Effects of Euglena gracilis EOD-1 Ingestion on Salivary IgA Reactivity and Health-Related Quality of Life in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
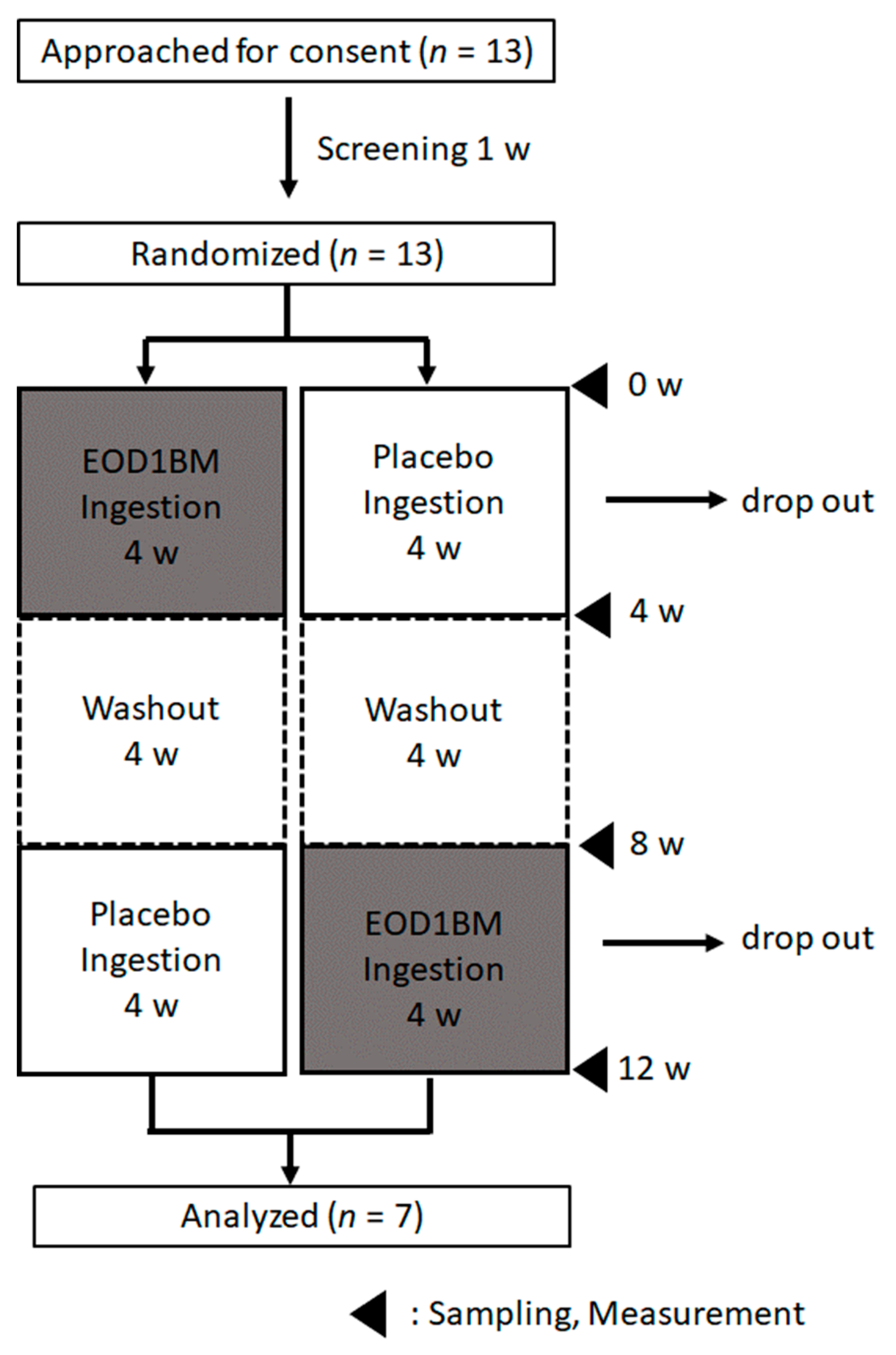
2.2. Ingestion Study
2.2.1. Target Population for Analysis
2.2.2. Test Method
2.2.3. Saliva Collection
2.2.4. Health-Related QOL Scale Measurement
2.3. Flow Cytometry Analysis for the Reactivity of Anti-EOD1PM Antibody
2.4. ELISA for Anti-B Glucan Antibody in Serum and Saliva
2.5. Statistical Analyses
3. Results
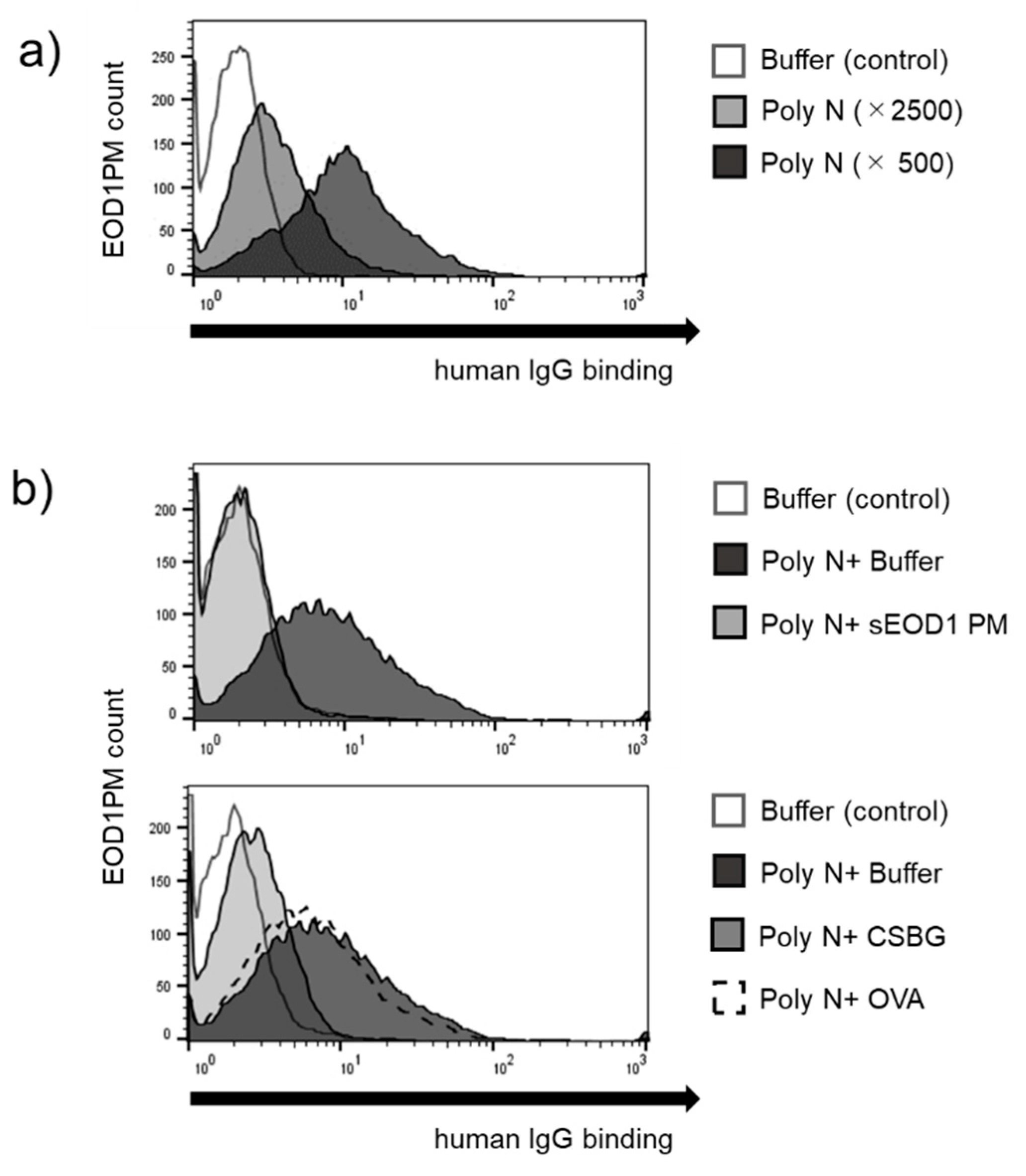
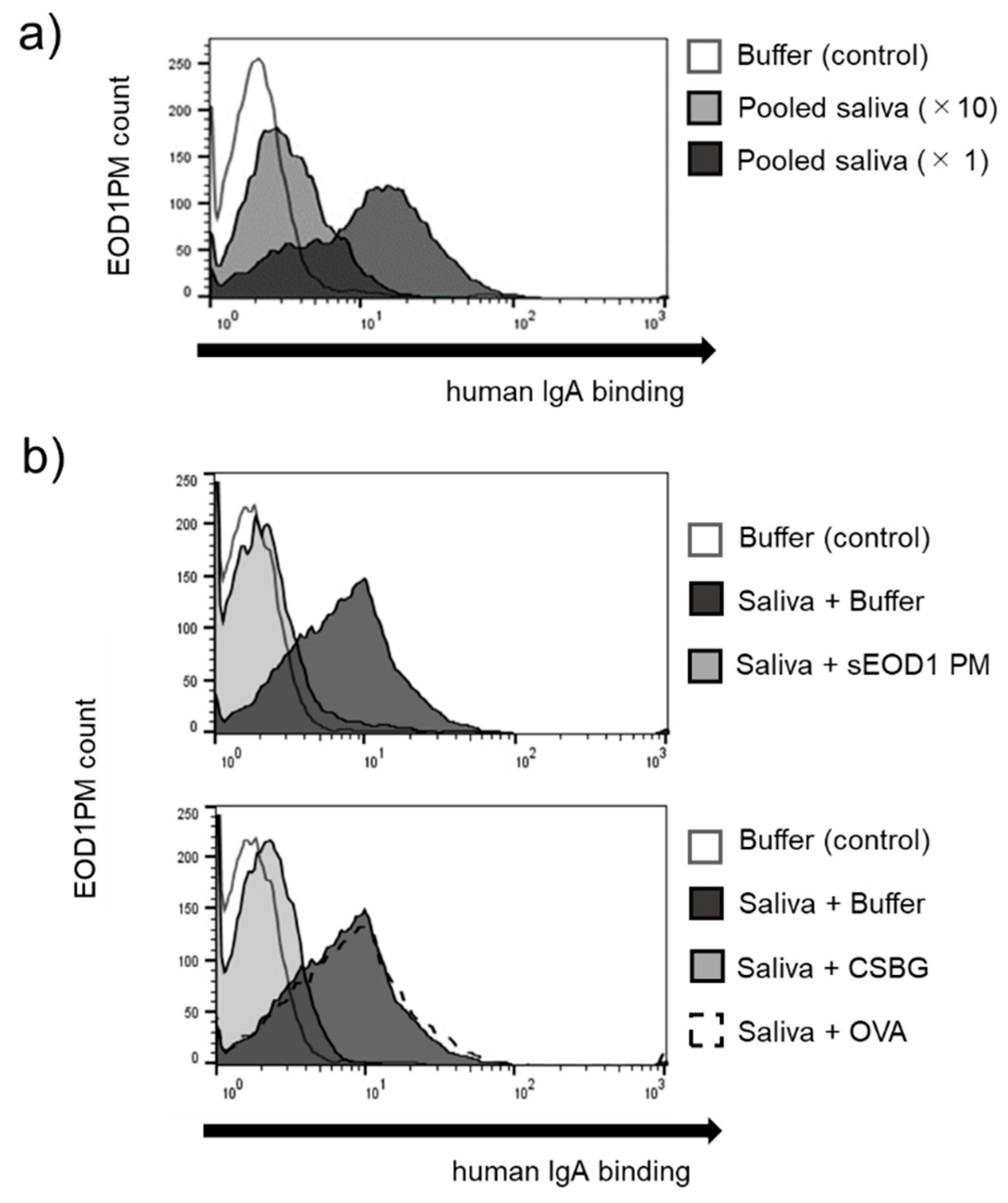
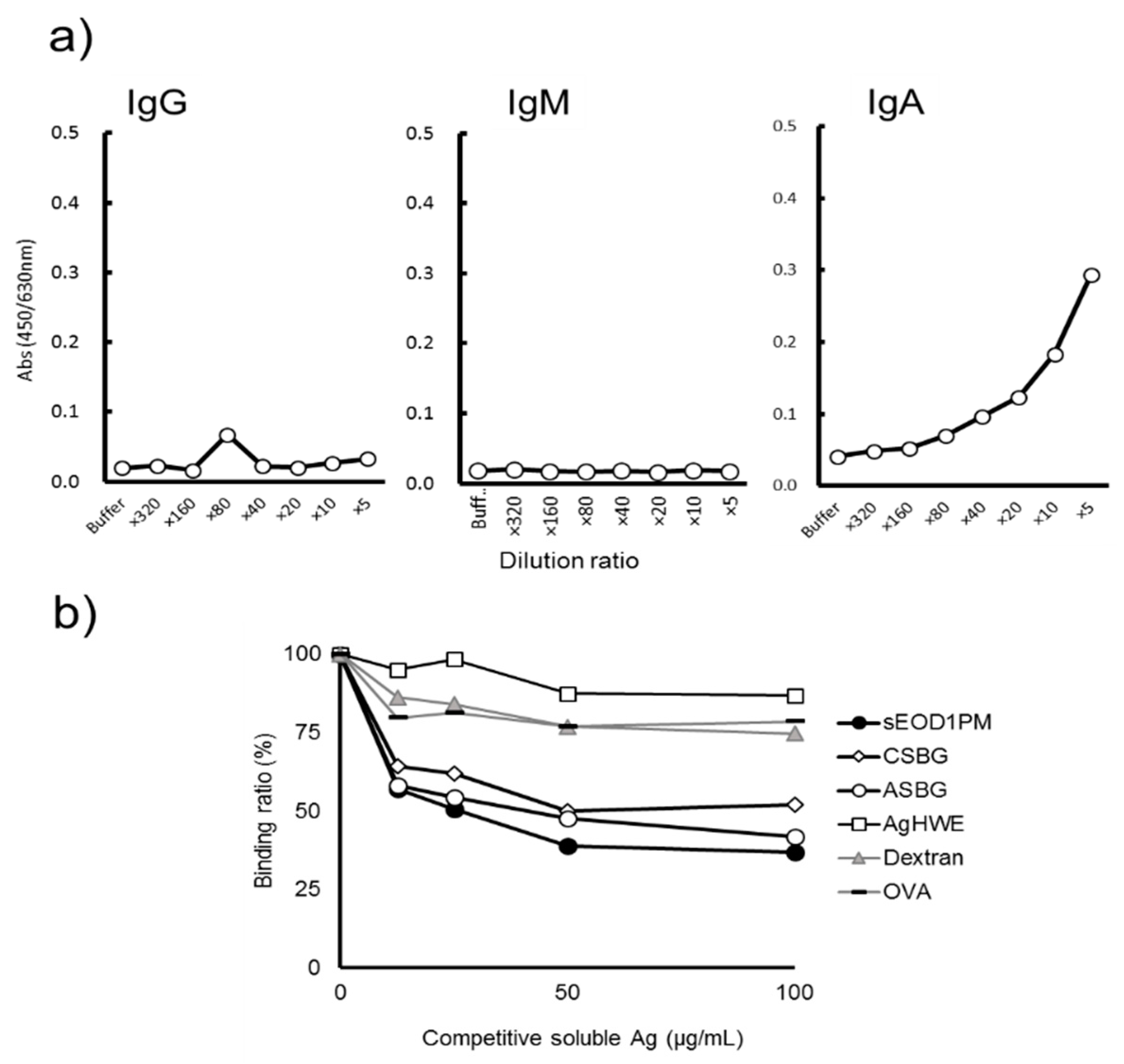
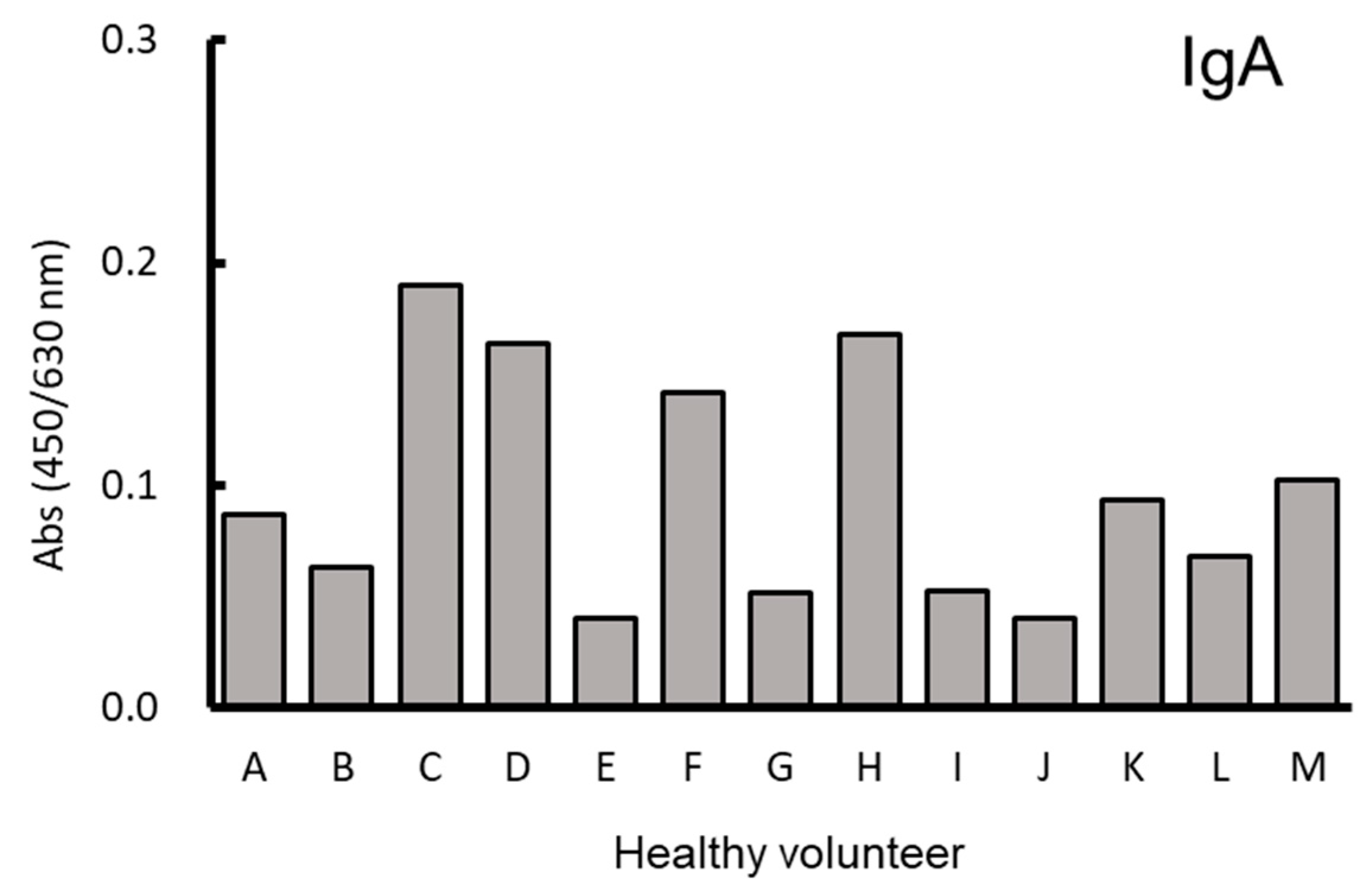
3.1. Binding of Human Serum and Saliva Immunoglobulin to Paramylon Granules
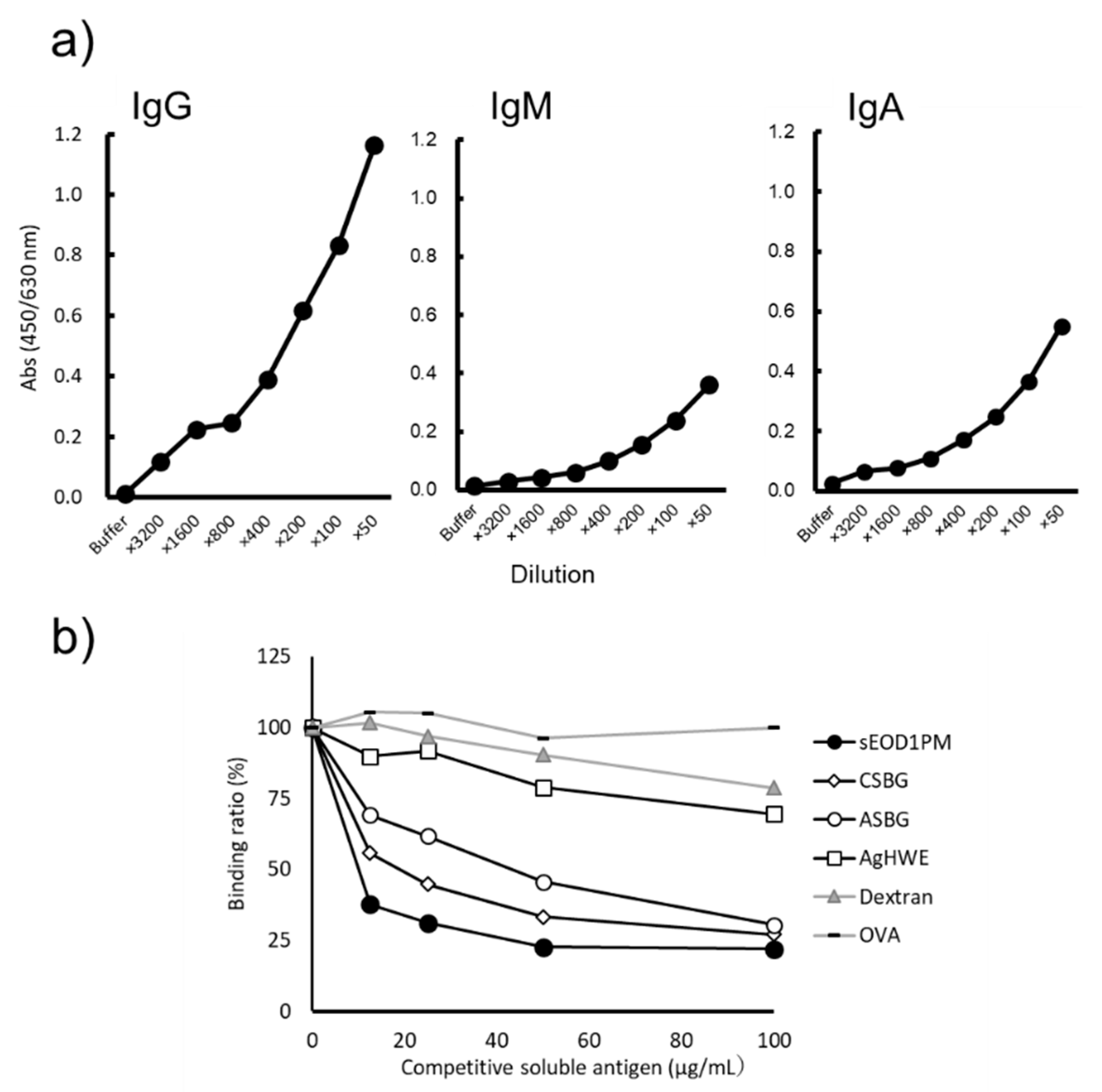
3.2. Anti-EOD1PM Antibody Reactivity to Solubilized EOD1PM in ELISA
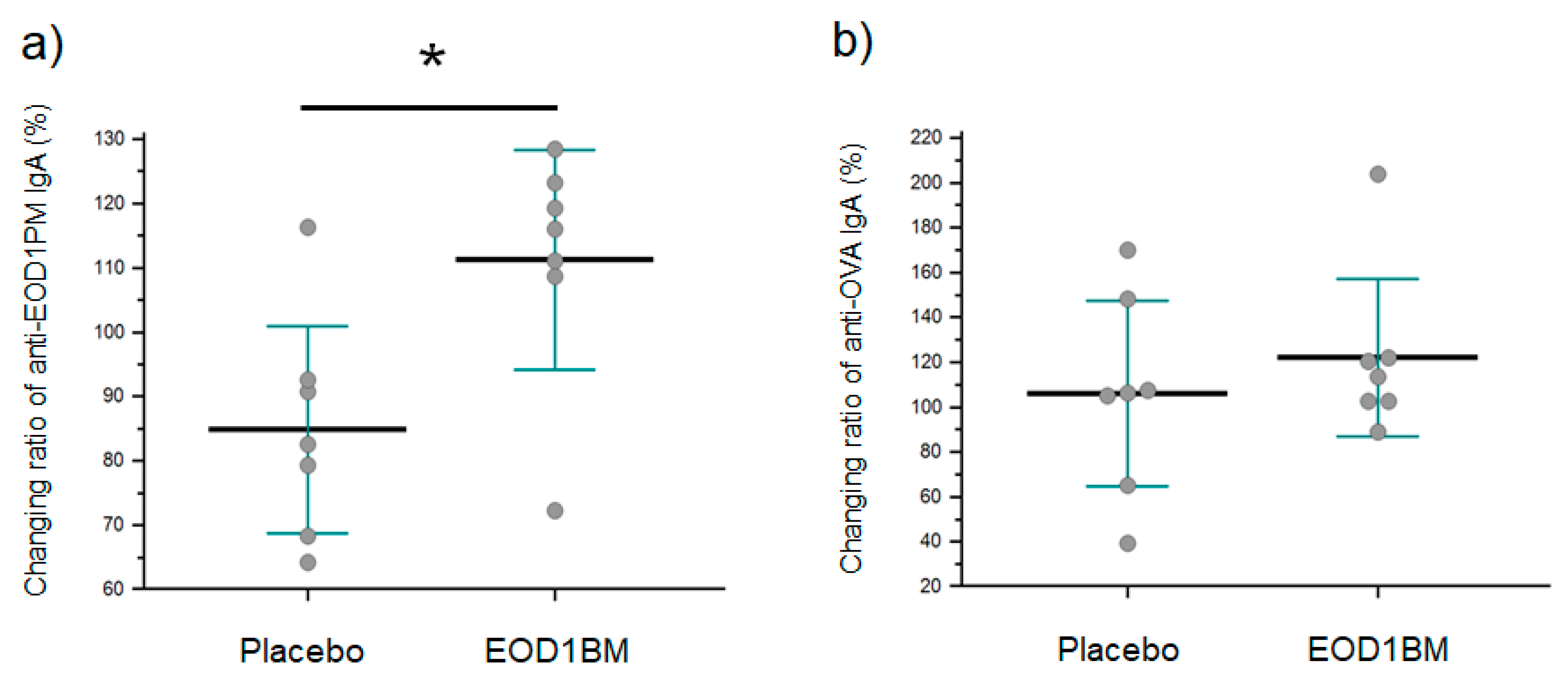
3.3. Influence of EOD1BM Ingestion on Salivary Anti-EOD1PM IgA Antibody Titer
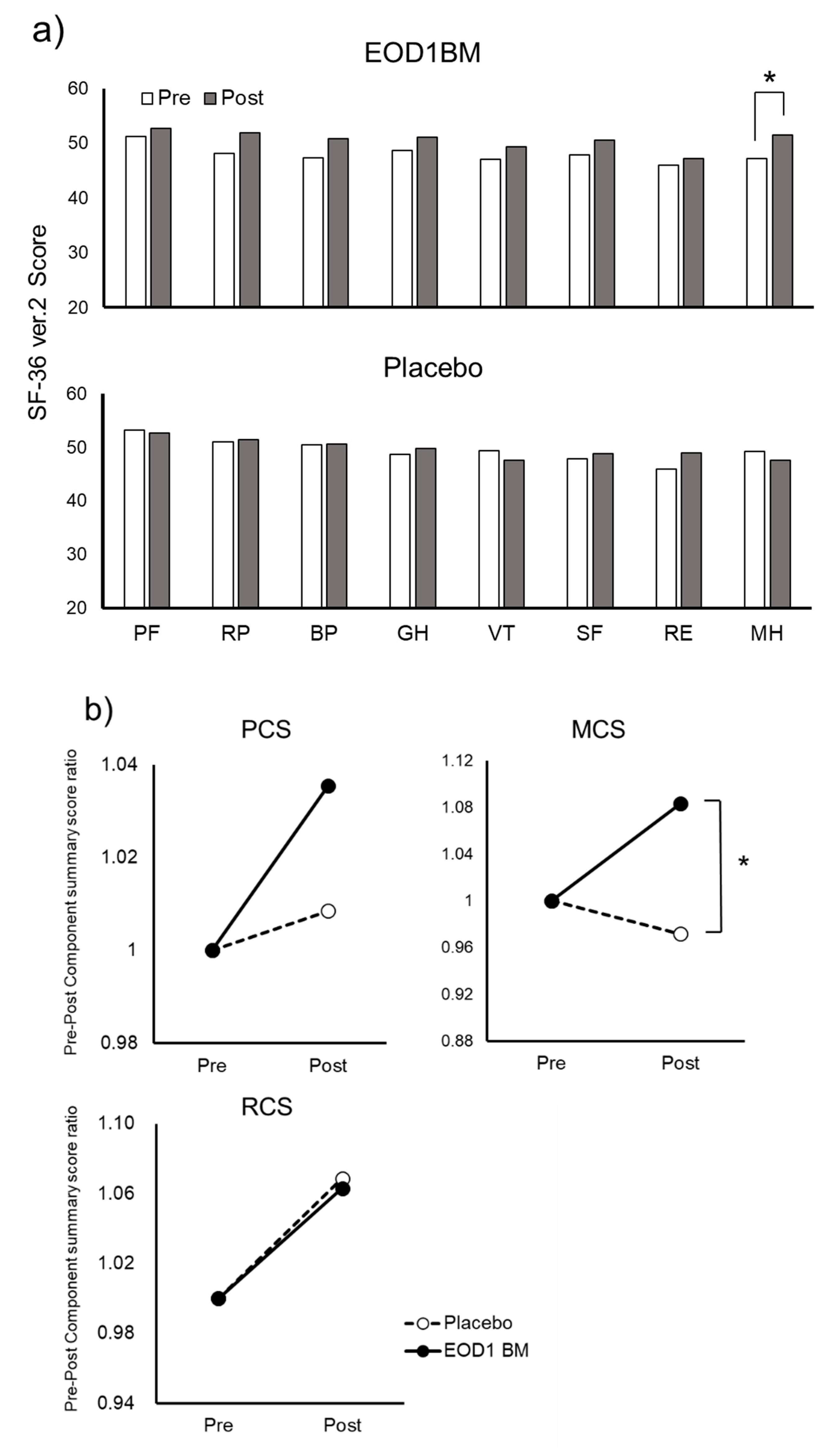
3.4. Effect of EOD1BM Ingestion on Health-Related QOL
