Additive-Enhanced SnO2 FBG Sensor with Optimized Annealing Time, Temperature, and Multilayer Coating for High-Performance Humidity Sensing
Abstract
1. Introduction
2. Materials and Methods
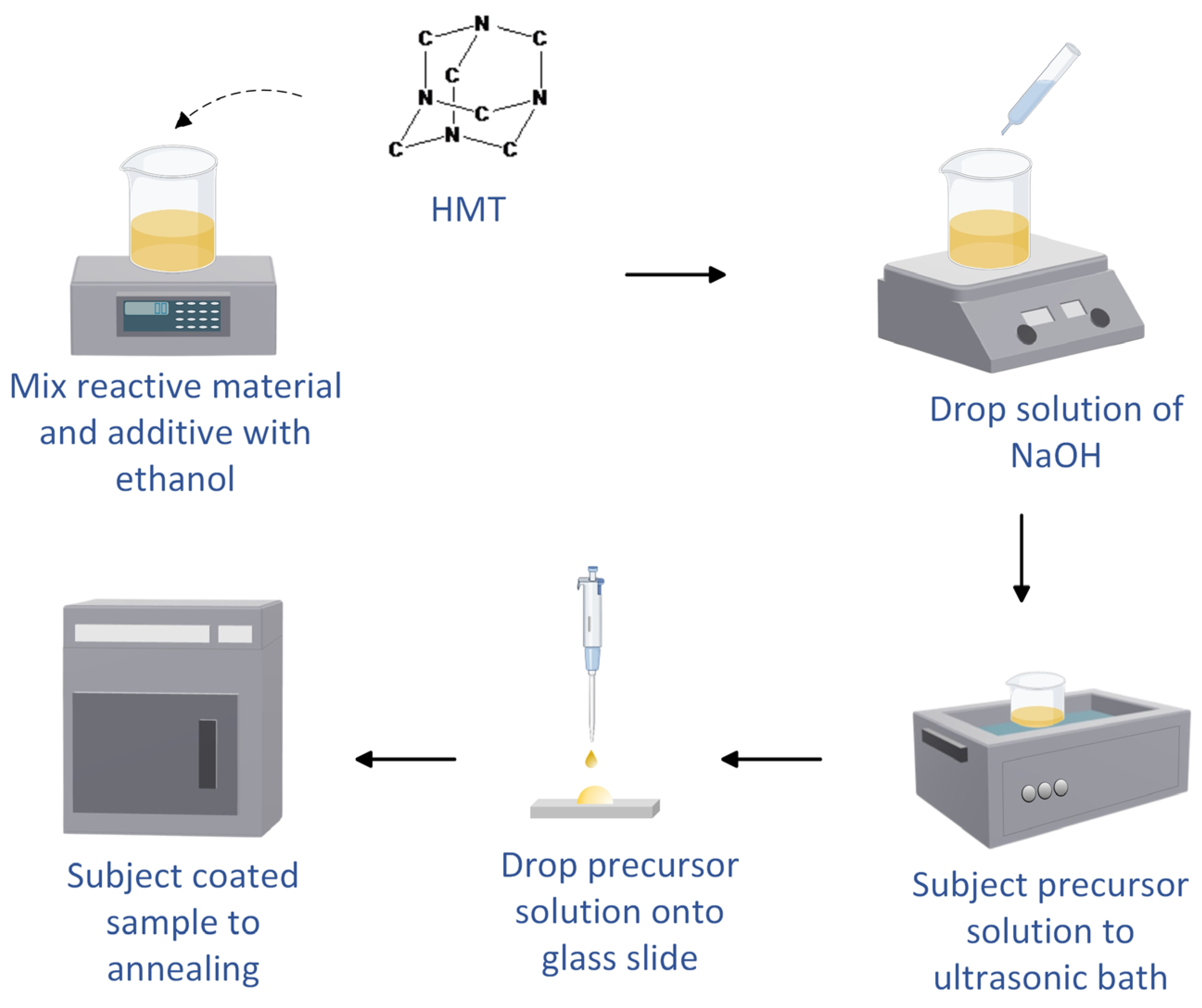
2.1. Additive-Enhanced SnO2 Synthesis
2.2. Investigation of Annealing Time and Temperature
2.3. Investigation of Multilayer and Hybrid Layer Coating
3. Results and Discussions
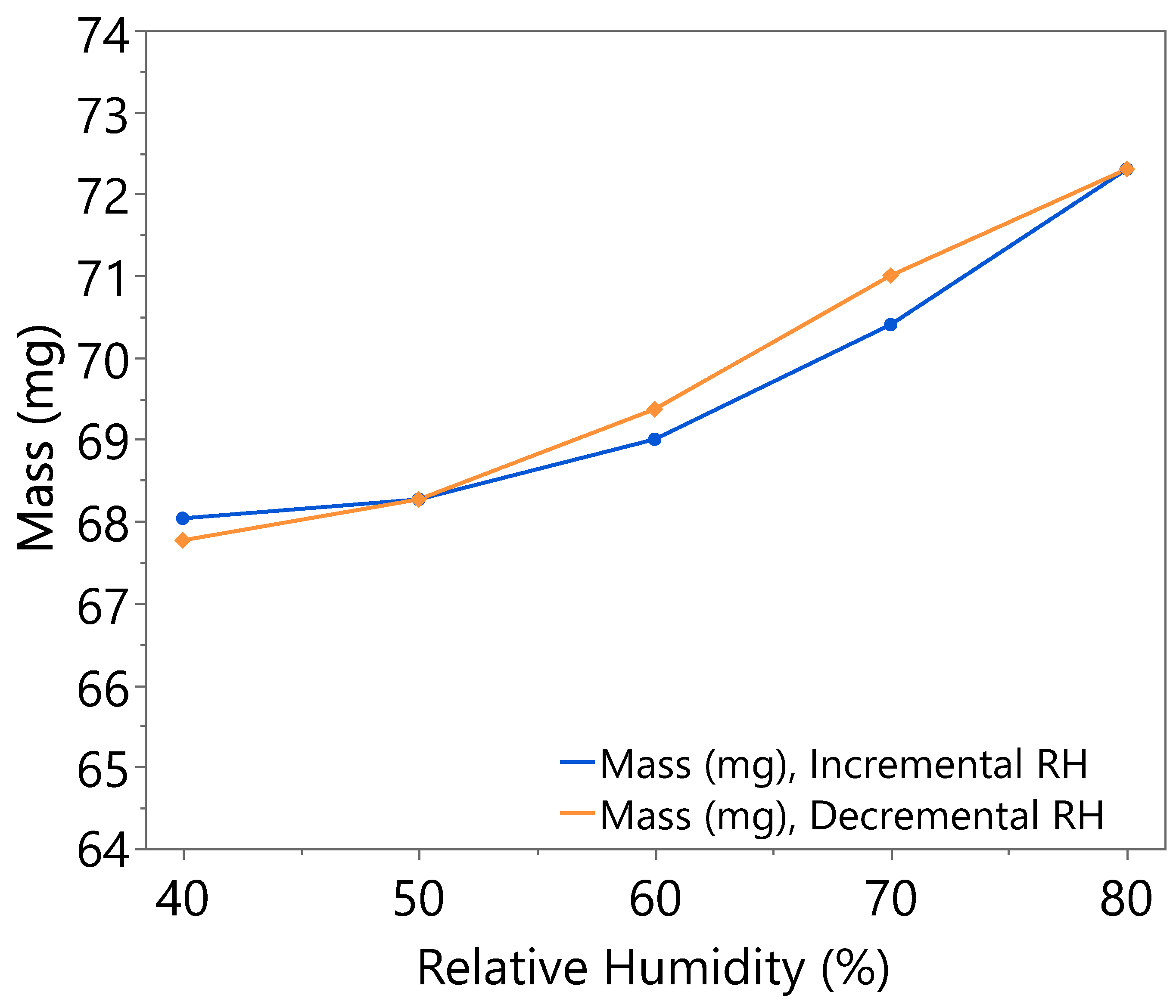
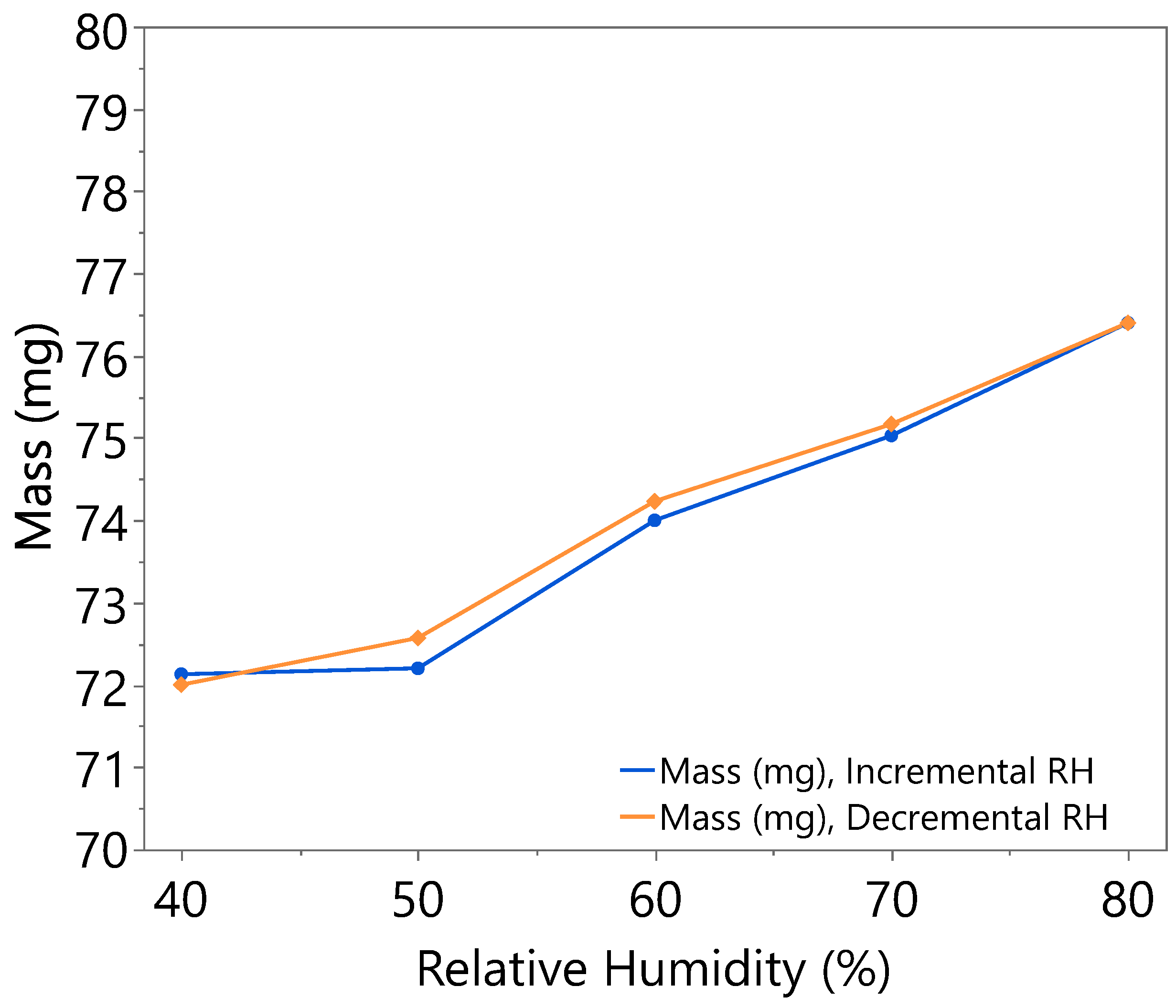
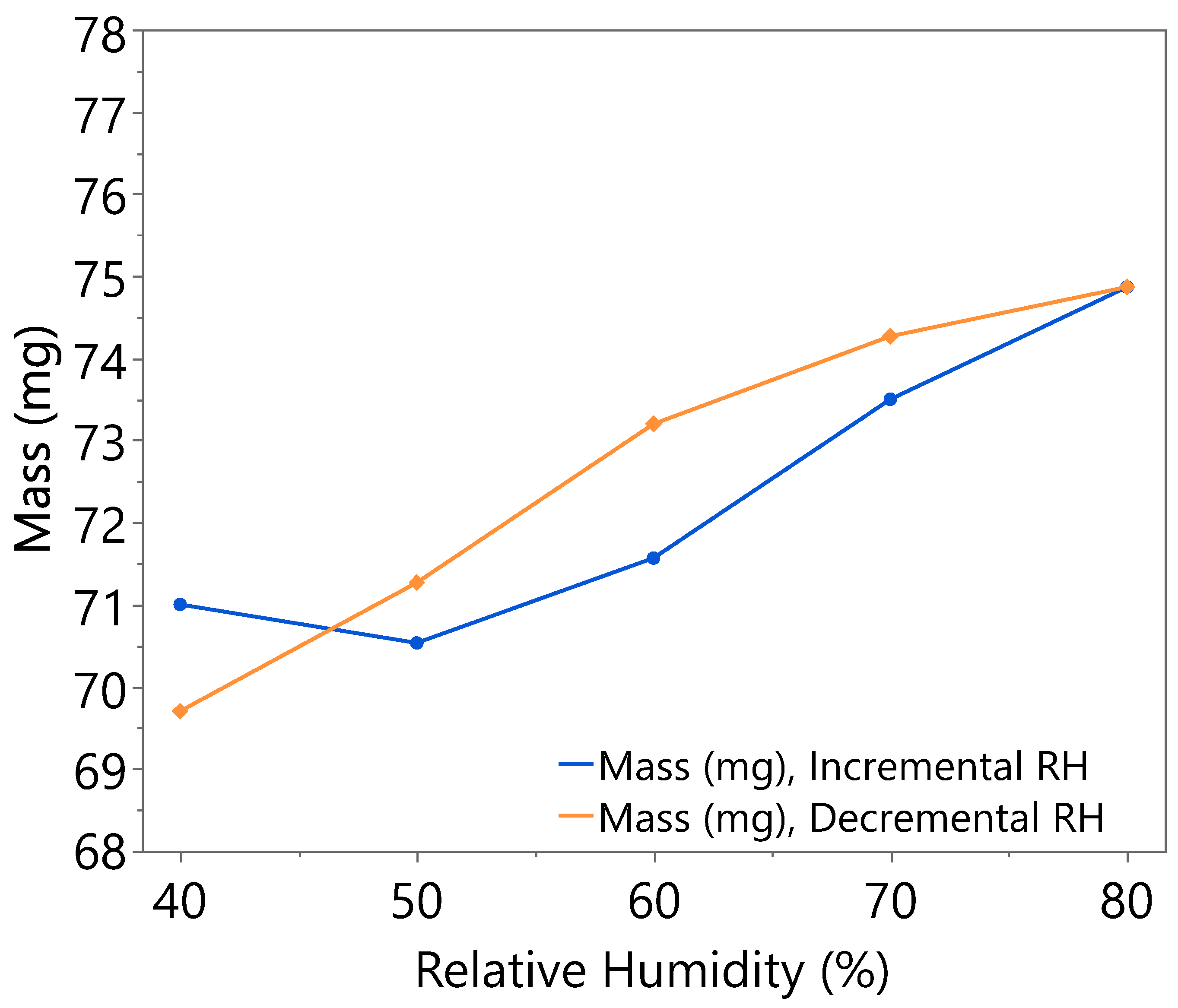
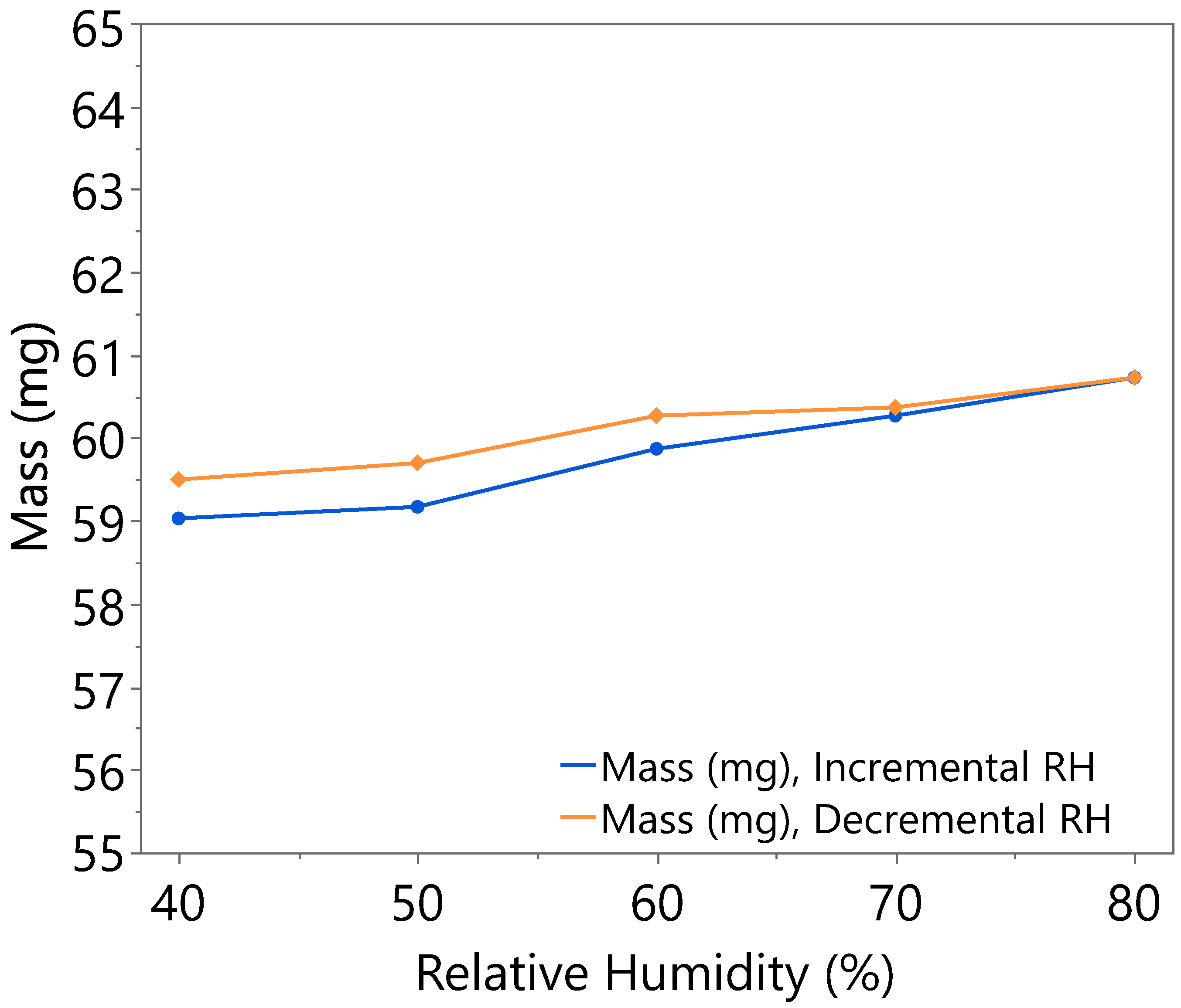
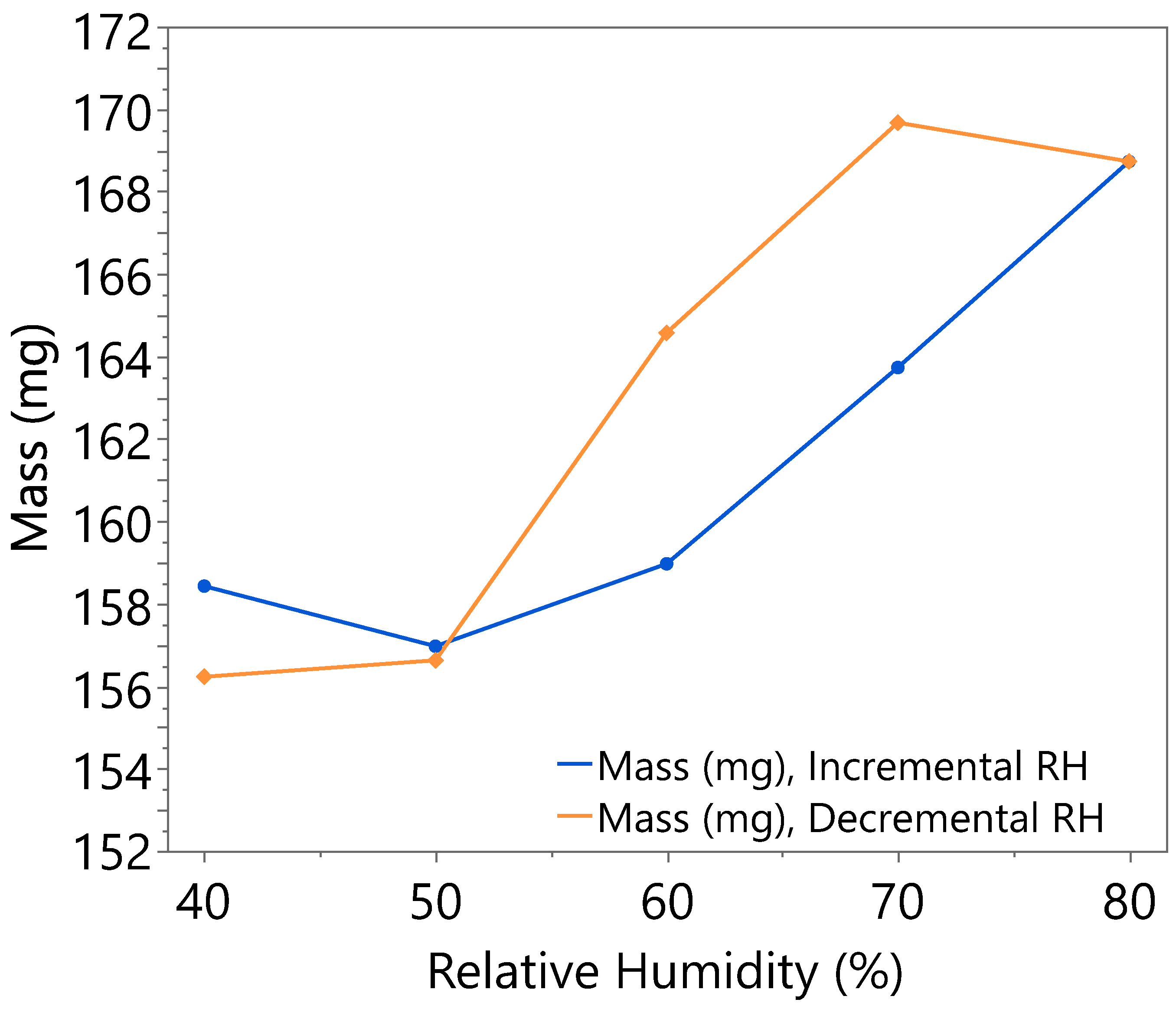
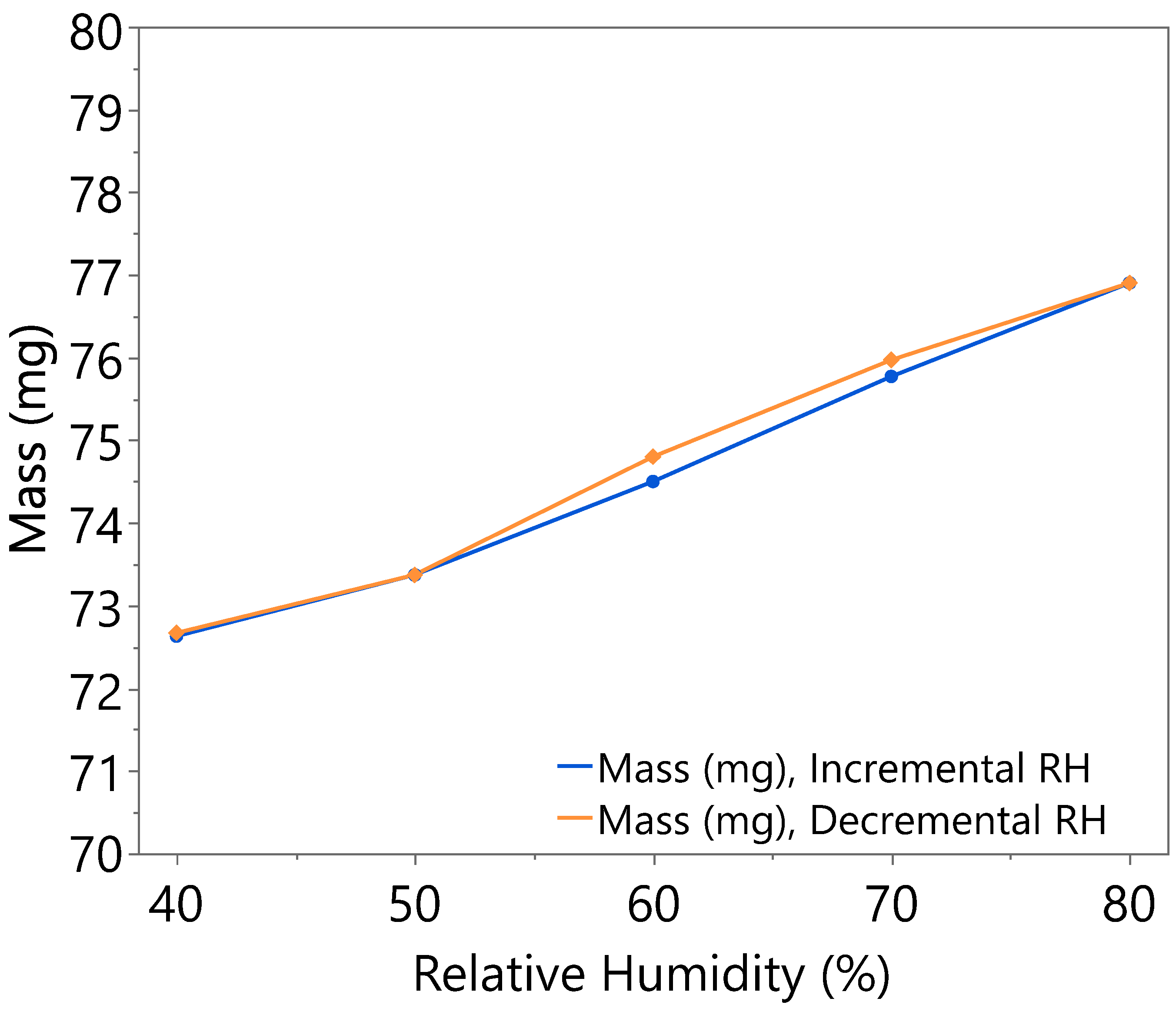
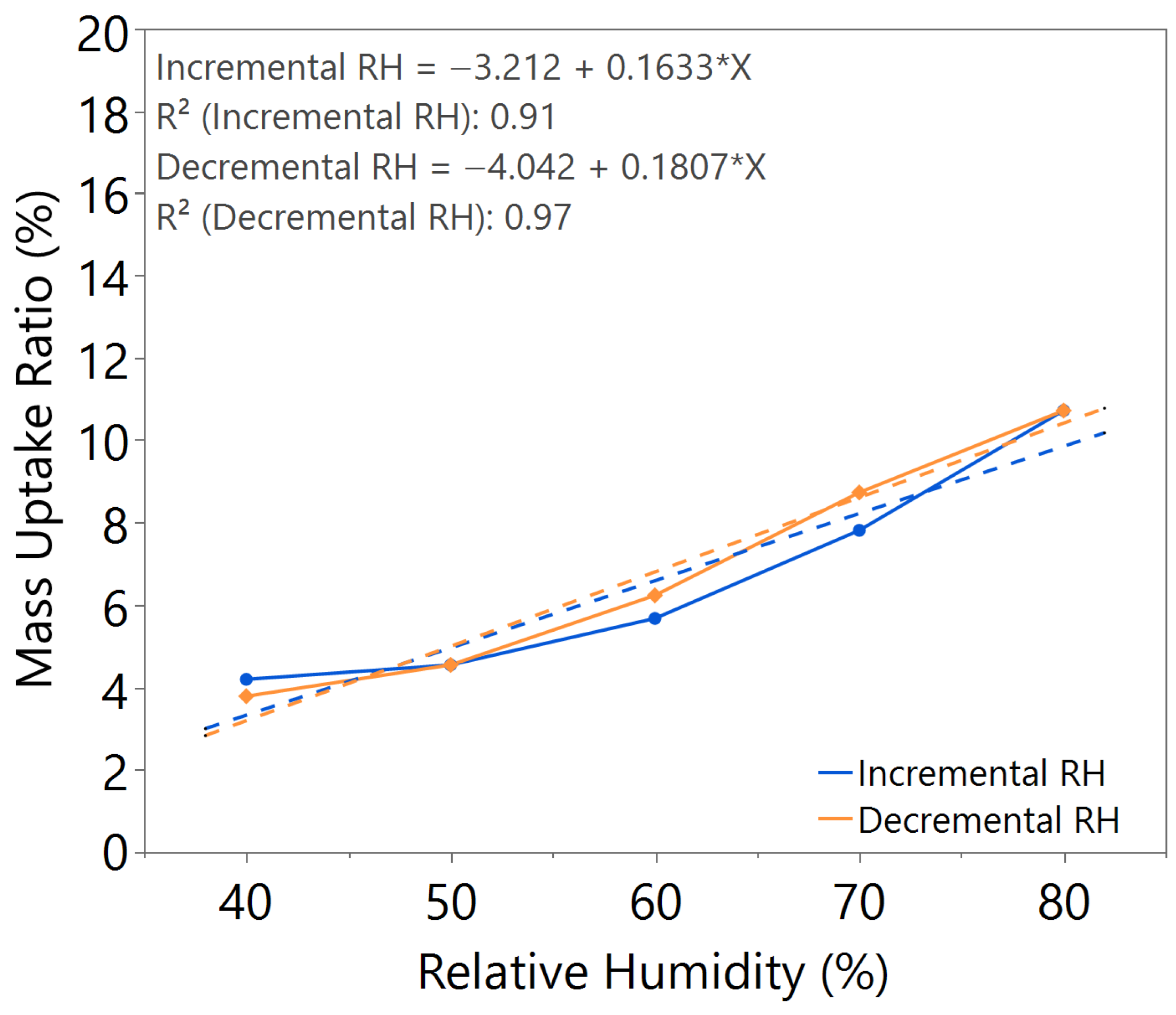
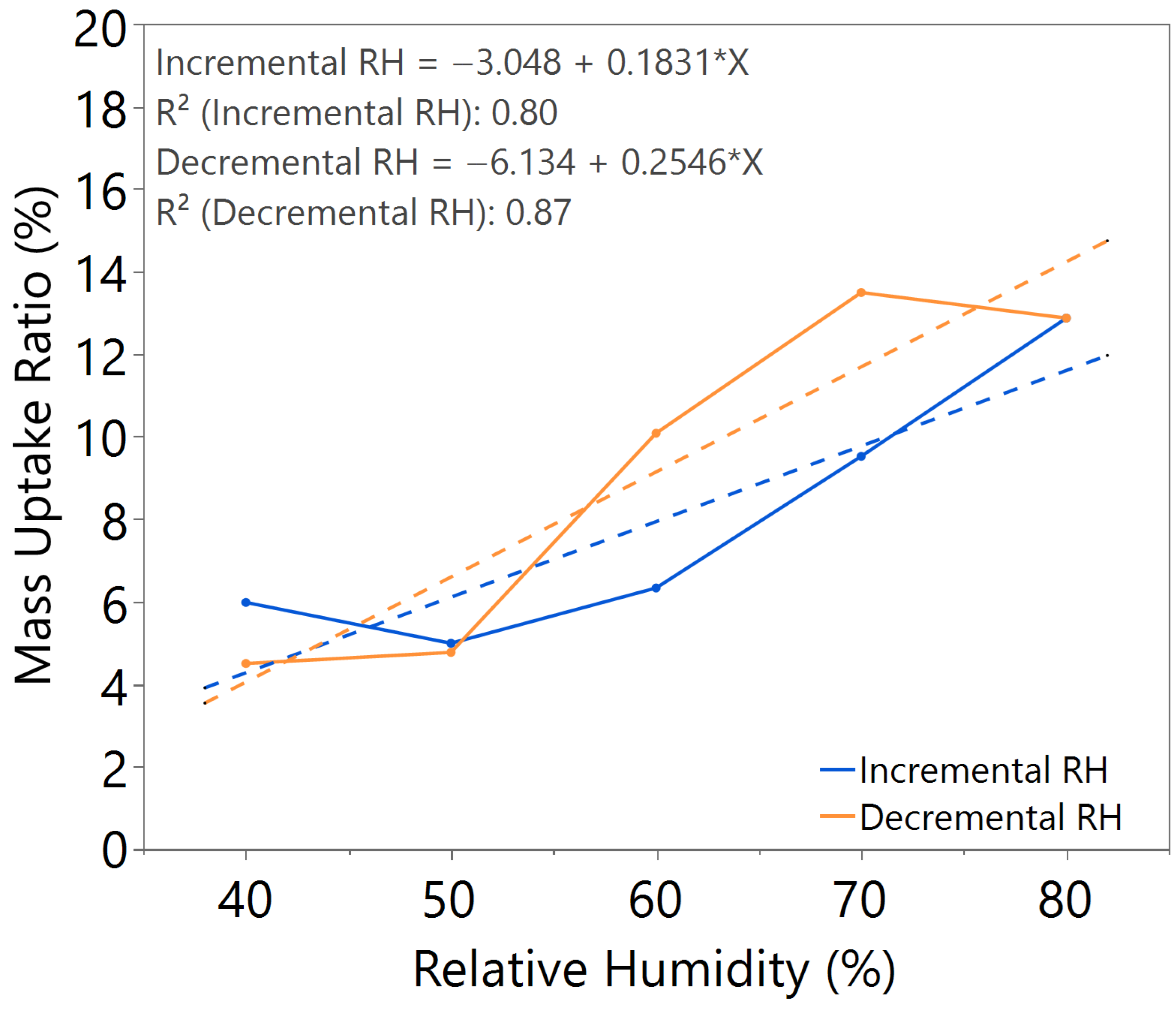
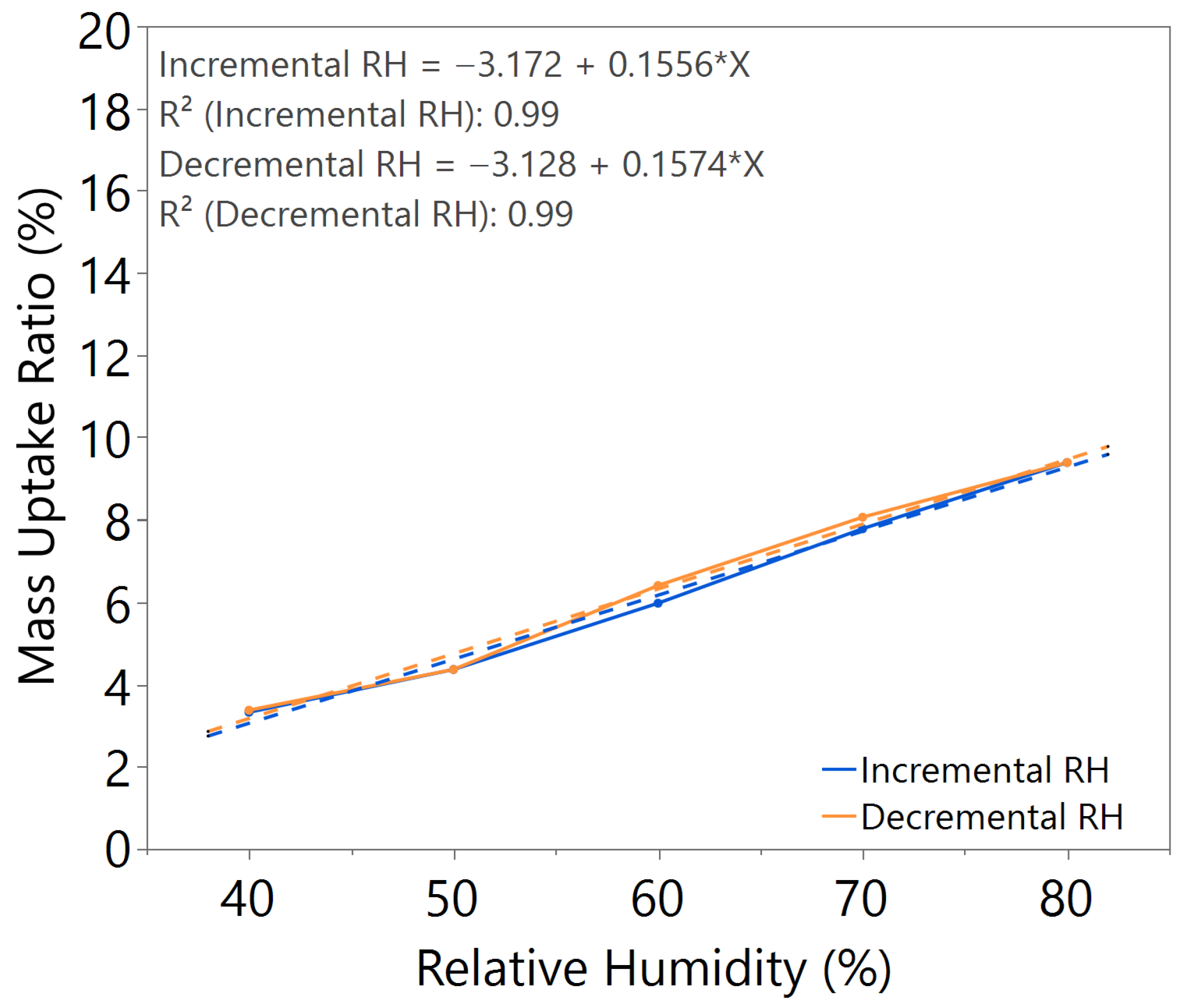
3.1. Hygroscopicity Test
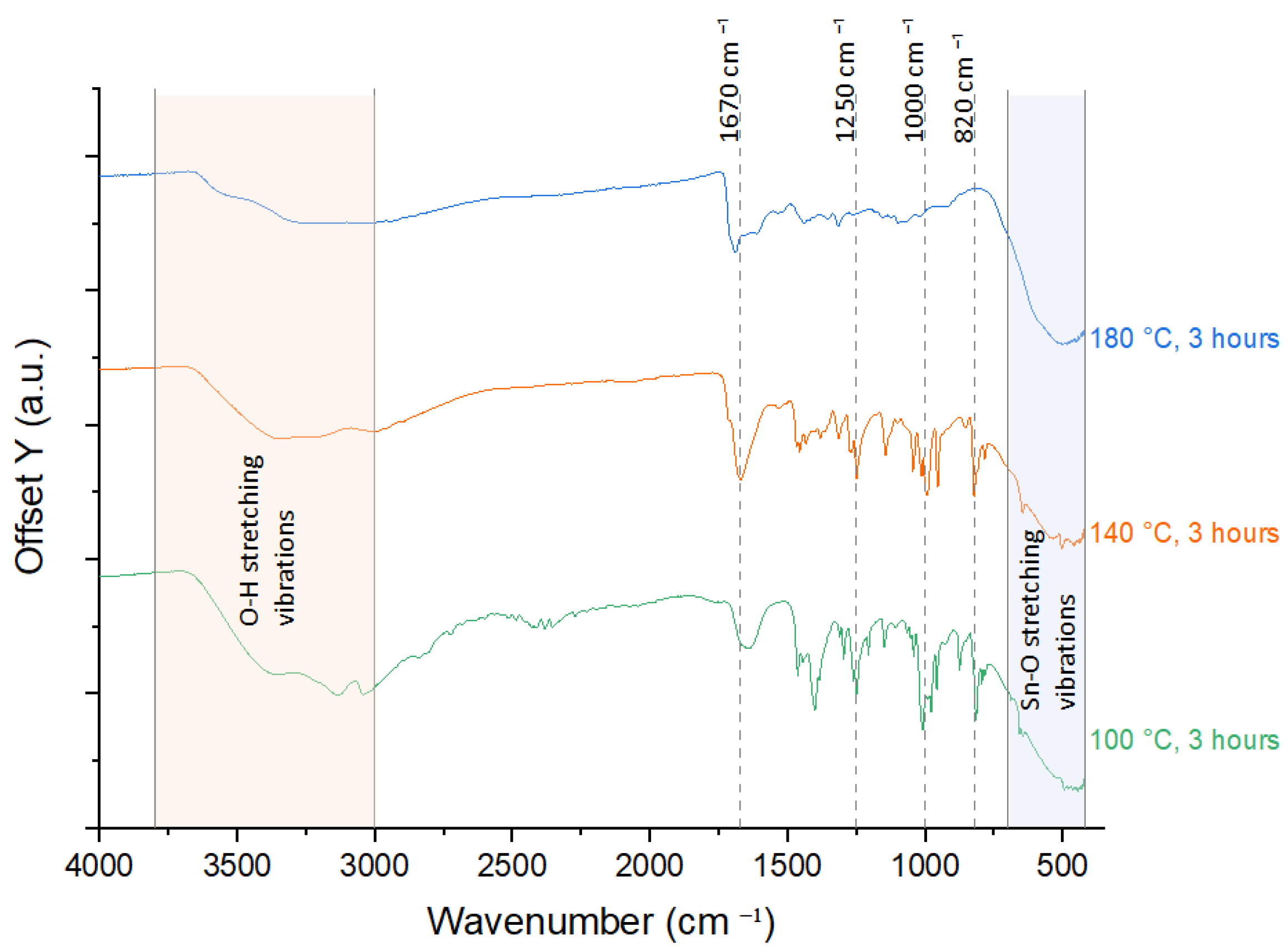
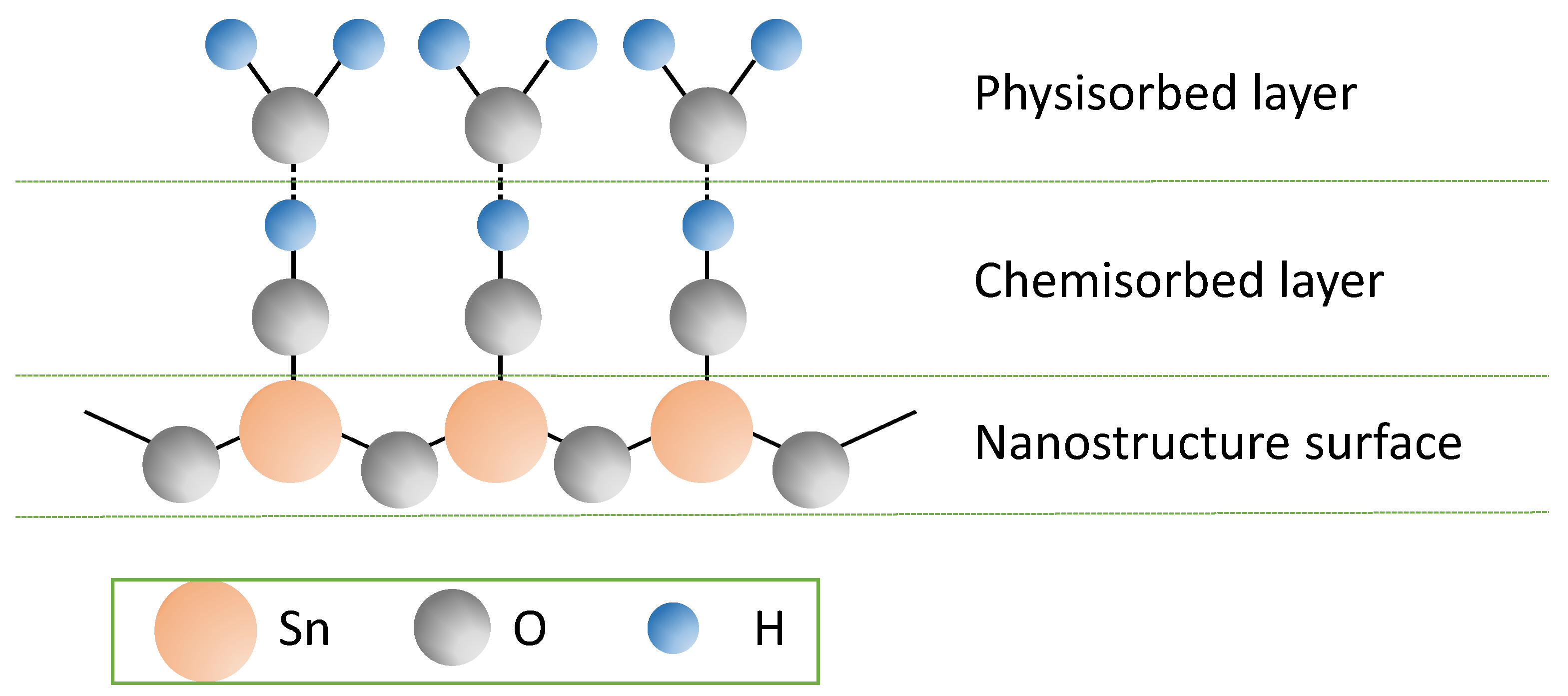
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
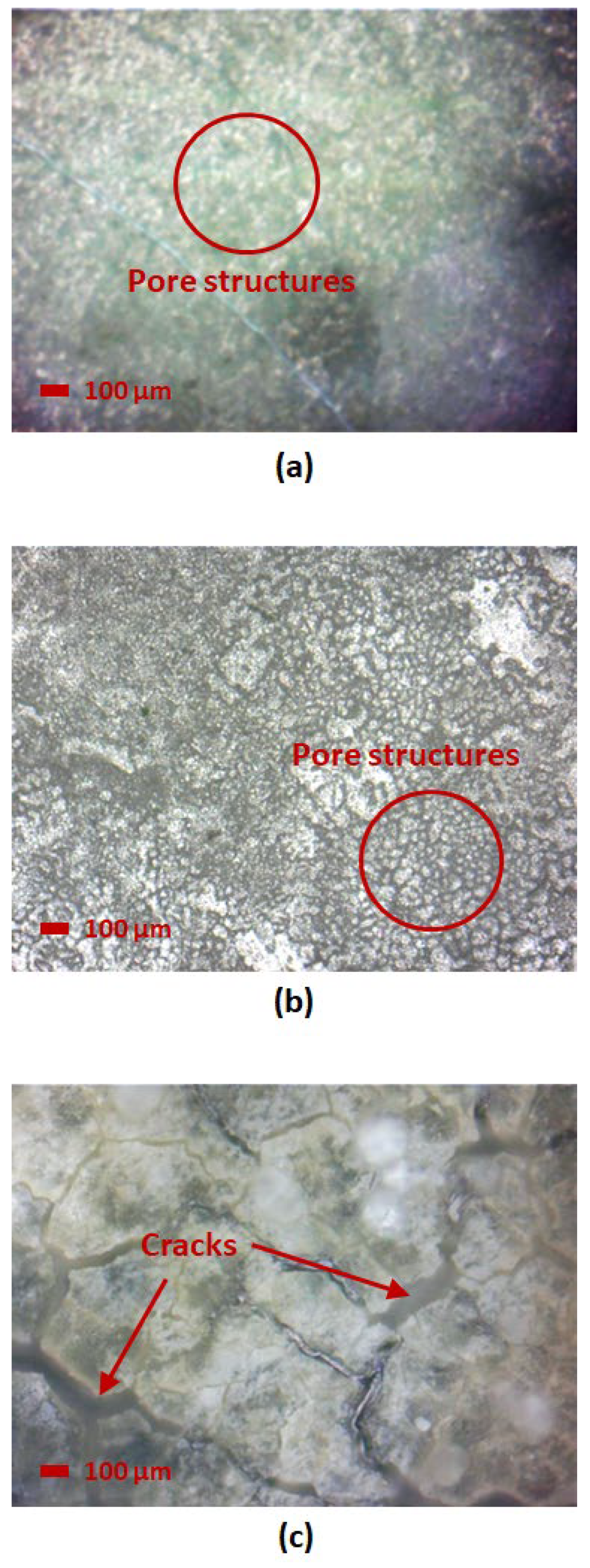
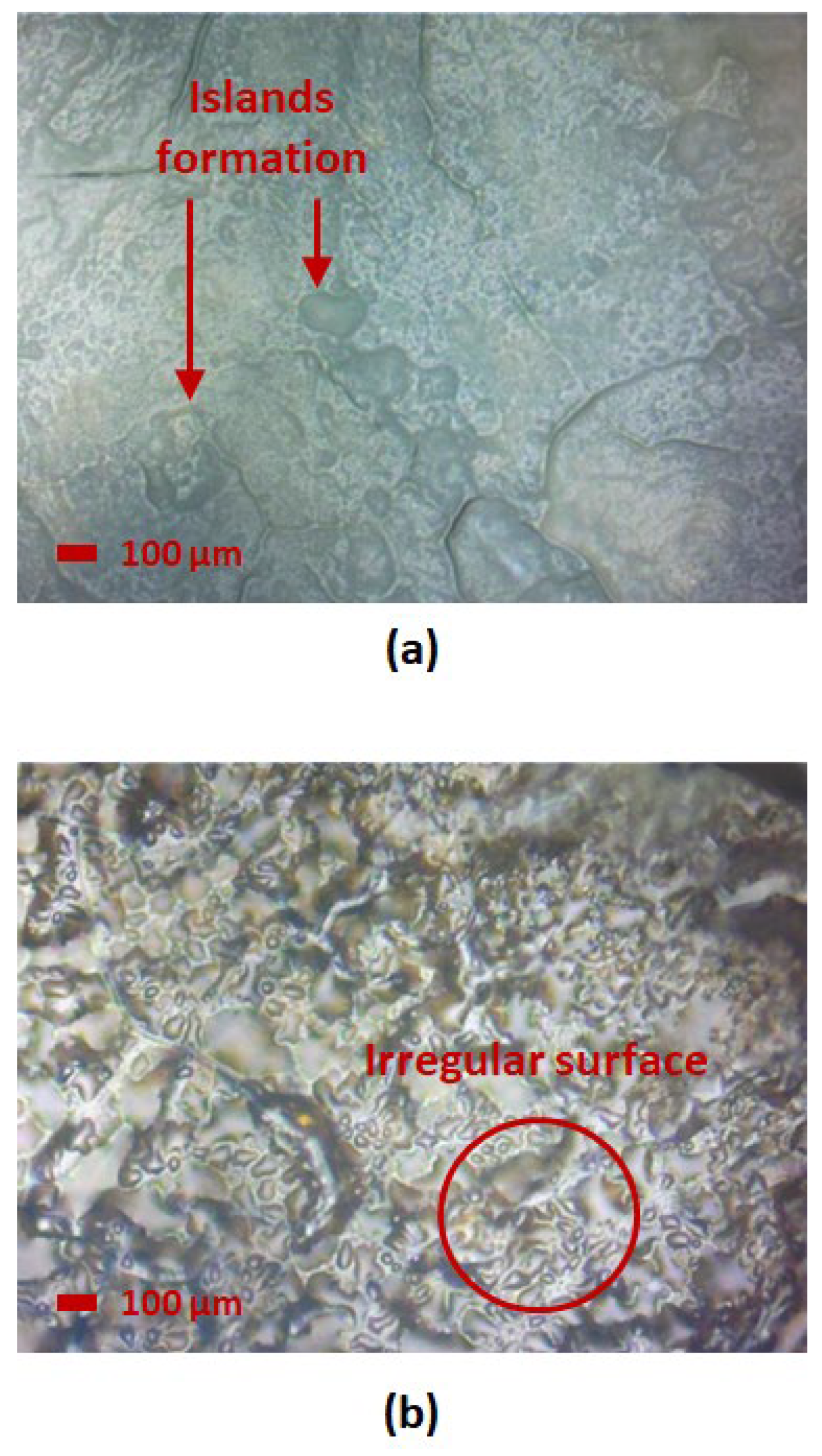
3.3. Microscope Image
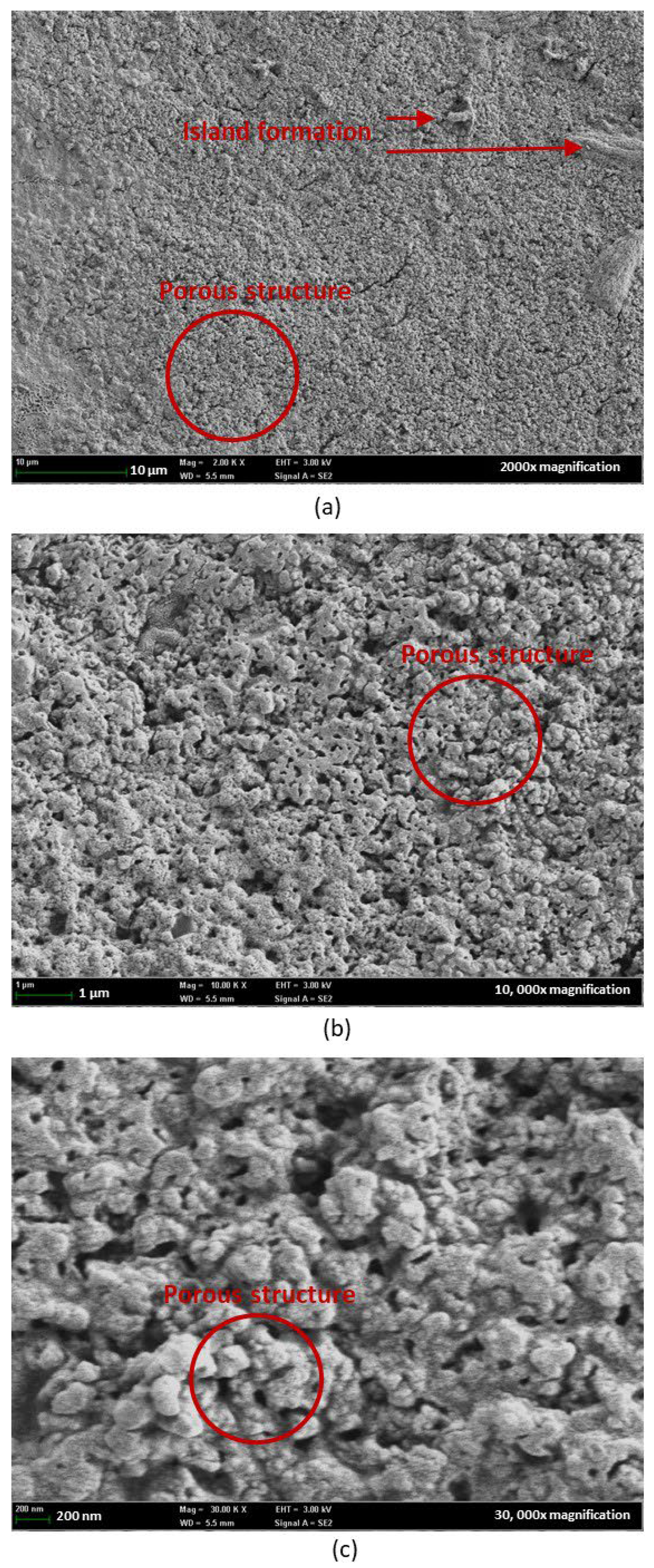
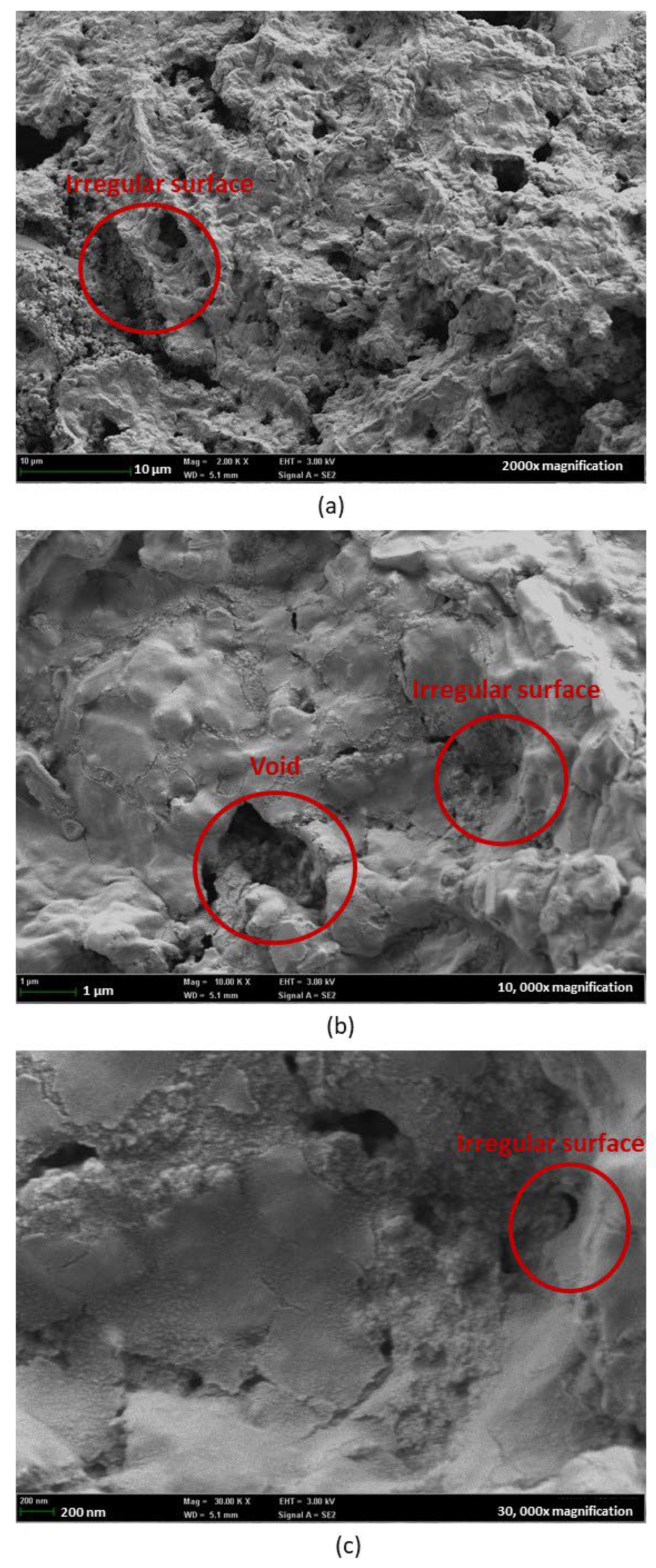
3.4. Field Emission Scanning Electron Microscope (FESEM)
3.5. Optical Spectrum Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Singh, M.; Moumen, A.; Duina, G.; Comini, E. 1D titanium dioxide: Achievements in chemical sensing. Materials 2020, 13, 2974. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Ansari, M.T.I.; Raghuwanshi, S.K. Design and development of titanium dioxide (TiO2)-coated eFBG sensor for the detection of petrochemicals adulteration. IEEE Trans. Instrum. Meas. 2021, 70, 7002508. [Google Scholar] [CrossRef]
