Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo—Evidence from Human Studies
Abstract
1. Introduction
2. Methods
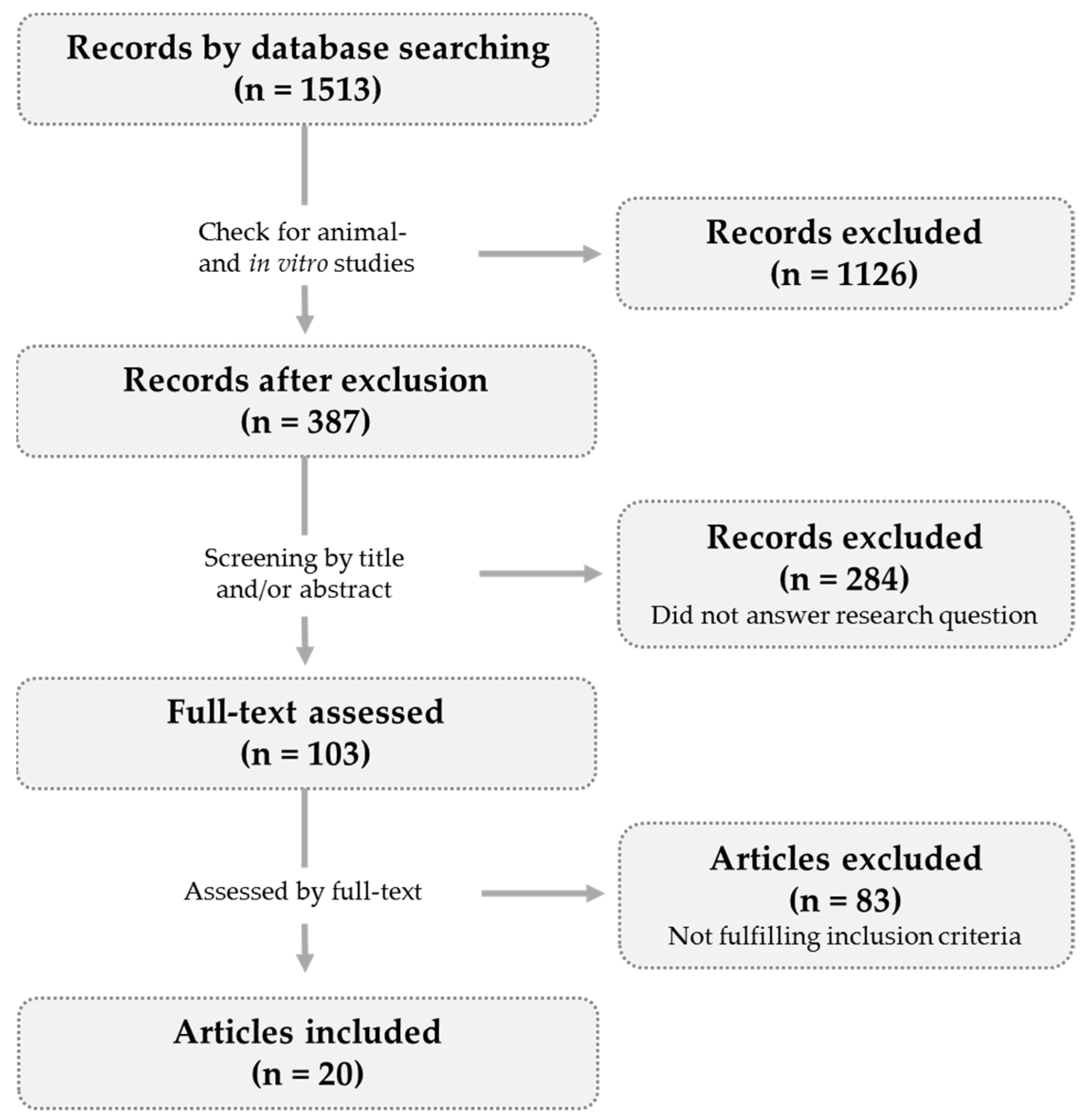
2.1. Article Selection
2.2. Data Presentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Almeida, M.; O’Brien, C.A. Basic biology of skeletal aging: Role of stress response pathways. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Akesson, K. Biochemical markers of bone turnover: A review. Acta Orthop. Scand. 2009, 66, 376–386. [Google Scholar] [CrossRef]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; van Rietbergen, B. Bone remodelling in humans is load-driven but not lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Turner, C.H.; Robling, A.G. Mechanical loading and bone formation. IBMS Bonekey 2004, 1, 15–23. [Google Scholar] [CrossRef]
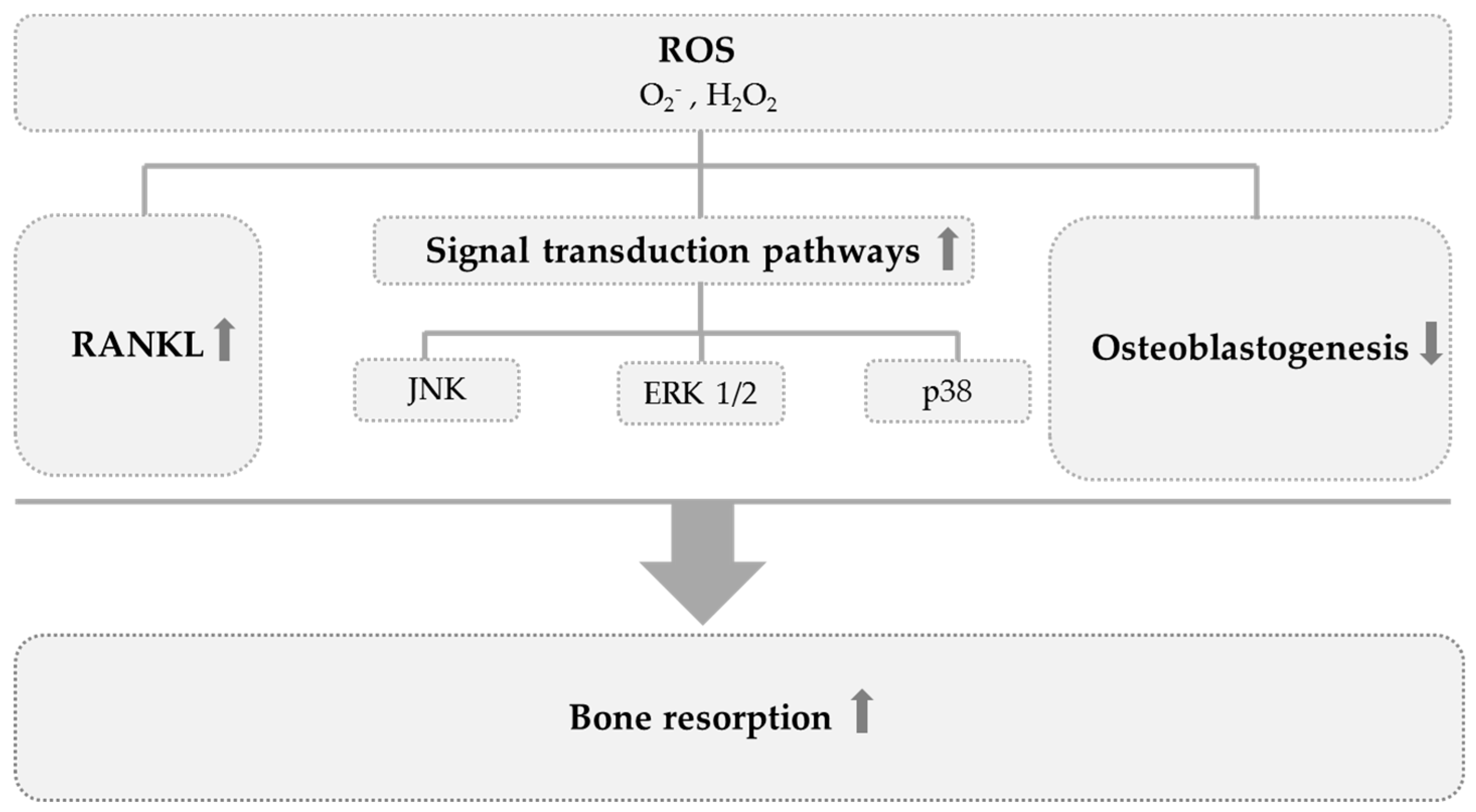
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef]
- Bai, X.C.; Lu, D.; Liu, A.L.; Zhang, Z.M.; Li, X.M.; Zou, Z.P.; Zeng, W.S.; Cheng, B.L.; Luo, S.Q. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J. Biol. Chem. 2005, 280, 17497–17506. [Google Scholar] [CrossRef]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Investig. 1990, 85, 632–639. [Google Scholar] [CrossRef]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.M.; Kim, H.H.; Lee, Z.H. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Key, L.L., Jr.; Wolf, W.C.; Gundberg, C.M.; Ries, W.L. Superoxide and bone resorption. Bone 1994, 15, 431–436. [Google Scholar] [PubMed]
- Yang, S.; Ries, W.L.; Key, L.L., Jr. Nicotinamide adenine dinucleotide phosphate oxidase in the formation of superoxide in osteoclasts. Calcif. Tissue Int. 1998, 63, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, A.N.; Tare, R.S. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin. Chim. Acta 2002, 318, 145–148. [Google Scholar] [CrossRef]
- Basu, S.; Michaelsson, K.; Olofsson, H.; Johansson, S.; Melhus, H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001, 288, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Cremonini, E.; Romani, A.; Fila, E.; Castaldini, M.C.; Ferrazzini, S.; Giganti, M.; Massari, L. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. Biomed. Res. Int. 2014, 2014, 569563. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, M.A.; Ruiz-Ramos, M.; Correa-Munoz, E.; Mendoza-Nunez, V.M. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet. Disord. 2007, 8, 124. [Google Scholar] [CrossRef]