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 494, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Horst, G.; Tonda, R.; Lumpkins, B.; Mathis, G. Evaluation of the effects of feeding dried algae containing beta-1, 3-glucan on broilers challenged with Eimeria. Poult. Sci. 2018, 97, 3494–3500. [Google Scholar] [CrossRef]
- Bianchi, V.A.; Castro, J.M.; Rocchetta, I.; Conforti, V.; Pascual, M.; Luquet, C.M. Modulating effects of orally supplied Euglena gracilis on the physiological responses of the freshwater mussel Diplodon chilensis, exposed to sewage water pollution in a Patagonian river (Argentina). Fish Shellfish Immunol. 2016, 51, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kawashima, J.; Nishida, N.; Onaka, N. Beta-Glucan Derived from Euglena (Paramylon); CMC Publishing Co., Ltd.: Tokyo, Japan, 2018; pp. 174–182. [Google Scholar]
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2017, 5, 205–214. [Google Scholar] [CrossRef]
- Sugiyama, A.; Hata, S.; Suzuki, K.; Yoshida, E.; Nakano, R.; Mitra, S.; Arashida, R.; Asayama, Y.; Yabuta, Y.; Takeuchi, T. Oral administration of paramylon, a beta-1, 3-d-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Vet. Med. Sci. 2010, 72, 755–763. