S-Doped FeOOH Layers as Efficient Hole Transport Channels for the Enhanced Photoelectrochemical Performance of Fe2O3
Abstract
:1. Introduction
2. Materials and Methods
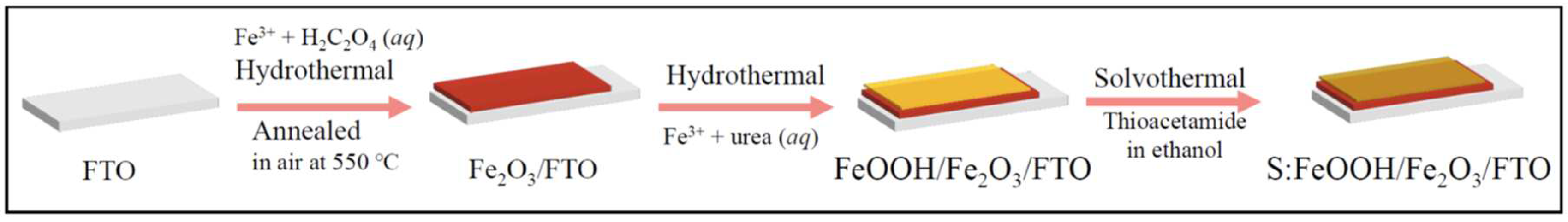
2.1. Synthesis of the Photoanodes
2.2. PEC Measurements
3. Results and Discussions
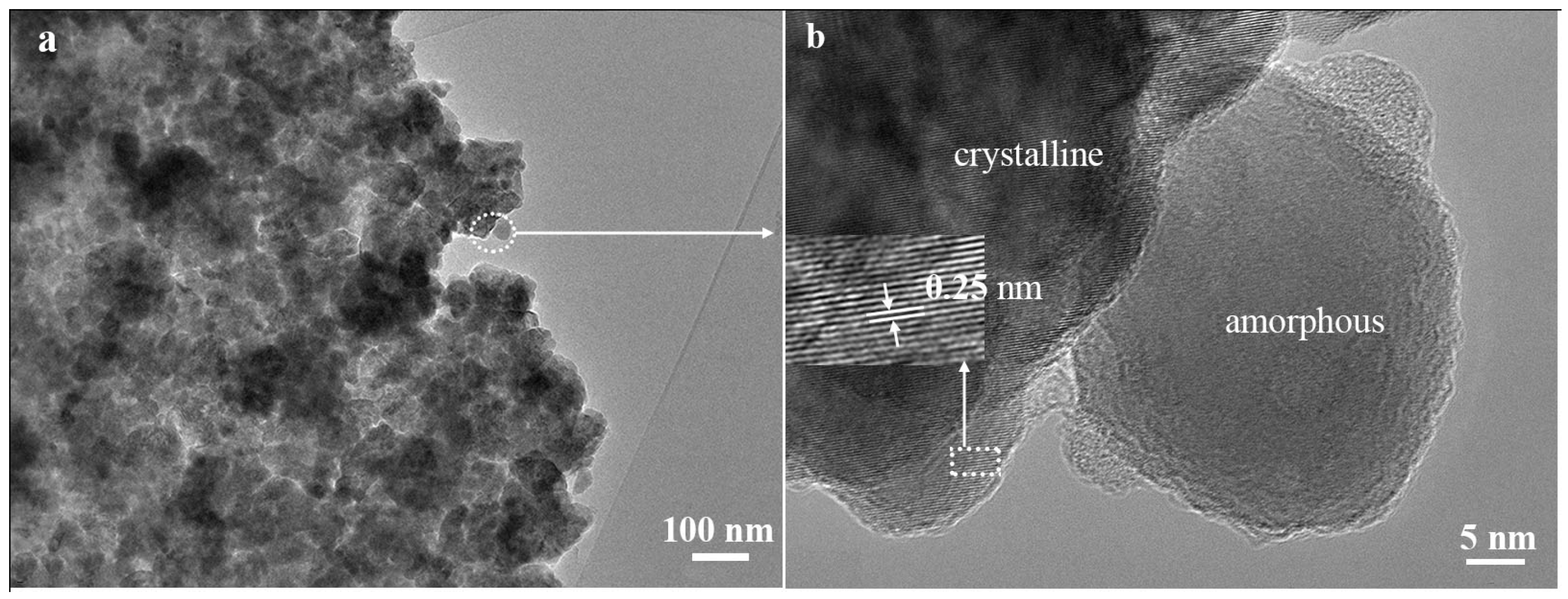
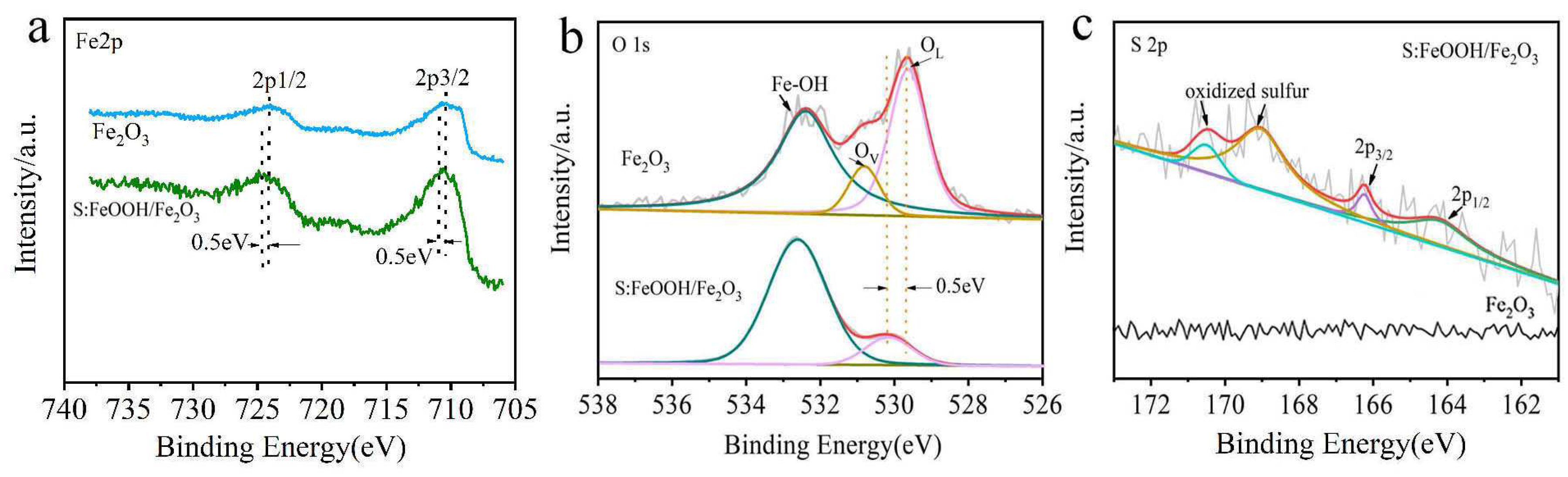
3.1. Material Characterization
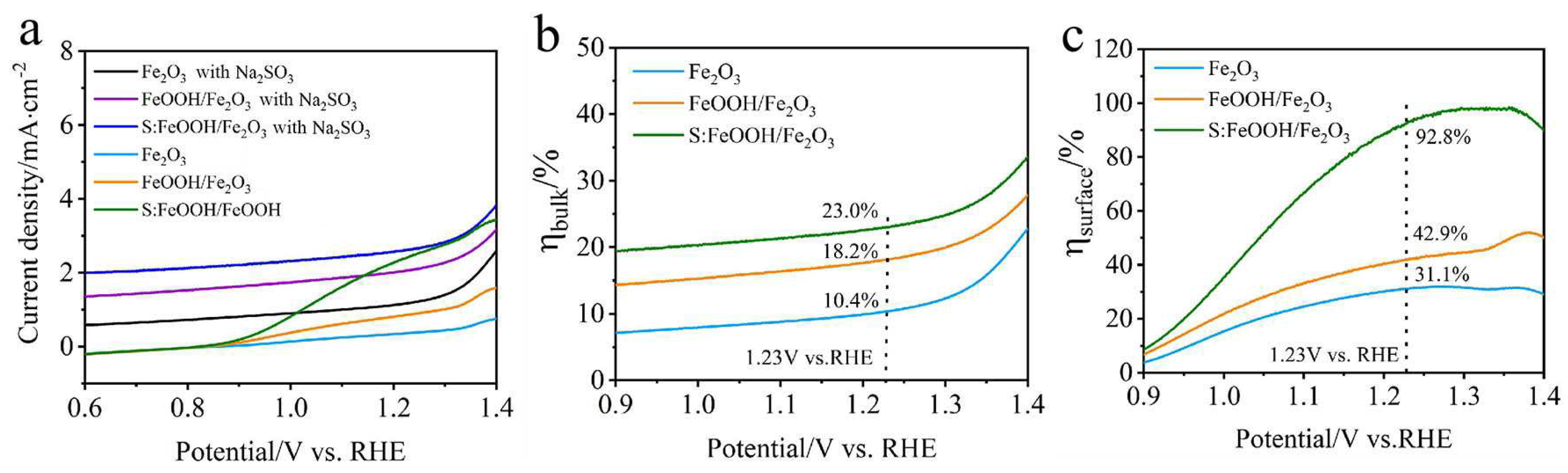
3.2. PEC Results
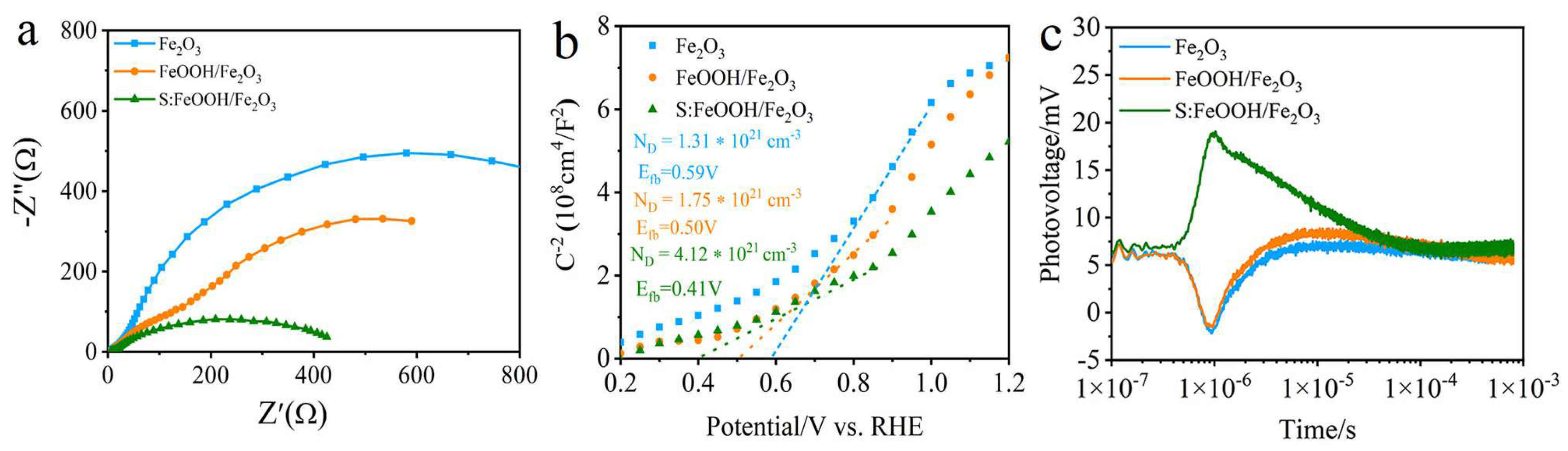
3.3. Charge Transfer Kinetics
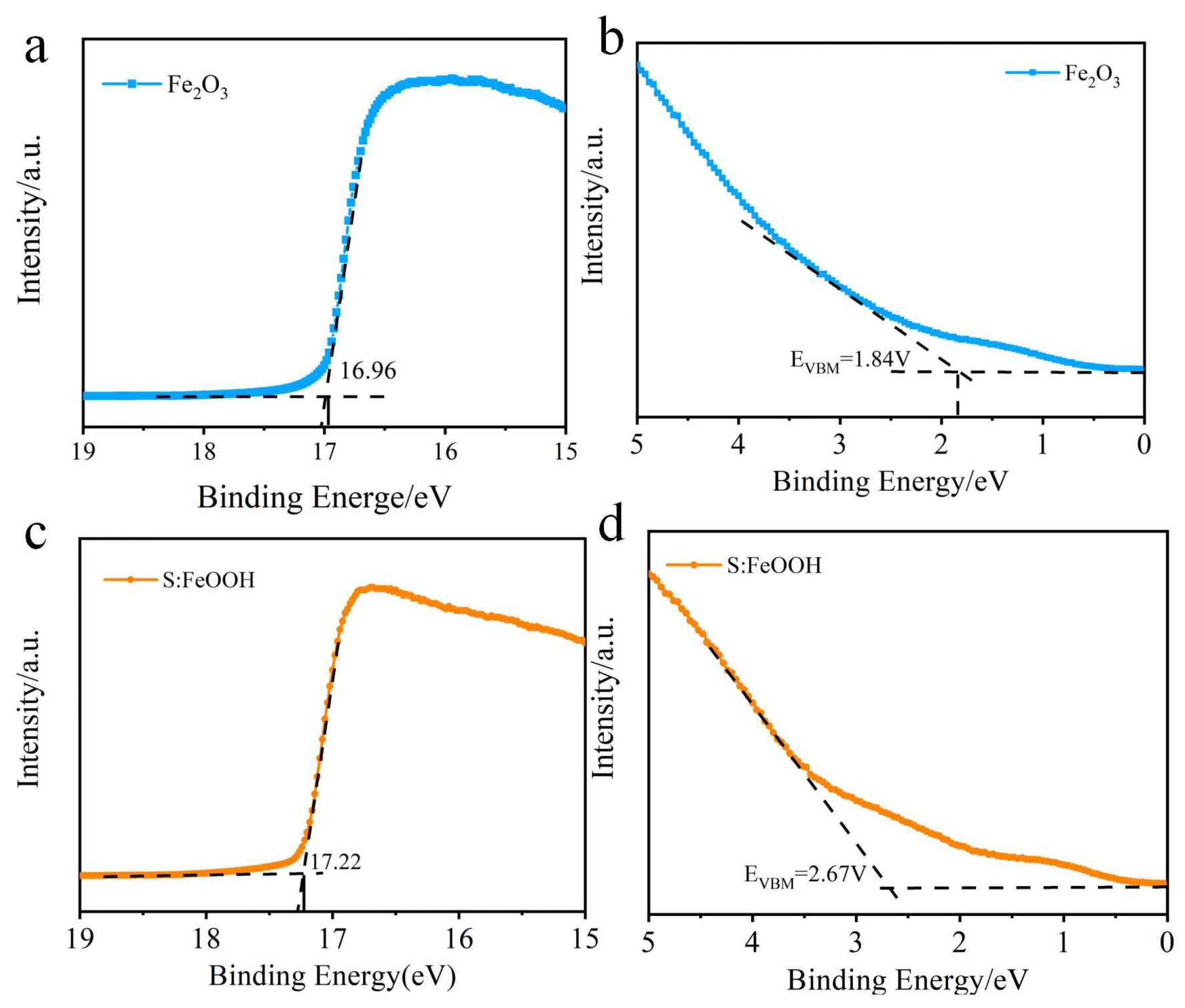
3.4. Mechanism Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, L.F.; Dai, L.L.; Burton, O.J.; Chen, L.; Andrei, V.; Zhang, Y.C.; Ren, D.; Cheng, J.; Wu, L.; Frohna, K.; et al. High carrier mobility along the [111] orientation in Cu2O photoelectrodes. Nature 2024, 628, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Zhang, C.X.; Geng, J.F.; Zong, S.H.; Wang, P.Q. Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy. Molecules 2025, 30, 630. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Liao, W.R.; Kang, Y.C.; Xiao, H.R.; Chen, Y.K.; Wang, Q.Y.; Luo, T.; Chen, J.; Li, H.; et al. Metal vacancies in semiconductor oxides enhance hole mobility for efficient photoelectrochemical water splitting. Nat. Catal. 2025, 8, 229–238. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wu, L.; Tang, D.J.; Xue, J.; Dang, K.; He, H.B.; Bai, S.M.; Ji, H.; Chen, C.; Zhang, Y.; et al. Transition from sequential to concerted proton-coupled electron transfer of water oxidation on semiconductor photoanodes. J. Am. Chem. Soc. 2023, 145, 23849–23858. [Google Scholar] [CrossRef]
- Maurya, O.; Khaladkar, S.R.; Sinha, B.; Bhanage, B.M.; Deshmukh, R.R.; Kim, J.H.; Kalekar, A. Effective transformation of hydrothermally grown TiO2 nanorods to nanotube arrays for improved PEC hydrogen evolution. Electrochim. Acta 2023, 471, 143391. [Google Scholar] [CrossRef]
- Arunachalam, P.; Amer, M.S.; AlOraij, H.A.; Al-Mayouf, A.M.; Hezam, M.; Al-Shalwi, M. Boosting the Photoelectrochemical Water Oxidation Performance of TiO2 Nanotubes by Surface Modification Using Silver Phosphate. Catalysts 2022, 12, 1440. [Google Scholar] [CrossRef]