- Yalin, S.; Bagis, S.; Polat, G.; Dogruer, N.; Cenk, A.S.; Hatungil, R.; Erdogan, C. Is there a role of free oxygen radicals in primary male osteoporosis? Clin. Exp. Rheumatol. 2005, 23, 689–692. [Google Scholar]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- de Haan, J.B.; Cristiano, F.; Iannello, R.; Bladier, C.; Kelner, M.J.; Kola, I. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum. Mol. Genet. 1996, 5, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.H.; Helfrich, M.H.; Wallace, H.M.; Ralston, S.H. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone 1996, 19, 223–226. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaëlsson, K.; Pettersson-Kymmer, U.; Eriksson, S.; Grodstein, F.; Wolk, A.; Bellavia, A.; Ahmed, L.A.; et al. Fruit and Vegetable Intake and Hip Fracture Incidence in Older Men and Women: The CHANCES Project. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Bellavia, A.; Orsini, N.; Wolk, A.; Michaëlsson, K. Fruit and vegetable intake and risk of hip fracture: A cohort study of Swedish men and women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Cao, W.-T.; Tian, H.-Y.; He, J.; Chen, G.-D.; Chen, Y.-M. Greater Intake of Fruit and Vegetables Is Associated with Greater Bone Mineral Density and Lower Osteoporosis Risk in Middle-Aged and Elderly Adults. PLoS ONE 2017, 12, e0168906. [Google Scholar] [CrossRef]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lee, S.-K.; Chun, O.K. Soy Isoflavones and Osteoporotic Bone Loss: A Review with an Emphasis on Modulation of Bone Remodeling. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Hamdi Kara, I.; Aydin, S.; Gemalmaz, A.; Aktürk, Z.; Yaman, H.; Bozdemir, N.; Kurdak, H.; Sitmapinar, K.; Devran Sencar, I.; Başak, O.; et al. Habitual tea drinking and bone mineral density in postmenopausal Turkish women: Investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study). Int. J. Vitam. Nutr. Res. 2007, 77, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Maghbooli, Z.; Shafaie, A.R.; Javadi, E.; Larijani, B. Relationship between Tea drinking and Bone Mineral Density in Iranian population. Iran. J. Public Health 2007, 57–62. [Google Scholar]