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Ohno, N. Modulation of host defense systems by beta-glucans. Nihon Saikingaku Zasshi 2000, 55, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Adachi, Y.; Ohno, N. Contribution of dectin-1 to the recognition of fungal cell wall products and the activation of innate immune response. Nihon Ishinkin Gakkai Zasshi 2006, 47, 185–194. [Google Scholar] [CrossRef]
- Ishibashi, K.; Yoshida, M.; Nakabayashi, I.; Shinohara, H.; Miura, N.N.; Adachi, Y.; Ohno, N. Role of anti-beta-glucan antibody in host defense against fungi. FEMS Immunol. Med. Microbiol. 2005, 44, 99–109. [Google Scholar] [CrossRef]
- Ishibashi, K.; Dogasaki, C.; Motoi, M.; Miura, N.; Adachi, Y.; Ohno, N. Anti-fungal cell wall β-glucan antibody in animal sera. Nihon Ishinkin Gakkai Zasshi 2010, 51, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Morita, M.; Motoi, M.; Liu, Y.; Miura, N.N.; Adachi, Y.; Ohno, N. Analysis of the titer and reactivity of antibody/ies against fungal cell wall beta-glucans in human sera. Int. J. Med. Mushrooms 2013, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Esfahani, A.; Jafarabadi, M.A.; Ziaei, J.E.; Movassaghpourakbari, A.; Farrin, N. Effect of Beta glucan on quality of life in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Adv. Pharm. Bull. 2014, 4, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.M.; Talbott, J.A. Baker’s yeast beta-glucan supplement reduces upper respiratory symptoms and improves mood state in stressed women. J. Am. Coll. Nutr. 2012, 31, 295–300. [Google Scholar] [CrossRef]