- Li, F.; Li, P.; Zhang, H. Preparation and research of a high-performance ZnO/SnO2 humidity sensor. Sensors 2021, 22, 293. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Kok, S.P.; Go, Y.I.; Xu, W.; Wong, M.D. Advances in Fibre Bragg Grating (FBG) Sensing: A Review of Conventional and New Approaches and Novel Sensing Materials in Harsh and Emerging Industrial Sensing. IEEE Sens. J. 2024, 24, 29485–29505. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. A facile nanocasting synthesis of mesoporous Ag-doped SnO2 nanostructures with enhanced humidity sensing performance. Sens. Actuators B 2016, 223, 750–760. [Google Scholar] [CrossRef]
- Riza, M.A.; Go, Y.I.; Maier, R.R.; Harun, S.W.; Anas, S.B. Hygroscopic materials and characterization techniques for fiber sensing applications: A review. Sens. Mater. 2020, 32, 3755–3772. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ramezani, M.; Darroudi, M. Bio-sensing applications of cerium oxide nanoparticles: Advantages and disadvantages. Biosens. Bioelectron. 2017, 96, 33–43. [Google Scholar] [CrossRef]
- Amara, U.; Mahmood, K.; Awais, M.; Khalid, M.; Nasir, M.; Riaz, S.; Hayat, A.; Nawaz, M.H. Nickel-doped iron oxide nanoparticle-conjugated porphyrin interface (porphyrin/Fe2O3@Ni) for the non-enzymatic detection of dopamine from lacrimal fluid. Dalton Trans. 2022, 51, 5098–5107. [Google Scholar] [CrossRef]