- Keramat, A.; Patwardhan, B.; Larijani, B.; Chopra, A.; Mithal, A.; Chakravarty, D.; Adibi, H.; Khosravi, A. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet. Disord. 2008, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.; Prince, R.L.; Kerr, D.A.; Devine, A.; Woodman, R.J.; Lewis, J.R.; Hodgson, J.M. Tea and flavonoid intake predict osteoporotic fracture risk in elderly Australian women: A prospective study. Am. J. Clin. Nutr. 2015, 102, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-F.; Wu, B.-H.; Fan, F.; Xie, H.-L.; Xue, W.-Q.; Zhu, H.-L.; Chen, Y.-M. Dietary patterns and the risk of hip fractures in elderly Chinese: A matched case-control study. J. Clin. Endocrinol. Metab. 2013, 98, 2347–2355. [Google Scholar] [CrossRef]
- Shen, C.-L.; Chyu, M.-C. Tea flavonoids for bone health: From animals to humans. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2016, 64, 1151–1157. [Google Scholar] [CrossRef]
- New, S.A.; Robins, S.P.; Campbell, M.K.; Martin, J.C.; Garton, M.J.; Bolton-Smith, C.; Grubb, D.A.; Lee, S.J.; Reid, D.M. Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am. J. Clin. Nutr. 2000, 71, 142–151. [Google Scholar] [CrossRef]
- Wood, A.D.; Macdonald, H.M. Interactions of Dietary Patterns, Systemic Inflammation, and Bone Health. In Nutritional Influences on Bone Health; Burckhardt, P., Dawson-Hughes, B., Weaver, C.M., Eds.; Springer: London, UK, 2013; pp. 19–30. [Google Scholar]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between dietary flavonoid intakes and bone health in a Scottish population. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef]
- Murphy, M.M.; Barraj, L.M.; Herman, D.; Bi, X.; Cheatham, R.; Randolph, R.K. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J. Acad. Nutr. Diet. 2012, 112, 222–229. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Hu, B.; Yu, B.; Tang, D.; Li, S.; Wu, Y. Daidzein promotes osteoblast proliferation and differentiation in OCT1 cells through stimulating the activation of BMP-2/Smads pathway. Genet. Mol. Res. GMR 2016, 15, 15028792. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Suh, K.S.; Sul, D.; Kim, B.-J.; Lee, S.K.; Jung, W.-W. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int. J. Mol. Med. 2012, 29, 169–177. [Google Scholar] [PubMed]
- Ko, C.H.; Siu, W.S.; Wong, H.L.; Shum, W.T.; Fung, K.P.; San Lau, C.B.; Leung, P.C. Pro-bone and antifat effects of green tea and its polyphenol, epigallocatechin, in rat mesenchymal stem cells in vitro. J. Agric. Food Chem. 2011, 59, 9870–9876. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Suzuki, K.; Muramatsu, M.; Nomura, A.; Inoue, F.; Into, T.; Yoshiko, Y.; Niida, S. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS ONE 2014, 9, e97177. [Google Scholar] [CrossRef] [PubMed]