- Ohno, N.; Uchiyama, M.; Tsuzuki, A.; Tokunaka, K.; Miura, N.N.; Adachi, Y.; Aizawa, M.W.; Tamura, H.; Tanaka, S.; Yadomae, T. Solubilization of yeast cell-wall β-(1→3)-d-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr. Res. 1999, 316, 161–172. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, N.N.; Adachi, Y.; Tamura, H.; Tanaka, S.; Ohno, N. The solubilization and biological activities of Aspergillus β-(1→3)-d-glucan. FEMS Immunol. Med. Microbiol. 2004, 42, 155–166. [Google Scholar] [CrossRef]
- Ohno, N.; Furukawa, M.; Miura, N.N.; Adachi, Y.; Motoi, M.; Yadomae, T. Antitumor β-glucan from the cultured fruit body of Agaricus blazei. Biol. Pharm. Bull. 2001, 24, 820–828. [Google Scholar] [CrossRef]
- Ojiri, Y.; Endoh, H.; Okumoto, T.; Atsuta, K.; Yoshinari, O.; Moriyama, H. Randomized, double-blind, placebo-controlled, crossover study to evaluate the effects of beta-1,3/1,6 glucan on stress associated with daily lifestyle in healthy subjects. Funct. Foods Health Dis. 2015, 5, 145–154. [Google Scholar] [CrossRef]