- Saritas, S. Temperature-dependent gas sensor application of chromium oxide structure. J. Mater. Sci. Mater. Electron. 2023, 34, 679. [Google Scholar] [CrossRef]
- Mounasamy, V.; Mani, G.K.; Madanagurusamy, S. Vanadium oxide nanostructures for chemiresistive gas and vapour sensing: A review on state of the art. Microchim. Acta 2020, 187, 253. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Z.; Ma, J.; Wang, L.; Yang, J.; Du, B.; Yu, Q.; Zu, X. Highly sensitive surface acoustic wave (SAW) humidity sensors based on sol–gel SiO2 films: Investigations on the sensing property and mechanism. Sens. Actuators B 2015, 215, 283–291. [Google Scholar] [CrossRef]
- Li, P.; Yang, F. Preparation and performance of TiO2/ZnO humidity sensor based on TiO2. Mater. Sci. Eng. B 2023, 298, 116902. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef]
- Kok, S.P.; Go, Y.I.; Wong, M.L.D. Effect of Hygroscopic and Electronic Materials, Challenges, and Sensing Requirements in Humidity-Controlled Industry. J. Electron. Mater. 2025, 54, 6960–6981. [Google Scholar] [CrossRef]
- Ungula, J.; Dejene, B.; Swart, H. Effect of annealing on the structural, morphological and optical properties of Ga-doped ZnO nanoparticles by reflux precipitation method. Results Phys. 2017, 7, 2022–2027. [Google Scholar] [CrossRef]
- Gao, M.; Xiao, W.; Miao, L.; Yang, Z.; Liang, W.; Ao, T.; Yang, Q.; Chen, W. Prussian blue and its analogs: A robust platform for efficient capacitive deionization. Desalination 2024, 574, 117278. [Google Scholar] [CrossRef]