- Nash, L.A.; Sullivan, P.J.; Peters, S.J.; Ward, W.E. Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food Res. 2015, 59, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.-C.; Gao, W.; Wang, J.-S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.-S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, W.; Wang, F.; Dong, J.; Wang, D.; Sun, B.; Wang, B. Stimulation of Wnt/beta-Catenin Signaling to Improve Bone Development by Naringin via Interacting with AMPK and Akt. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.; Zha, X.; Xu, Z.; Li, L.; Liu, Y.; Xu, L.; Cui, L.; Xu, D.; Zhu, B. Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and p38 MAPK pathways. Mol. Cell. Biochem. 2015, 407, 41–50. [Google Scholar] [CrossRef]
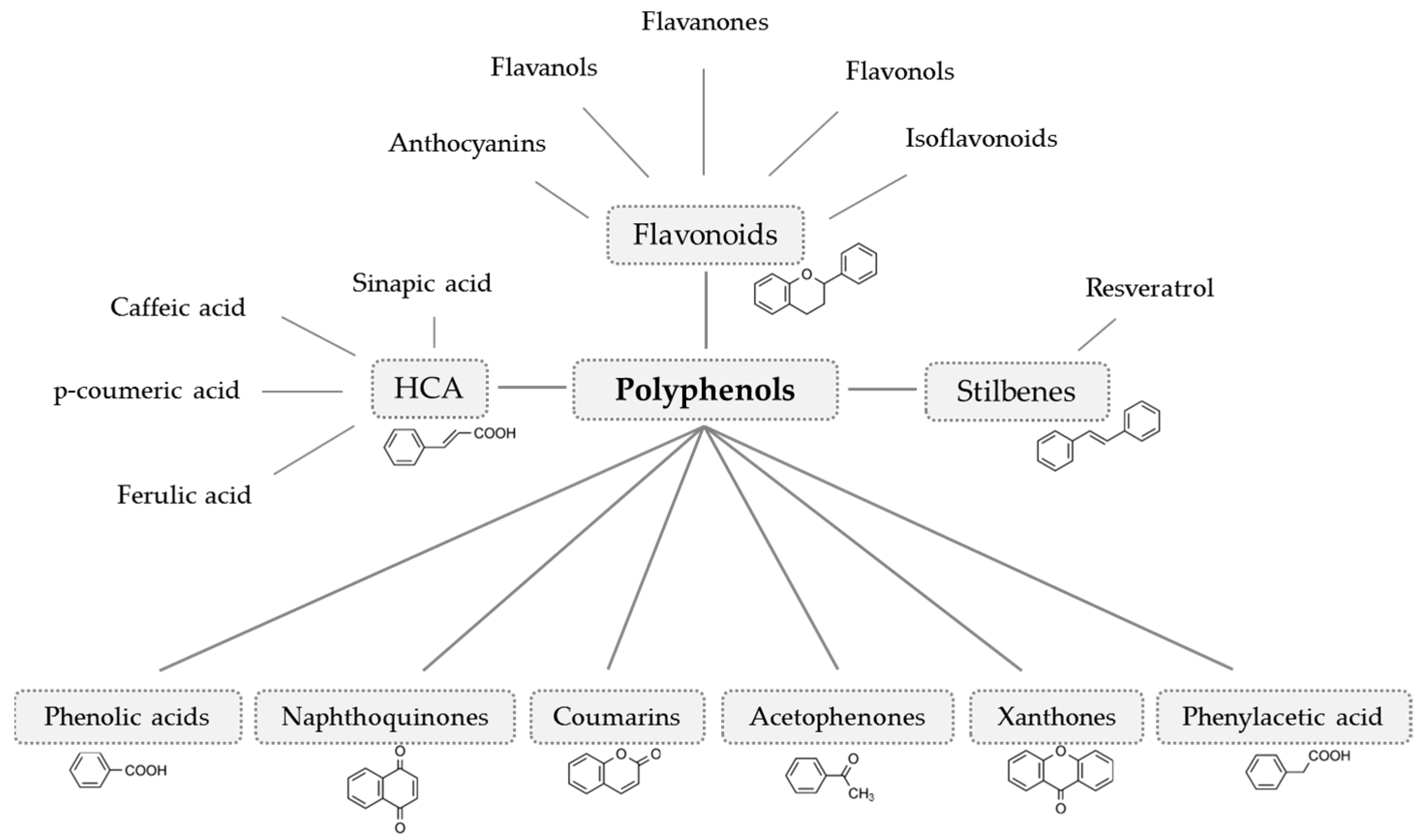
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Br. J. Nutr. 2008, 99, 8. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols - nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Cao, J.; Chen, W.; Zhang, Y.; Zhang, Y.; Zhao, X. Content of Selected Flavonoids in 100 Edible Vegetables and Fruits. Food Sci. Technol. Res. 2010, 16, 395–402. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and Lignans in Legumes: Nutritional and Health Aspects in Humans 11The method development and synthesis of the standards and deuterium-labelled compounds was supported by National Institutes of Health Grants No. 1 R01 CA56289-01 and No. 2 R01 CA56289-04, and analytical work by the EU research contract FAIR-CT95-0894. J. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar]
- Kim, H.-S.; Suh, K.S.; Ko, A.; Sul, D.; Choi, D.; Lee, S.K.; Jung, W.-W. The flavonoid glabridin attenuates 2-deoxy-D-ribose-induced oxidative damage and cellular dysfunction in MC3T3-E1 osteoblastic cells. Int. J. Mol. Med. 2013, 31, 243–251. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Anthocyanins - nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Stalmach, A. Bioavailability of Dietary Anthocyanins and Hydroxycinnamic Acid. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Elsevier Acad. Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Celep, G.S.; Rastmanesh, R.; Marotta, F. Microbial Metabolism of Polyphenols and Health. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Elsevier Acad. Press: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Martinez-Ortega, M.V.; Carcia-Parrilla, M.C.; Troncoso, A.M. Resveratrol content in wines and musts from the south of Spain. Die Nahr. 2000, 44, 253–256. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-Term Supplementation of Green Tea Extract Does Not Modify Adiposity or Bone Mineral Density in a Randomized Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 256–264. [Google Scholar] [CrossRef]
- Shen, C.-L.; Chyu, M.-C.; Yeh, J.K.; Zhang, Y.; Pence, B.C.; Felton, C.K.; Brismee, J.-M.; Arjmandi, B.H.; Doctolero, S.; Wang, J.-S. Effect of green tea and Tai Chi on bone health in postmenopausal osteopenic women: A 6-month randomized placebo-controlled trial. Osteoporos. Int. 2012, 23, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.-Y.; Chiu, H.-F.; Lee, H.-H.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Consumption of onion juice modulates oxidative stress and attenuates the risk of bone disorders in middle-aged and post-menopausal healthy subjects. Food Funct. 2016, 7, 902–912. [Google Scholar] [CrossRef]
- Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Horcajada, M.N.; Offord-Cavin, E.; Peacock, M.; Weaver, C.M. Effect of Hesperidin with and Without a Calcium (Calcilock) Supplement on Bone Health in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2016, 101, 923–927. [Google Scholar] [CrossRef]
- Alekel, D.L.; van Loan, M.D.; Koehler, K.J.; Hanson, L.N.; Stewart, J.W.; Hanson, K.B.; Kurzer, M.S.; Peterson, C.T. The soy isoflavones for reducing bone loss (SIRBL) study: A 3-y randomized controlled trial in postmenopausal women. Am. J. Clin. Nutr. 2010, 91, 218–230. [Google Scholar] [CrossRef]
- Shedd-Wise, K.M.; Alekel, D.L.; Hofmann, H.; Hanson, K.B.; Schiferl, D.J.; Hanson, L.N.; van Loan, M.D. The soy isoflavones for reducing bone loss study: 3-yr effects on pQCT bone mineral density and strength measures in postmenopausal women. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2011, 14, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic Activity of Genistein Aglycone in Postmenopausal Women: Evidence from a Post-Hoc Analysis of a Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008, 23, 715–720. [Google Scholar] [CrossRef]