- Keogh, G.F.; Cooper, G.J.; Mulvey, T.B.; McArdle, B.H.; Coles, G.D.; Monro, J.A.; Poppitt, S.D. Randomized controlled crossover study of the effect of a highly beta-glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 2003, 78, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fukuwatari, Y.; Okumura, K.; Takeda, K.; Ishibashi, K.; Furukawa, M.; Ohno, N.; Mori, K.; Gao, M.; Motoi, M. Immunomodulating activity of Agaricus brasiliensis KA21 in mice and in human volunteers. Evid.-Based Complement. Altern. Med. 2008, 5, 205–219. [Google Scholar] [CrossRef]
- Fukuhara, S.; Ware, J.E., Jr.; Kosinski, M.; Wada, S.; Gandek, B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1045–1053. [Google Scholar] [CrossRef]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Fukuhara, S.; Suzukamo, Y. Manual of SF-36v2 Japanese Version; Institute for Health Outcomes and Process Evaluation Research: Kyoto, Japan, 2004; pp. 7–145. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, A.M. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Carpenter, K.C.; Davidson, T.; McFarlin, M.A. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J. Diet. Suppl. 2013, 10, 171–183. [Google Scholar] [CrossRef]
- Cohen-Kedar, S.; Baram, L.; Elad, H.; Brazowski, E.; Guzner-Gur, H.; Dotan, I. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur. J. Immunol. 2014, 44, 3729–3740. [Google Scholar] [CrossRef]
- Jesenak, M.; Urbancikova, I.; Banovcin, P. Respiratory tract infections and the role of biologically active polysaccharides in their management and prevention. Nutrients 2017, 9, 779. [Google Scholar] [CrossRef]
- Borchani, C.; Fonteyn, F.; Jamin, G.; Destain, J.; Willems, L.; Paquot, M.; Blecker, C.; Thonart, P. Structural characterization, technological functionality, and physiological aspects of fungal β-d-glucans. Crit. Rev. Food Sci. Nutr. 2016, 56, 1746–1752. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Ishibashi, K.; Yamanaka, D.; Adachi, Y.; Kanzaki, K.; Okita, K.; Iwakura, Y.; Ohno, N. Modulation of an innate immune response by soluble yeast β-glucan prepared by a heat degradation method. Int. J. Biol. Macromol. 2017, 104, 367–376. [Google Scholar] [CrossRef]
- Masuzawa, S.; Yoshida, M.; Ishibashi, K.I.; Saito, N.; Akashi, M.; Yoshikawa, N.; Suzuki, T.; Nameda, S.; Miura, N.N.; Adachi, Y.; et al. Solubilized candida cell wall β-glucan, CSBG, is an epitope of natural human antibody. Drug Dev. Res. 2003, 58, 179–189. [Google Scholar] [CrossRef]