- Fu, H.; Qi, Q.; Li, Y.; Pan, J.; Zhong, C. Oxygen-Vacancy-Induced Enhancement of BiVO4 Bifunctional Photoelectrochemical Activity for Overall Water Splitting. Nanomaterials 2024, 14, 1270. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, X.; Pei, Y.; Han, W. Enhancing Photoelectrocatalytic Efficiency of BiVO4 Photoanodes by Crystal Orientation Control. Nanomaterials 2024, 14, 1870. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.F.; Zhang, S.C. Enhanced PEC performance of hematite photoanode coupled with bimetallic oxyhydroxide NiFeOOH through a simple electroless method. Appl. Catal. B 2020, 265, 118580. [Google Scholar] [CrossRef]
- Li, Y.; Mei, Q.; Liu, Z.J.; Hu, X.S.; Zhou, Z.H.; Huang, J.W.; Bai, B.; Liu, H.; Ding, F.; Wang, Q.Z. Fluorine-doped iron oxyhydroxide cocatalyst: Promotion on the WO3 photoanode conducted photoelectrochemical water splitting. Appl. Catal. B 2022, 304, 120995. [Google Scholar] [CrossRef]
- Li, W.L.; Geng, H.C.; Yao, L.; Cao, K.S.; Sheng, P.T.; Cai, Q.Y. Photoelectrocatalytic Hydrogen Generation Enabled by CdS Passivated ZnCuInSe Quantum Dot-Sensitized TiO2 Decorated with Ag Nanoparticles. Nanomaterials 2019, 9, 393. [Google Scholar] [CrossRef]
- Pech-Rodríguez, W.J.; Sahin, N.E.; Suarez-Velázquez, G.G.; Meléndez-González, P.C. Semiconductor-Based Photoelectrocatalysts in Water Splitting: From the Basics to Mechanistic Insights—A Brief Review. Materials 2025, 18, 1952. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Zhao, Y.C.; Li, Y.D. Improved photoelectrochemical water splitting performance of Sn-doped hematite photoanode with an amorphous cobalt oxide layer. Int. J. Hydrog. Energ. 2024, 51, 1176–1183. [Google Scholar] [CrossRef]
- Xu, C.Y.; Wang, H.X.; Guo, H.Y.; Liang, K.; Zhang, Y.M.; Li, W.C.; Chen, J.; Lee, J.S.; Zhang, H. Parallel multi-stacked photoanodes of Sbdoped p–n homojunction hematite with near-theoretical solar conversion efficiency. Nat. Commun. 2024, 15, 9712. [Google Scholar] [CrossRef] [PubMed]