- Brink, E.; Coxam, V.; Robins, S.; Wahala, K.; Cassidy, A.; Branca, F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: A randomized, double-blind, placebo controlled study. Am. J. Clin. Nutr. 2008, 87, 761–770. [Google Scholar] [CrossRef]
- Kenny, A.M.; Mangano, K.M.; Abourizk, R.H.; Bruno, R.S.; Anamani, D.E.; Kleppinger, A.; Walsh, S.J.; Prestwood, K.M.; Kerstetter, J.E. Soy proteins and isoflavones affect bone mineral density in older women: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 234–242. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Fraser, W.D.; Thatcher, N.J.; Kilpatrick, E.S.; Atkin, S.L. Soy Reduces Bone Turnover Markers in Women During Early Menopause: A Randomized Controlled Trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.Y.; Tsai, K.S.; Tu, S.T.; Wu, J.S.; Chang, C.I.; Chen, C.L.; Shaw, N.S.; Peng, H.Y.; Wang, S.Y.; Wu, C.H. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: A 2-year randomized double-blind placebo-controlled study. Osteoporos. Int. 2012, 23, 1571–1580. [Google Scholar] [CrossRef]
- Vupadhyayula, P.M.; Gallagher, J.C.; Templin, T.; Logsdon, S.M.; Smith, L.M. Effects of soy protein isolate on bone mineral density and physical performance indices in postmenopausal women—A 2-year randomized, double-blind, placebo-controlled trial. Menopause 2009, 16, 320–328. [Google Scholar] [CrossRef]
- Wong, W.W.; Lewis, R.D.; Steinberg, F.M.; Murray, M.J.; Cramer, M.A.; Amato, P.; Young, R.L.; Barnes, S.; Ellis, K.J.; Shypailo, R.J.; et al. Soy isoflavone supplementation and bone mineral density in menopausal women: A 2-y multicenter clinical trial. Am. J. Clin. Nutr. 2009, 90, 1433–1439. [Google Scholar] [CrossRef]
- Hooshmand, S.; Chai, S.C.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br. J. Nutr. 2011, 106, 923–930. [Google Scholar] [CrossRef]
- Hooshmand, S.; Brisco, J.R.Y.; Arjmandi, B.H. The effect of dried plum on serum levels of receptor activator of NF-κB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: A randomised controlled trial. Br. J. Nutr. 2014, 112, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: A randomized, controlled trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Simonavice, E.; Liu, P.-Y.; Ilich, J.Z.; Kim, J.-S.; Arjmandi, B.; Panton, L.B. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl. Physiol. Nutr. Metab. 2014, 39, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Ornstrup, M.J.; Harslof, T.; Kjaer, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Eastell, R.; Hannon, R.A. Biomarkers of bone health and osteoporosis risk. Proc. Nutr. Soc. 2008, 67, 157–162. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