- Riza, M.A.; Go, Y.I.; Maier, R.R.; Harun, S.W.; Anas, S.B.A. Characterization of hysteresis free, low-temperature hydrothermally synthesized zinc oxide for enhanced humidity sensing. Sens. Int. 2021, 2, 100106. [Google Scholar] [CrossRef]
- Park, W.-T.; Son, I.; Park, H.-W.; Chung, K.-B.; Xu, Y.; Lee, T.; Noh, Y.-Y. Facile routes to improve performance of solution-processed amorphous metal oxide thin film transistors by water vapor annealing. ACS Appl. Mater. Interfaces 2015, 7, 13289–13294. [Google Scholar] [CrossRef] [PubMed]
- Muthee, D.K.; Dejene, B.F. Effect of annealing temperature on structural, optical, and photocatalytic properties of titanium dioxide nanoparticles. Heliyon 2021, 7, e07269. [Google Scholar] [CrossRef] [PubMed]
- Sikarwar, S.; Yadav, B.; Singh, S.; Dzhardimalieva, G.; Pomogailo, S.; Golubeva, N.D.; Pomogailo, A. Fabrication of nanostructured yttria stabilized zirconia multilayered films and their optical humidity sensing capabilities based on transmission. Sens. Actuators B 2016, 232, 283–291. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent advances in graphene-based humidity sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356. [Google Scholar] [CrossRef]
- Li, S.; Wan, H.; Lin, J.; Min, J. Physicochemical interactions between amorphous metal oxide and polymer in metal–polymer hybrid materials. Mater. Des. 2023, 230, 111993. [Google Scholar] [CrossRef]