- Humayun, A.; Manivelan, N.; Prabakar, K. Charge Transfer in n-FeO and p-α-Fe2O3 Nanoparticles for Efficient Hydrogen and Oxygen Evolution Reaction. Nanomaterials 2024, 14, 1515. [Google Scholar] [CrossRef] [PubMed]
- Baldovi, H.G. Optimization of α-Fe2O3 Nanopillars Diameters for Photoelectrochemical Enhancement of α-Fe2O3-TiO2 Heterojunction. Nanomaterials 2021, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Suman; Singh, S.; Ankita; Kumar, A.; Kataria, N.; Kumar, S.; Kumar, P. Photocatalytic activity of α-Fe2O3@CeO2 and CeO2@α-Fe2O3 core-shell nanoparticles for degradation of Rose Bengal dye. J. Environ. Chem. Eng. 2021, 9, 106266. [Google Scholar] [CrossRef]
- Sun, J.; Xia, W.; Zheng, Q.; Zen, X.; Liu, W.; Li, G.; Wang, P. Increased active sites on irregular morphological α-Fe2O3 nanorods for enhanced photoelectrochemical performance. ACS Omega 2020, 5, 12339–12345. [Google Scholar] [CrossRef]
- Yan, K.; Qiu, Y.; Xia, S.; Gon, J.; Zhao, S.; Xu, J. Self-driven hematite-based photoelectrochemical water splitting cells with three-dimensional nanobowl heterojunction and high-photovoltage perovskite solar cells. Mater. Today Energy 2017, 6, 128–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhu, S.S.; Liu, Y.Y.; Zhang, X.; Wagn, J.J.; Braun, A. Covalent S-O bonding enables enhanced photoelectrochemical performance of Cu2S/Fe2O3 heterojunction for water splitting. Small 2021, 17, 2100320. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Li, L.; Zong, X.; Sun, H.; Tao, R.; Fan, X. Fe2O3 nanorods/CuO nanoparticles p-n heterojunction photoanode: Effective charge separation and enhanced photoelectrochemical properties. J. Colloid Interface Sci. 2021, 602, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Tayyeb, A.; Ryu, H.; Jang, J.I.; Lee, B.K. Simultaneous enhancement of charge separation and hole transportation in a W: α-Fe2O3/MoS2 photoanode: A collaborative approach of MoS2 as a heterojunction and W as a metal dopant. ACS Appl. Mater. Interfaces 2021, 13, 39215–39229. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Chen, Y.; Zhang, R.; Li, C.; Zhang, K.; Li, M.; Lin, Y.; Wang, D.; Zou, X.; et al. Interface engineering Z-scheme Ti-Fe2O3/In2O3 photoanode for highly efficient photoelectrochemical water splitting. Appl. Catal. B 2021, 290, 120058. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Park, J.; Jung, M.; Ji, S.G.; Lee, H.; Seo, J.H.; Kwak, M.J.; Il Seok, S.; Lee, J.H.; Jang, J.H. NiFeOx decorated Ge-hematite/perovskite for an efficient water splitting system. Nat. Commun. 2021, 12, 4309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Long, R.; Prezhdo, O.V. Why silicon doping accelerates electron polaron diffusion in hematite. J. Am. Chem. Soc. 2019, 141, 20222–20233. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Leng, W.; Wu, D. Ti doped α-Fe2O3 electrodes for water oxidation. J. Alloys Compd. 2022, 927, 166975. [Google Scholar] [CrossRef]
- Nyarige, J.S.; Paradzah, A.T.; Krüger, T.P.J.; Diale, M. Mono-Doped and Co-Doped Nanostructured Hematite for Improved Photoelectrochemical Water Splitting. Nanomaterials 2022, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; García-Rodríguez, R.; Cameron, P.; Eslava, S. Role of cobalt–iron (oxy) hydroxide (CoFeOx) as oxygen evolution catalyst on hematite photoanodes. Energy Environ. Sci. 2018, 11, 2972–2984. [Google Scholar] [CrossRef]
- Chang, Y.; Han, M.; Ding, Y.; Wei, H.; Zhang, D.; Luo, H.; Li, X.; Yan, X. Interface Engineering of CoFe-LDH Modified Ti:α-Fe2O3 Photoanode for Enhanced Photoelectrochemical Water Oxidation. Nanomaterials 2023, 13, 2579. [Google Scholar] [CrossRef]
- Wu, H.H.; Ding, H.H.; Ma, J.; Li, X.; Huai, S.S.; Zhang, C.M.; Li, P.; Xia, J.J.; Fang, X.L.; Wang, X.F. P-N homojunction and heteroatom active site engineering over Fe2O3 nanorods for highly efficient photoelectrochemical water splitting. Int. J. Hydrog. Energ. 2024, 88, 965–976. [Google Scholar] [CrossRef]