| Participants | Intervention | Control Group | Duration | Power Analysis | Effects on Bone | Jadad Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age (Year) | Gender | Health Status | (Powder/Food Item) | ||||||
| Flavanols | ||||||||||
| Dostal et al. 2016 [69] | 121 | 50–70 | Female | Overweight/obese, postmenopausal, high breast cancer risk | GTE (843 mg EGCG/d) | Overweight/obese, postmenopausal women with high breast cancer risk | 1 year | Yes (80%) | Total body BMD ↔ | 5 |
| Shen et al. 2012 [70] | 171 | >50 | Female | Postmenopausal, osteopenic | GTE (500 mg/d) | Postmenopausal, osteopenic women | 6 months | Yes (85-90%) | bAP ↑ TRAP ↔ bAP/TRAP ratio ↑ | 5 |
| Flavonols | ||||||||||
| Law et al. 2016 [71] | 30 | 40–80 | Female, male | Healthy | Onion juice (100 ml/d) | Healthy men and women | 8 weeks | No | Total body BMD ↔ bAP ↓ PTH ↔ Calcium ↔ | 5 |
| Flavanones | ||||||||||
| Martin et al. 2016 [72] | 12 | >50 | Female | Postmenopausal, healthy | Hesperidin (500 mg) | Postmenopausal, healthy women | 3 months | Yes (80%) | bAP ↔ DPD ↔ | 5 |
| Isoflavonoids | ||||||||||
| Alekel et al. 2010; Shedd-Wise et al. 2011 [73,74] | 255 | 46–65 | Female | Postmenopausal, healthy | Soy isoflavonoids (80 and 120 mg/d) | Postmenopausal, healthy women | 3 years | Yes (94%) | Total body BMD ↔ spine BMD ↔ femur BMD ↔ neck BMD ↔ | 5 |
| Arcoraci et al. 2017; Marini et al. 2008 [75,76,77] | 389 | 49–67 | Female | Postmenopausal, osteopenic | Genistein (54 mg/d) | Postmenopausal, osteopenic women | 2 years | Yes (80%) | Femur BMD ↑ spine BMD ↑ PYD ↓ DPD ↓ bAP ↑ RANKL ↓ OPG ↑ | 5 |
| Brink et al. 2008 [78] | 237 | 53±3 | Female | Early postmenopausal, healthy | Isoflavonoid enriched foods (110 mg isoflavonoid aglycones/d) | Early postmenopausal, healthy women | 1 year | Yes (84%) | Total body BMD ↔ bone markers ↔ | 5 |
| Kenny et al. 2009 [79] | 131 | >60 | Female | Postmenopausal, healthy | Isoflavonoids (105 mg/d) | Postmenopausal, healthy women | 1 year | No | Total body BMD ↔ femur BMD ↔ spine BMD ↔ wrist BMD ↔ | 4 |
| Sathyapalan et al. 2016 [80] | 200 | >50 | Female | Early postmenopausal | Isoflavonoids (66 mg/d) | Early postmenopausal women | 6 months | Yes (95%) | ßCTX ↓ P1NP ↓ | 5 |
