mean (SD) | 7.33 (7.96) | 6.06 (7.33) | 8.54 (8.39) | 0.016 |
| Disease Activity (%) | ||||
| Remission | 55 (27.09) | 28 (28.28) | 27 (25.96) | 0.145 |
| Mild | 69 (33.99) | 40 (40.40) | 29 (27.88) | |
| Moderate | 34 (16.75) | 14 (14.14) | 20 (19.23) | |
| Severe | 45 (22.17) | 17 (17.17) | 28 (26.92) | |
| Body-mass Index (BMI) (%) | ||||
| underweight | 26 (12.62) | 13 (12.87) | 13 (12.38) | 0.964 |
| normal weight | 101 (49.03) | 47 (46.53) | 54 (51.43) | |
| pre-obesity | 53 (25.73) | 28 (27.72) | 25 (23.81) | |
| obesity class I | 16 (7.77) | 8 (7.92) | 8 (7.62) | |
| obesity class II | 5 (2.43) | 2 (1.98) | 3 (2.86) | |
| obesity class III | 5 (2.43) | 3 (2.97) | 2 (1.90) | |
| Parameter | Chron’s Disease (n = 101) | Ulcerative Colitis (n = 105) | ||||
|---|---|---|---|---|---|---|
| Vitamin D Deficiency | No Vitamin D Deficiency | p | Vitamin D Deficiency | No Vitamin D Deficiency | p | |
| Disease Activity (%) | ||||||
| Remission | 42.9 | 57.1 | 0.001 | 22.7 | 77.3 | 0.106 |
| Mild | 20.0 | 80.0 | 9.1 | 90.9 | ||
| Moderate | 78.6 | 21.4 | 41.2 | 58.8 | ||
| Severe | 47.1 | 52.9 | 33.3 | 66.7 | ||
| BMI classes (%) | ||||||
| underweight | 30.8 | 69.2 | 0.625 | 25.0 | 75.0 | 0.013 |
| normal weight | 31.9 | 68.1 | 14.3 | 85.7 | ||
| pre-obesity | 46.4 | 53.6 | 26.3 | 73.7 | ||
| obesity class I | 50.0 | 50.0 | 62.5 | 37.5 | ||
| obesity class II | 50.0 | 50.0 | 33.3 | 66.7 | ||
| obesity class III | 66.7 | 33.3 | 100.0 | 0.0 | ||
| Parameter | Crohn’s Disease—OR (SE) | Ulcerative Colitis—OR (SE) |
|---|---|---|
| BMI (kg/m2) | 1.05 (0.04) | 1.12 *** (0.05) |
| Phase Angle | 0.66 ** (0.11) | 0.49 *** (0.10) |
| Disease Duration | 1.03 (0.04) | 0.97 (0.04) |
| Disease Activity | ||
| Remission | 3.35 ** (2.04) | 1.44 (1.10) |
| Moderate | 11.22 *** (9.09) | 2.67 (1.98) |
| Severe | 2.23 (1.58) | 2.69 (1.92) |
| Gender (male) | 0.41 (0.22) | 0.64 (0.38) |
| LR chi2(5) | 25.89 | 28.81 |
| p-value | 0.000 | 0.000 |
| Num. Obs | 98 | 84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients 2019, 11, 2583. https://doi.org/10.3390/nu11112583
Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GAD. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients. 2019; 11(11):2583. https://doi.org/10.3390/nu11112583
Chicago/Turabian StyleMentella, Maria Chiara, Franco Scaldaferri, Marco Pizzoferrato, Antonio Gasbarrini, and Giacinto Abele Donato Miggiano. 2019. "The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD" Nutrients 11, no. 11: 2583. https://doi.org/10.3390/nu11112583
APA StyleMentella, M. C., Scaldaferri, F., Pizzoferrato, M., Gasbarrini, A., & Miggiano, G. A. D. (2019). The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients, 11(11), 2583. https://doi.org/10.3390/nu11112583