- Jeong, I.K.; Mahadik, M.A.; Hwang, J.B.; Chae, W.S.; Choi, S.H.; Jang, J.S. Lowering the onset potential of Zr-doped hematite nanocoral photoanodes by Al co-doping and surface modification with electrodeposited Co-Pi. J. Colloid Interface Sci. 2021, 581, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.W.; Zhang, R.; Chai, Z.C.; Pu, J.Y.; Kang, R.; Wu, G.Q.; Zeng, X.H. Synergies of Zn/P-co-doped α-Fe2O3 photoanode for improving photoelectrochemical water splitting performance. Int. J. Hydrog. Energ. 2024, 59, 22–29. [Google Scholar] [CrossRef]
- Ji, M.H.; Chen, Y.X.; Chen, R.; Li, K.X.; Zhao, H.-P.; Shi, H.Y.; Wang, H.L.; Jiang, X.; Lu, C.Z. A novel α-Fe2O3 photoanode with multilayered In2O3/Co–Mn nanostructure for efficient photoelectrochemical water splitting. Int. J. Hydrog. Energ. 2024, 51, 66–77. [Google Scholar] [CrossRef]
- Kim, N.; Ju, S.; Ha, J.; Choi, H.; Sung, H.; Lee, H. Hierarchical Co–Pi Clusters/Fe2O3 Nanorods/FTO Micropillars 3D Branched Photoanode for High-Performance Photoelectrochemical Water Splitting. Nanomaterials 2022, 12, 3664. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Liu, M.; Liu, C.; Zhou, W.; Wang, D.; Liu, L.; Ye, J. Ultrathin FeOOH nanosheets as an efficient cocatalyst for photocatalytic water oxidation. J. Mater. Chem. A 2019, 7, 9222–9229. [Google Scholar] [CrossRef]
- Wang, T.; Gao, L.; Wang, P.; Long, X.; Chai, H.; Li, F.; Jin, J.J. Dual-doping in the bulk and the surface to ameliorate the hematite anode for photoelectrochemical water oxidation. J. Colloid Interface Sci. 2022, 624, 60−69. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Miao, X.; Zhao, L.; Zhu, C. Deposition of FeOOH Layer on Ultrathin Hematite Nanoflakes to Promote Photoelectrochemical Water Splitting. Micromachines 2024, 15, 387. [Google Scholar] [CrossRef]
- Li, R.X.; Zhan, F.Q.; Wen, G.C.; Wang, B.; Qi, J.H.; Liu, Y.S.; Feng, C.C.; La, P.Q. Facile Synthesis of a Micro–Nano-Structured FeOOH/BiVO4/WO3 Photoanode with Enhanced Photoelectrochemical Performance. Catalysts 2024, 14, 828. [Google Scholar] [CrossRef]