- Feldman, S.; Schwartz, H.I.; Kalman, D.S.; Mayers, A.; Kohrman, H.M.; Clemens, R.; Krieger, D.R. Randomized phase II clinical trials of Wellmune WGP® for immune support during cold and flu season. J. Appl. Res. 2009, 9, 30–43. [Google Scholar]
- Talbott, S.M.; Talbott, J.A.; Talbott, T.L.; Dingler, E. β-Glucan supplementation, allergy symptoms, and quality of life in self-described ragweed allergy sufferers. Food Sci. Nutr. 2013, 1, 90–101. [Google Scholar] [CrossRef]
- Kamiya, T.; Tang, C.; Kadoki, M.; Oshima, K.; Hattori, M.; Saijo, S.; Adachi, Y.; Ohno, N.; Iwakura, Y. β-Glucans in food modify colonic microflora by inducing antimicrobial protein, calprotectin, in a Dectin-1-induced-IL-17F-dependent manner. Mucosal Immunol. 2018, 11, 763–773. [Google Scholar] [CrossRef]
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.H.; Kim, E.; Kim, S.I.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting from Here to There. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
| Pre | Post | Pre-Post Ratio | |||
|---|---|---|---|---|---|
| s-IgA concentration (μg/mL) | Placebo | 31.1 ± 10.7 | 22.9 ± 7.6 | 0.77 ± 0.82 |  |
| EOD1BM | 23.1 ± 10.5 | 27.0 ± 4.2 | 1.41 ± 0.26 | ||
| s-IgA secretion rate (μg/min) | Placebo | 48.6 ± 11.1 | 41.6 ± 13.5 | 0.88 ± 0.28 |  |