- Goldoni, A.; Alijani, V.; Sangaletti, L.; D’Arsiè, L. Advanced promising routes of carbon/metal oxides hybrids in sensors: A review. Electrochim. Acta 2018, 266, 139–150. [Google Scholar] [CrossRef]
- Braunfelds, J.; Senkans, U.; Skels, P.; Janeliukstis, R.; Salgals, T.; Redka, D.; Lyashuk, I.; Porins, J.; Spolitis, S.; Haritonovs, V. FBG-based sensing for structural health monitoring of road infrastructure. J. Sens. 2021, 2021, 8850368. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Yan, Y.; Xiao, K.; Wang, Z.; Zheng, D.; Leal-Junior, A.; Kumar, S.; Ortega, B.; Marques, C. Wearable photonic smart wristband for cardiorespiratory function assessment and biometric identification. Opto-Electron. Adv. 2025, 8, 240254. [Google Scholar] [CrossRef]
- Rao, G.; Feng, W.; Zhang, J.; Wang, S.; Chen, L.; Guo, Z.; Chen, W. Simulation approach to decomposition kinetics and thermal hazards of hexamethylenetetramine. J. Therm. Anal. Calorim. 2019, 135, 2447–2456. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Moisture Absorption Properties and Equilibrium Conditioning of Polymer Matrix Composite Materials; American Society for Testing and Materials: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared spectrum characteristics and quantification of OH groups in coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Xiao, P.; Gu, J.; Wan, C.; Wang, S.; He, J.; Zhang, J.; Huang, Y.; Kuo, S.-W.; Chen, T. Ultrafast formation of free-standing 2D carbon nanotube thin films through capillary force driving compression on an air/water interface. Chem. Mater. 2016, 28, 7125–7133. [Google Scholar] [CrossRef]