- Lu, X.Y.; Ye, K.H.; Zhang, S.Q.; Zhang, J.; Yang, J.D.; Huang, Y.C.; Ji, H.B. Amorphous type FeOOH modified defective BiVO4 photoanodes for photoelectrochemical water oxidation. Chem. Eng. J. 2022, 428, 131027. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, J.; Li, H.; Park, J.; Cho, I.S.; Han, H.S.; Zheng, X. One-step hydrothermal deposition of Ni:FeOOH onto photoanodes for enhanced water oxidation. ACS Energy Lett. 2016, 1, 624–632. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Wang, J.; Li, C.; Zheng, R.; Zhang, H.; Liu, J.; Zhang, X. Cobalt and vanadium co-doped FeOOH nanoribbons: An iron-rich electrocatalyst for efficient water oxidation. Mater. Chem. Front. 2021, 5, 6485–6490. [Google Scholar] [CrossRef]
- She, H.; Yue, P.; Huang, J.; .Wang, L.; Wang, Q. One-step hydrothermal deposition of F:FeOOH onto BiVO4 photoanode for enhanced water oxidation. Chem. Eng. J. 2020, 392, 123703. [Google Scholar] [CrossRef]
- Zhang, R.; Ning, X.; Wang, Z.; Zhao, H.; He, Y.; Han, Z.; Du, P.; Lu, X. Significantly promoting the photogenerated charge separation by introducing an oxygen vacancy regulation strategy on the FeNiOOH co-catalyst. Small 2022, 18, 2107938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Balogun, M.S.; Tong, Y.; Huang, Y. Oxygen vacancy–based metal oxides photoanodes in photoelectrochemical water splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Park, G.; Kim, Y.I.; Kim, Y.H.; Park, M.; Jang, K.Y.; Song, H.; Nam, K.M. Preparation and phase transition of FeOOH nanorods: Strain effects on catalytic water oxidation. Nanoscale 2017, 9, 4751−4758. [Google Scholar] [CrossRef]
- Mukai, K.; Suzuki, T.M.; Uyama, T.; Nonaka, T.; Morikawa, T.; Yamada, I. High-pressure synthesis of ε-FeOOH from β-FeOOH and its application to the water oxidation catalyst. RSC Adv. 2020, 10, 44756−44767. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Tang, J.; Tang, W.; Tang, L.; Yu, J.; Wang, J.; Yang, Y.; Ni, T.; Li, C.; Luo, J. A strategy to improve electrochemical water oxidation of FeOOH by modulating the electronic structure. Appl. Mater. Today 2021, 25, 101252. [Google Scholar] [CrossRef]