| Tai et al. 2012 [81] | 431 | 45–65 | Female | Postmenopausal with bone loss | Isoflavonoids (300 mg/d) | Postmenopausal women with bone loss | 2 years | Yes (80%) | Femur BMD ↔ Bone markers ↔ | 5 |
| Vupadhyayula et al. 2009 [82] | 203 | >50 | Female | Postmenopausal, healthy | Isoflavonoids (90 mg/d) | Postmenopausal, healthy women | 2 years | Yes (80%) | Spine BMD ↔ Femur BMD ↔ | 4 |
| Wong et al. 2009 [83] | 403 | 40–60 | Female | Climacteric, healthy | Soy isoflavonoids (80 and 120 mg/d) | Climacteric, healthy women | 2 years | Yes (80%) | Total Body BMD ↑ (120 mg/d) Bone markers ↔ | 5 |
| Anthocyanins | ||||||||||
| Hooshmand et al. 2011 and 2014 [84,85] | 160 | >50 | Female | Postmenopausal, osteopenic | Dried plums (100 g/d) | Postmenopausal, osteopenic women | 1 year | No | Ulna BMD ↑ Spine BMD ↑ OPG ↔ Sclerostin ↔ | 3 |
| Hooshmand et al. 2016 [86] | 48 | 65–79 | Female | Postmenopausal, osteopenic | Dried plums (50 and 100 g/d) | Postmenopausal, osteopenic women | 6 months | No | Total BMD ↑ TRAP ↓ | 3 |
| Simonavice et al. 2014 [87] | 27 | 64 ± 7 | Female | Postmenpausal, breast cancer survivors | Dried plums (90 g/d) | Postmenpausal women, breast cancer survivors | 6 months | Yes (80%) | Spine BMD ↔ Femur BMD ↔ Forearm BMD ↔ Bone markers ↔ | 3 |
| Stilbenes | ||||||||||
| Ornstrup et al. 2014 [88] | 74 | 49 ± 6 | Male | Obese, metabolic syndrome | Resveratrol (150 and 1000 mg/d) | Obese men with metabolic syndrome | 16 weeks | Yes (80%) | Spine BMD ↑ (1000 mg/d) bAP ↑ (1000 mg/d) OPG ↔ P1NP ↔ CTX ↔ NTX ↔ | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austermann, K.; Baecker, N.; Stehle, P.; Heer, M. Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo—Evidence from Human Studies. Nutrients 2019, 11, 871. https://doi.org/10.3390/nu11040871
Austermann K, Baecker N, Stehle P, Heer M. Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo—Evidence from Human Studies. Nutrients. 2019; 11(4):871. https://doi.org/10.3390/nu11040871
Chicago/Turabian StyleAustermann, Katharina, Natalie Baecker, Peter Stehle, and Martina Heer. 2019. "Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo—Evidence from Human Studies" Nutrients 11, no. 4: 871. https://doi.org/10.3390/nu11040871
APA StyleAustermann, K., Baecker, N., Stehle, P., & Heer, M. (2019). Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo—Evidence from Human Studies. Nutrients, 11(4), 871. https://doi.org/10.3390/nu11040871