- Carlsson, L.A.; Du, E. Water uptake in polymer composites with voids. In Durability of Composites in a Marine Environment 2; Springer: Cham, Switzerland, 2017; pp. 33–57. [Google Scholar]
- Riza, M.A.; Go, Y.I.; Harun, S.W.; Anas, S.B.A. Effect of additive concentration on crystalline surface of ZnO nanostructures morphology for enhanced humidity sensing. Sens. Int. 2023, 4, 100211. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, X.; Qian, M.; Xiang, Z.; Hu, X. An optical fiber sensor based on polyimide coated fiber Bragg grating for measurement of relative humidity. Opt. Fiber Technol. 2021, 61, 102406. [Google Scholar] [CrossRef]
- Wang, M.-S.; Lei, M.; Wang, Z.-Q.; Zhao, X.; Xu, J.; Yang, W.; Huang, Y.; Li, X. Scalable preparation of porous micron-SnO2/C composites as high performance anode material for lithium ion battery. J. Power Sources 2016, 309, 238–244. [Google Scholar] [CrossRef]
- Velumani, M.; Meher, S.; Alex, Z. Composite metal oxide thin film based impedometric humidity sensors. Sens. Actuators B 2019, 301, 127084. [Google Scholar] [CrossRef]


| Metal Oxide | Target Applications | Advantages | Ref |
|---|---|---|---|
| TiO2 | Chemical/gas sensing | Chemically and thermally stable | [4] |
| ZnO | Humidity sensing | Chemically and thermally stable | [9] |