- Zhang, B.B.; Yu, S.Q.; Dai, Y.; Huang, X.J.; Chou, L.J.; Lu, G.X.; Dong, G.J.; Bi, Y.P. Nitrogen-incorporation activates NiFeOx catalysts for efficiently boosting oxygen evolution activity and stability of BiVO4 photoanodes. Nat. Commun. 2021, 12, 6969. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, M.P.; Ghorpade, U.V.; Shin, S.W.; Suryawanshi, U.P.; Shim, H.J.; Kang, S.H.; Kim, J.H. Facile, Room Temperature, Electroless Deposited (Fe1−x,Mnx)OOH Nanosheets as Advanced Catalysts: The Role of Mn Incorporation. Small 2018, 14, 1801226. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, Y.; Bai, J.; Li, J.H.; Zhou, C.H.; Li, L.; Xie, C.Y.; Zhou, T.S.; Zhu, H.; Zhou, B.X. Ni doped amorphous FeOOH layer as ultrafast hole transfer channel for enhanced PEC performance of BiVO4. J. Colloid Interface Sci. 2023, 644, 509–518. [Google Scholar] [CrossRef]
- He, Y.R.; Zhang, R.F.; Wang, Z.; Ye, H.Q.; Zhao, H.H.; Lu, B.Z.; Du, P.Y.; Lu, X.Q. Unveiling the Influence of Sulfur Doping on Photoelectrochemical Performance in BiVO4/FeOOH Heterostructures. Anal. Chem. 2024, 96, 100–116. [Google Scholar] [CrossRef]
- Shi, J.; Hara, Y.; Sun, C.; Anderson, M.A.; Wang, X. Three-Dimensional High-Density Hierarchical Nanowire Architecture for High-Performance Photoelectrochemical Electrodes. Nano Lett. 2011, 11, 3413–3419. [Google Scholar] [CrossRef]
- Wang, H.F.; Yuan, M.L.; Zhang, J.X.; Bai, Y.L.; Zhang, K.; Li, B.; Zhang, G.J. Rational element-doping of FeOOH-based electrocatalysts for efficient ammonia electrosynthesis. EES Catal. 2024, 2, 324–334. [Google Scholar] [CrossRef]
- Wang, R.Q.; Li, D.Y.; Wang, H.L.; Liu, C.L.; Xu, L.J. Preparation, Characterization, and Performance Analysis of S-Doped Bi2MoO6 Nanosheets. Nanomaterials 2019, 9, 1341. [Google Scholar] [CrossRef]
- 55 Faria, D.L.A.D.; Silva, S.V.; Oliveira, M.T.D. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhao, Z.F.; Xu, Z.K.; Li, L.; Lin, S.Y. Efficient and durable S-doped Ni/FeOOH electrocatalysts for oxygen evolution reactions. Dalton Trans. 2023, 52, 1113–1121. [Google Scholar] [CrossRef]
- Quang, N.D.; Van, P.C.; Majumder, S.; Jeong, J.R.; Kim, D.; Kim, C. Rational construction of S-doped FeOOH onto Fe2O3 nanorods for enhanced water oxidation. J. Colloid Interface Sci. 2022, 616, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Li, C.C.; Liu, S.S.; Wang, T.; Gong, J.L. Gradient doping of phosphorus in Fe2O3 nanoarray photoanodes for enhanced charge separation. Chem. Sci 2017, 8, 91–100. [Google Scholar] [CrossRef]