| EOD1BM | 34.2 ± 16.5 | 50.9 ± 13.0 * | 1.91 ± 1.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, K.-i.; Nishioka, M.; Onaka, N.; Takahashi, M.; Yamanaka, D.; Adachi, Y.; Ohno, N. Effects of Euglena gracilis EOD-1 Ingestion on Salivary IgA Reactivity and Health-Related Quality of Life in Humans. Nutrients 2019, 11, 1144. https://doi.org/10.3390/nu11051144
Ishibashi K-i, Nishioka M, Onaka N, Takahashi M, Yamanaka D, Adachi Y, Ohno N. Effects of Euglena gracilis EOD-1 Ingestion on Salivary IgA Reactivity and Health-Related Quality of Life in Humans. Nutrients. 2019; 11(5):1144. https://doi.org/10.3390/nu11051144
Chicago/Turabian StyleIshibashi, Ken-ichi, Machiko Nishioka, Nobuteru Onaka, Madoka Takahashi, Daisuke Yamanaka, Yoshiyuki Adachi, and Naohito Ohno. 2019. "Effects of Euglena gracilis EOD-1 Ingestion on Salivary IgA Reactivity and Health-Related Quality of Life in Humans" Nutrients 11, no. 5: 1144. https://doi.org/10.3390/nu11051144
APA StyleIshibashi, K.-i., Nishioka, M., Onaka, N., Takahashi, M., Yamanaka, D., Adachi, Y., & Ohno, N. (2019). Effects of Euglena gracilis EOD-1 Ingestion on Salivary IgA Reactivity and Health-Related Quality of Life in Humans. Nutrients, 11(5), 1144. https://doi.org/10.3390/nu11051144