| ZnO/SnO2 composite | Humidity sensing | Improved surface area, increased oxygen vacancies | [5] |
| CeO2 | Biosensing | Mechanically stable, fast electron transfer kinetics | [10] |
| Ni-doped Fe2O3 | Biosensing | Non-toxicity, anti-interference | [11] |
| Cr2O3 | Gas sensing | Corrosion resistant | [12] |
| V2O5 | Gas sensing | Chemically and thermally stable | [13] |
| SiO2 | Humidity sensing | Porous nature | [14] |
| TiO2/ZnO composite | Humidity sensing | Increased oxygen vacancies, good linearity | [15] |
| SnO2-HMT | Humidity sensing | Enhanced hygroscopicity, negligible hysteresis | This work |
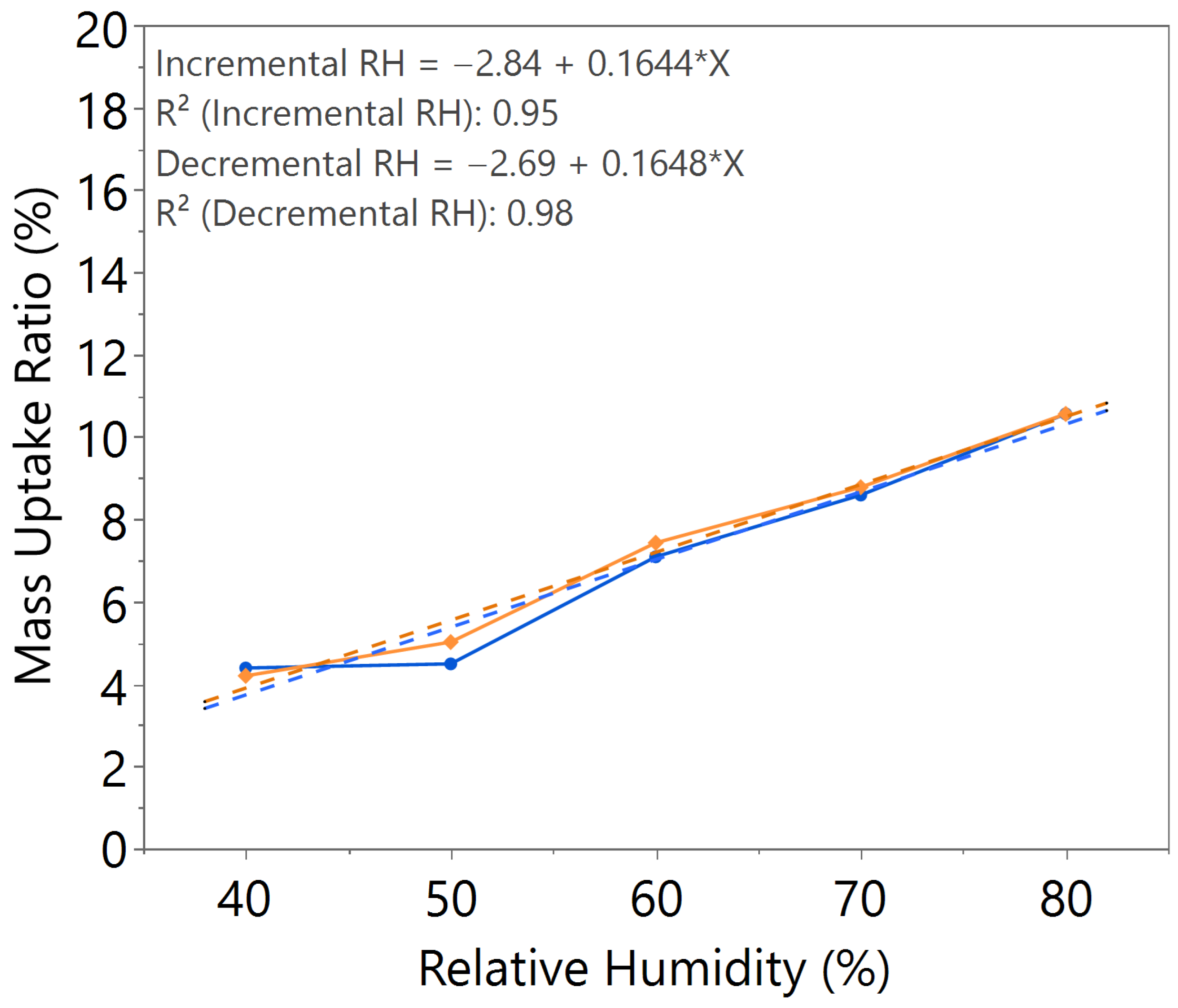
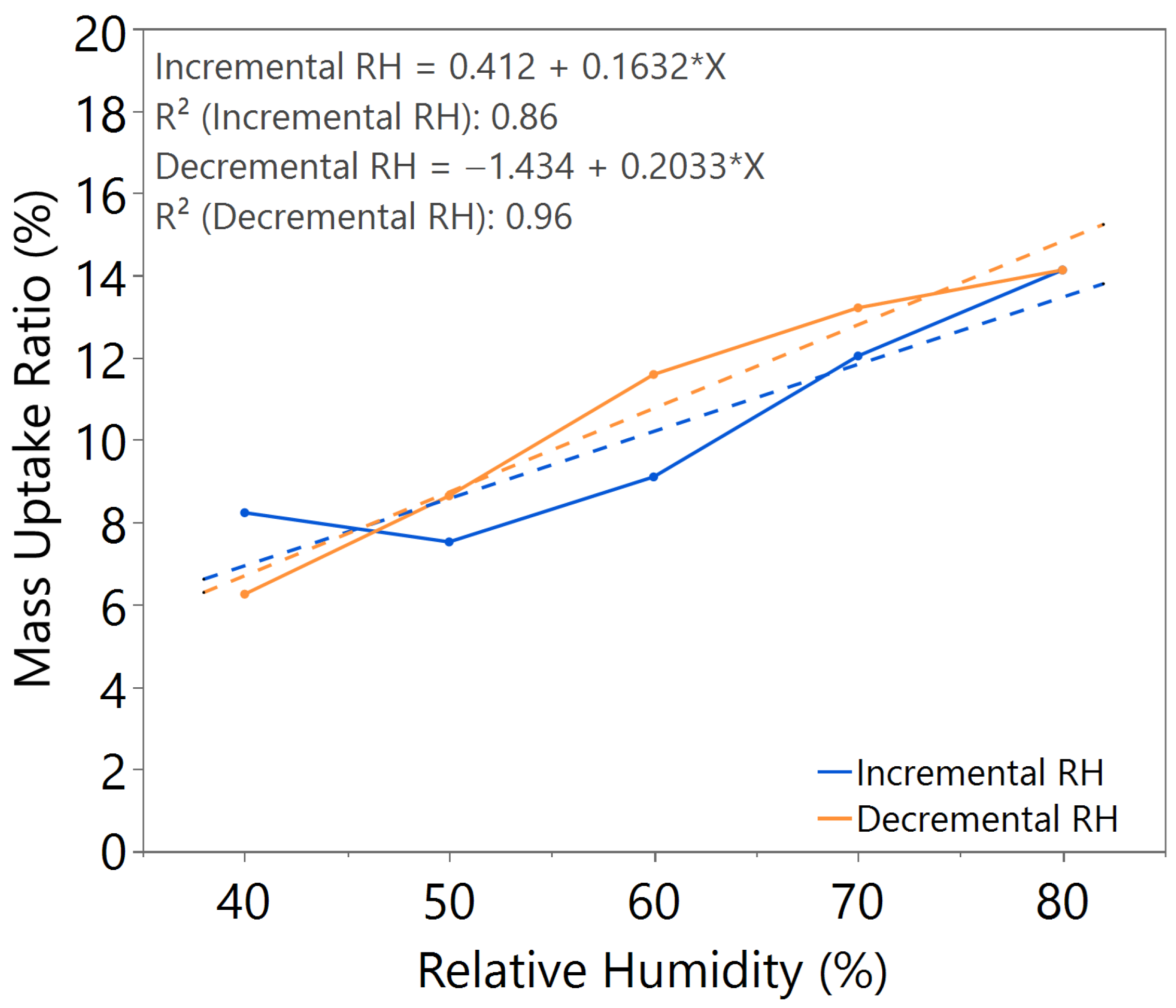
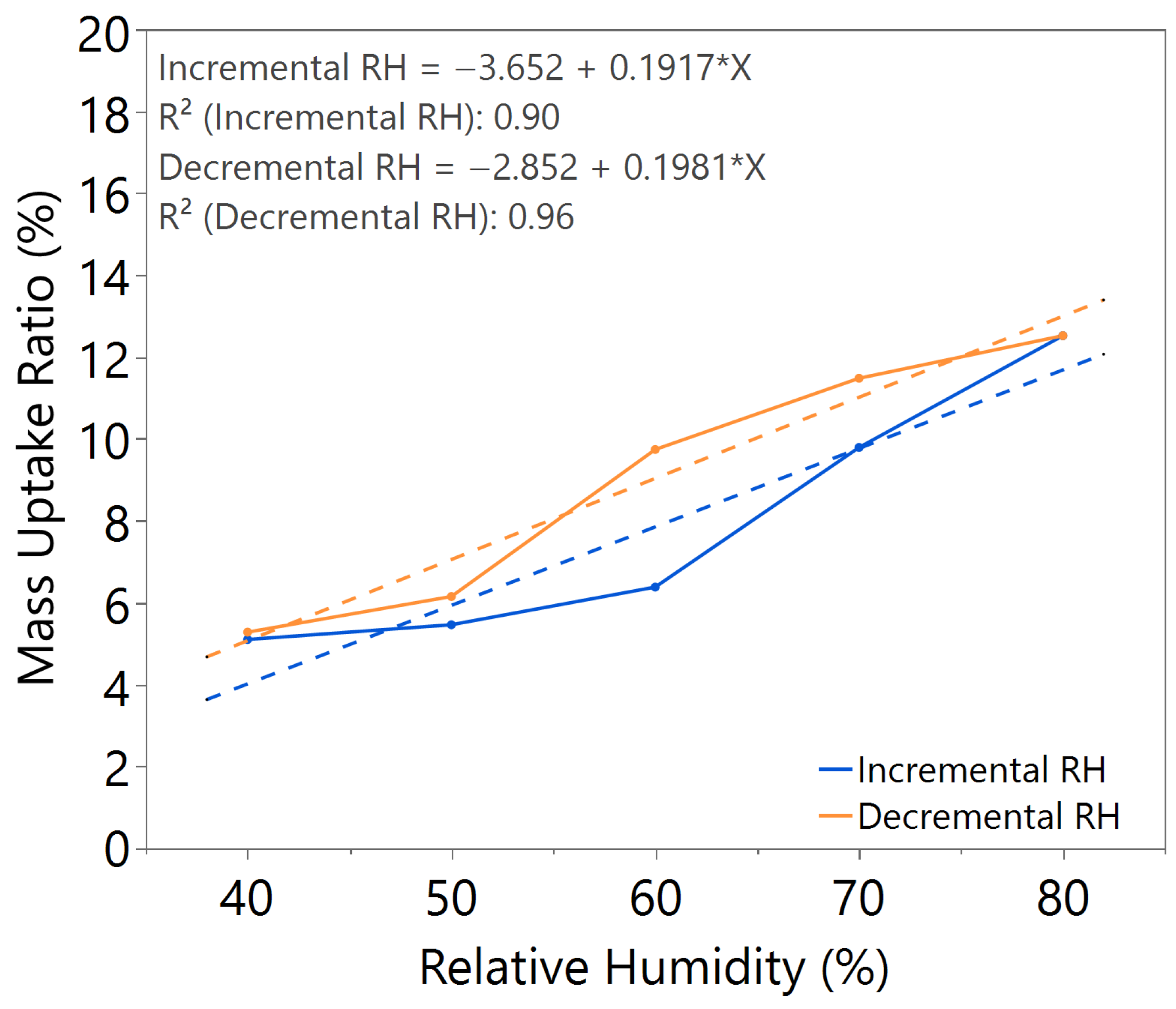
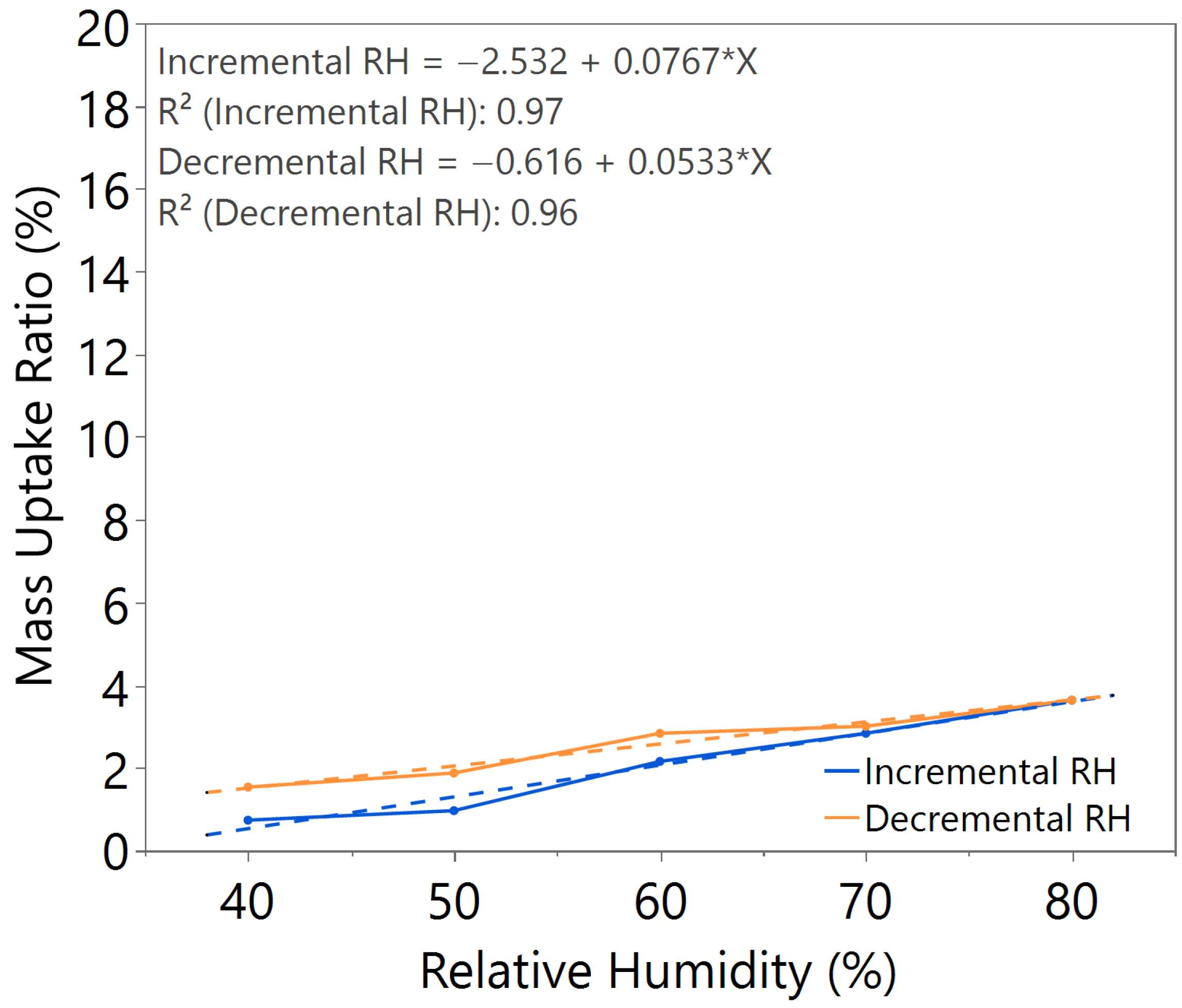
| Annealing Time (hours) | Annealing Temperature (°C) | m value (Incremental RH) | m value (Decremental RH) | Hysteresis |
|---|---|---|---|---|
| 2.5 | 140 | 0.1633 | 0.1807 | Slightly higher |
| 3.0 | 140 | 0.1644 | 0.1648 | Negligible |
| 3.5 | 140 | 0.1632 | 0.2033 | Significant |
| 3.0 | 100 | 0.1917 | 0.1981 | Significant |
| 3.0 | 180 | 0.0767 | 0.0533 | Slightly higher |
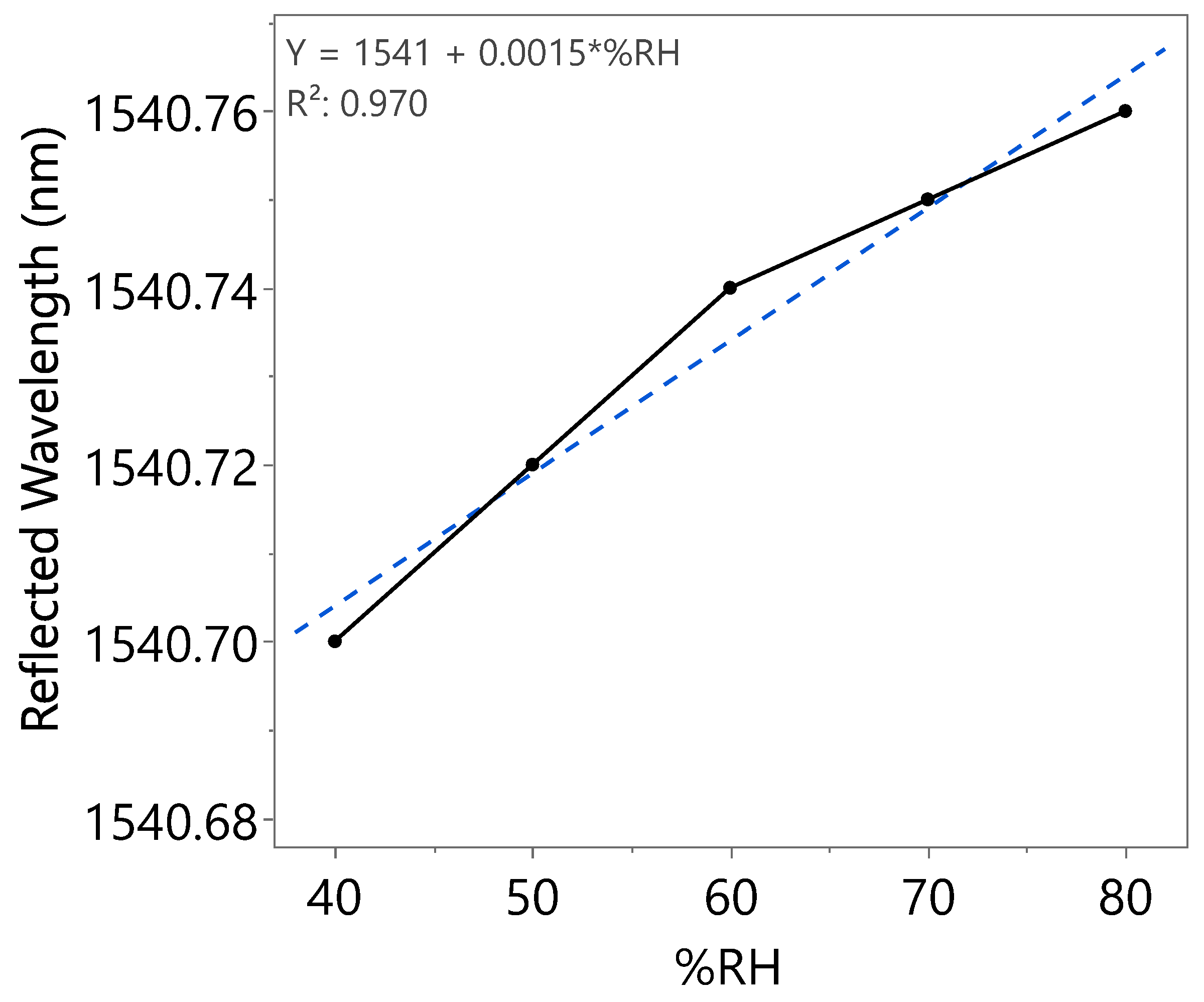
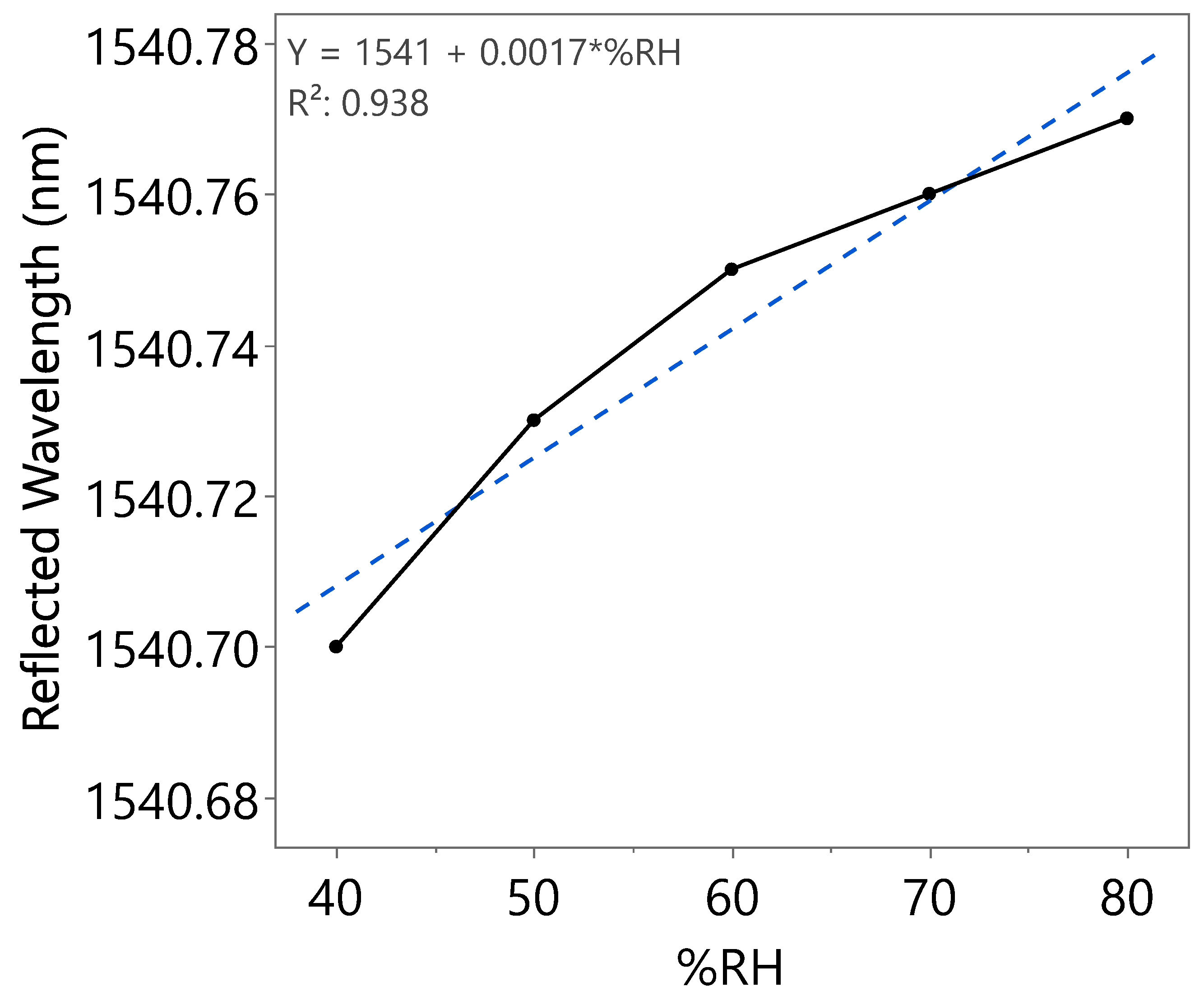
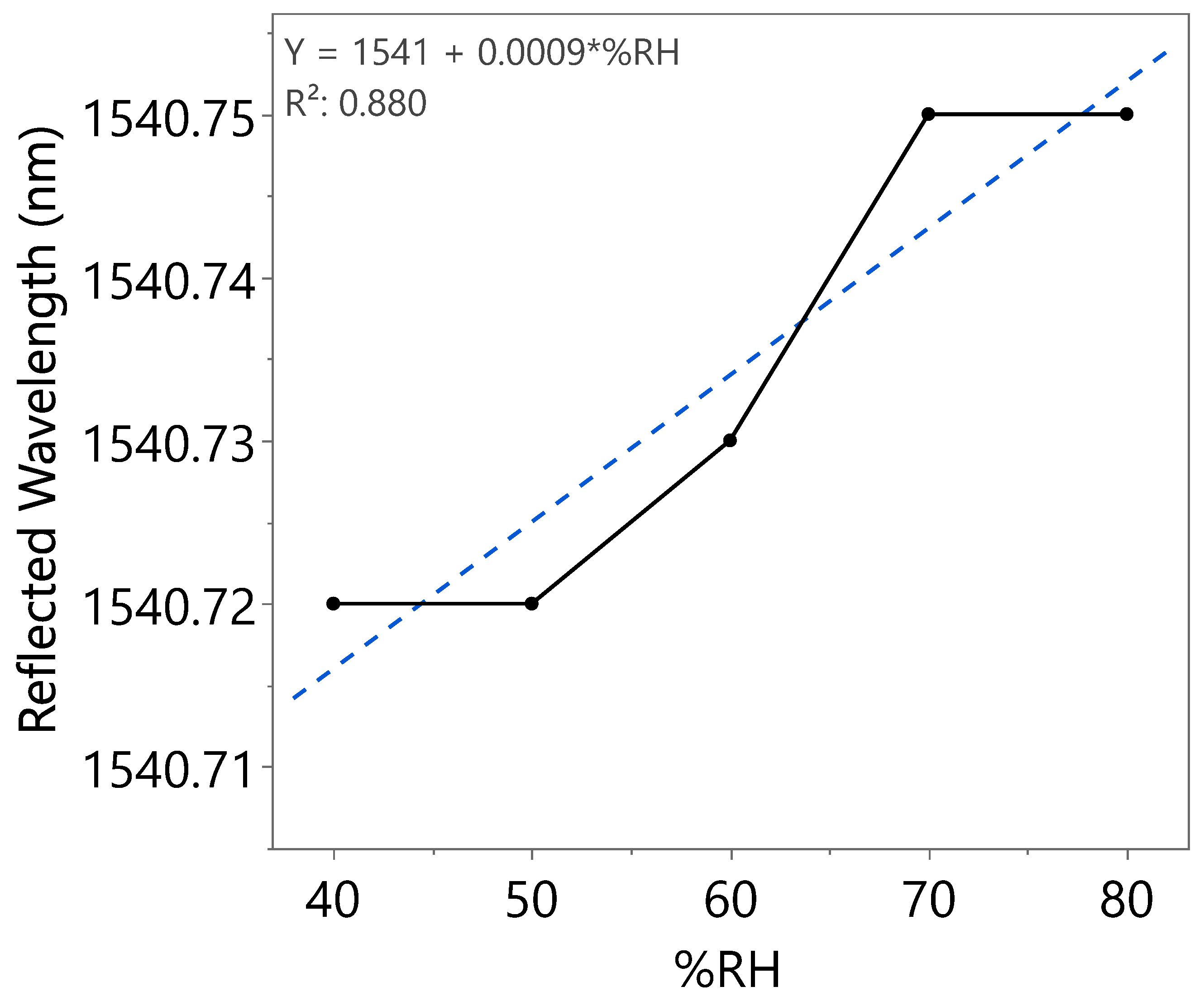
| Humidity Cycle | %RH | Reflected Wavelength (nm) | Bragg Wavelength Shift (pm) | Sensitivity (pm) |
|---|---|---|---|---|
| First | 40 | 1540.70 | 0 | 1.5 |
| 50 | 1540.72 | 20 | ||
| 60 | 1540.74 | 40 | ||
| 70 | 1540.75 | 50 | ||