- Zhu, W.W.; Wei, Y.Q.; Liu, Z.C.; Zhang, Y.C.; He, H.C.; Yang, S.G.; Li, Z.D.; Zou, Z.G.; Zhou, Y. Construction of unique heterojunction photoanodes through in situ quasi-epitaxial growth of FeVO4 on Fe2O3 nanorod arrays for enhanced photoelectrochemical performance. Catal. Sci. Technol. 2022, 12, 4372–4379. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, X.; Li, J.; Zhang, H.; Chen, S.; Han, W.; Yang, D. Surface modification of hematite photoanode by NiFe layered double hydroxide for boosting photoelectrocatalytic water oxidation. J. Alloy Compd. 2018, 764, 341–346. [Google Scholar] [CrossRef]
- Wu, Q.; Meng, D.; Zhang, Y.; Zhao, Q.; Bu, Q.; Wang, D.; Zou, X.; Lin, Y.; Li, S.; Xie, T. Acid-treated Ti4+ doped hematite photoanode for efficient solar water oxidation—Insight into surface states and charge separation. J. Alloys Compd. 2019, 782, 943–951. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, Y.; Jing, B.; Liu, X.; Wang, D. S-Doped FeOOH Layers as Efficient Hole Transport Channels for the Enhanced Photoelectrochemical Performance of Fe2O3. Nanomaterials 2025, 15, 767. https://doi.org/10.3390/nano15100767
Zhou Y, Zhang Y, Jing B, Liu X, Wang D. S-Doped FeOOH Layers as Efficient Hole Transport Channels for the Enhanced Photoelectrochemical Performance of Fe2O3. Nanomaterials. 2025; 15(10):767. https://doi.org/10.3390/nano15100767
Chicago/Turabian StyleZhou, Yanhong, Yiran Zhang, Boyang Jing, Xiaoyuan Liu, and Debao Wang. 2025. "S-Doped FeOOH Layers as Efficient Hole Transport Channels for the Enhanced Photoelectrochemical Performance of Fe2O3" Nanomaterials 15, no. 10: 767. https://doi.org/10.3390/nano15100767
APA StyleZhou, Y., Zhang, Y., Jing, B., Liu, X., & Wang, D. (2025). S-Doped FeOOH Layers as Efficient Hole Transport Channels for the Enhanced Photoelectrochemical Performance of Fe2O3. Nanomaterials, 15(10), 767. https://doi.org/10.3390/nano15100767