| 80 | 1540.76 | 60 | ||
| Second | 40 | 1540.70 | 0 | 1.7 |
| 50 | 1540.73 | 30 | ||
| 60 | 1540.75 | 50 | ||
| 70 | 1540.76 | 60 | ||
| 80 | 1540.77 | 70 | ||
| Third | 40 | 1540.72 | 0 | 0.9 |
| 50 | 1540.72 | 0 | ||
| 60 | 1540.73 | 10 | ||
| 70 | 1540.75 | 30 | ||
| 80 | 1540.75 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kok, S.P.; Go, Y.I.; Anas, S.B.A.; Wong, M.L.D.; Chan, K.Y. Additive-Enhanced SnO2 FBG Sensor with Optimized Annealing Time, Temperature, and Multilayer Coating for High-Performance Humidity Sensing. Nanomaterials 2025, 15, 1508. https://doi.org/10.3390/nano15191508
Kok SP, Go YI, Anas SBA, Wong MLD, Chan KY. Additive-Enhanced SnO2 FBG Sensor with Optimized Annealing Time, Temperature, and Multilayer Coating for High-Performance Humidity Sensing. Nanomaterials. 2025; 15(19):1508. https://doi.org/10.3390/nano15191508
Chicago/Turabian StyleKok, Soo Ping, Yun Ii Go, Siti Barirah Ahmad Anas, M. L. Dennis Wong, and Kah Yoong Chan. 2025. "Additive-Enhanced SnO2 FBG Sensor with Optimized Annealing Time, Temperature, and Multilayer Coating for High-Performance Humidity Sensing" Nanomaterials 15, no. 19: 1508. https://doi.org/10.3390/nano15191508
APA StyleKok, S. P., Go, Y. I., Anas, S. B. A., Wong, M. L. D., & Chan, K. Y. (2025). Additive-Enhanced SnO2 FBG Sensor with Optimized Annealing Time, Temperature, and Multilayer Coating for High-Performance Humidity Sensing. Nanomaterials, 15(19), 1508. https://doi.org/10.3390/nano15191508








