Research Status of Agricultural Nanotechnology and Its Application in Horticultural Crops
Abstract
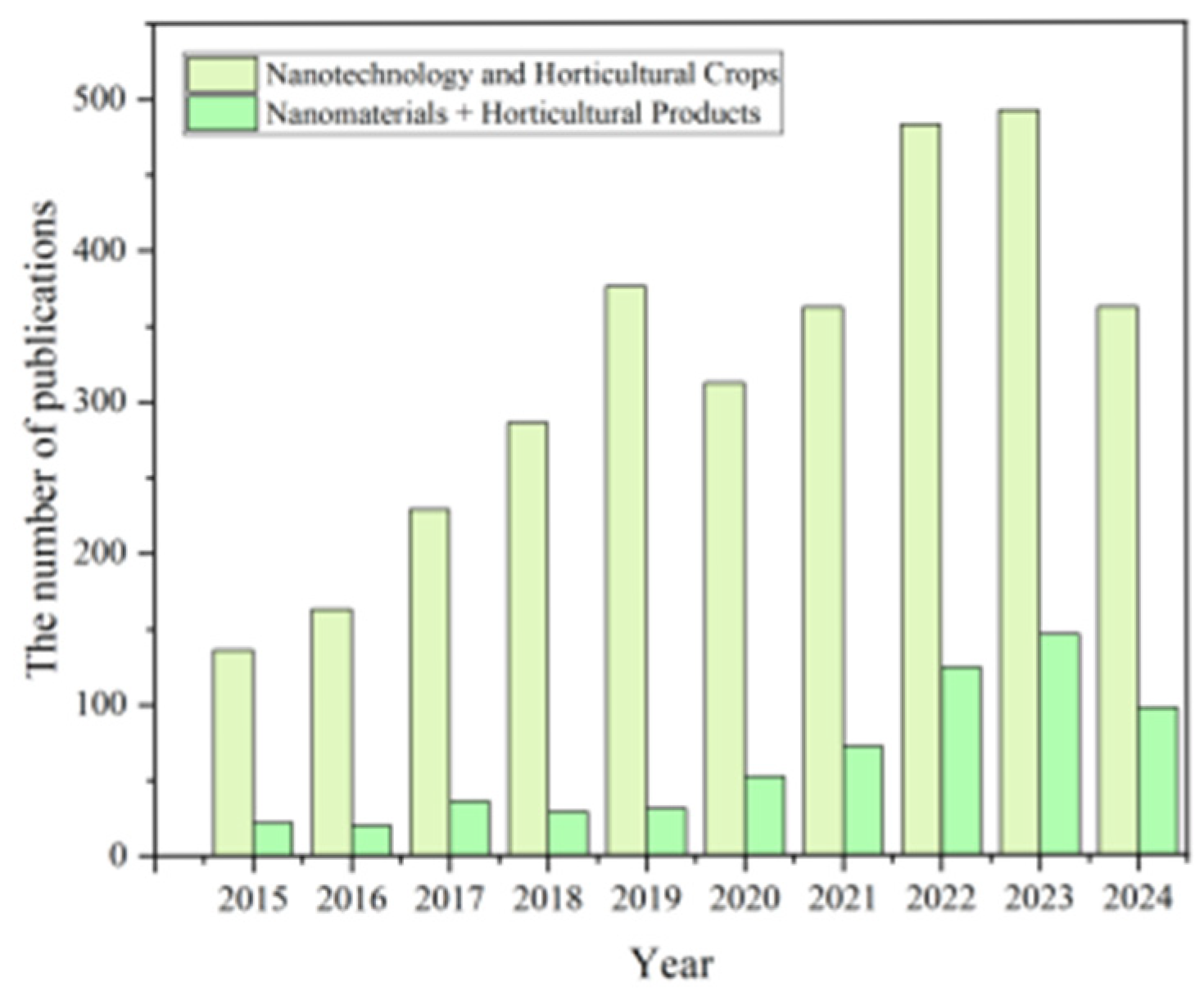
1. Introduction
2. Preparation and Performance Regulation of Agricultural Nanomaterials
2.1. Classification and Preparation Methods of Nanomaterials
2.1.1. Inorganic Nanomaterials
2.1.2. Organic Nanomaterials
2.2. Principles and Methods for Performance Enhancement
2.2.1. Gene Delivery
2.2.2. Crop Growth and Development
2.2.3. Detection and Removal of Pollutants
2.2.4. Detection of Quality and Bioactive Substances
2.2.5. Preservation of Fruits and Vegetables
3. Application Progress
3.1. Gene Delivery
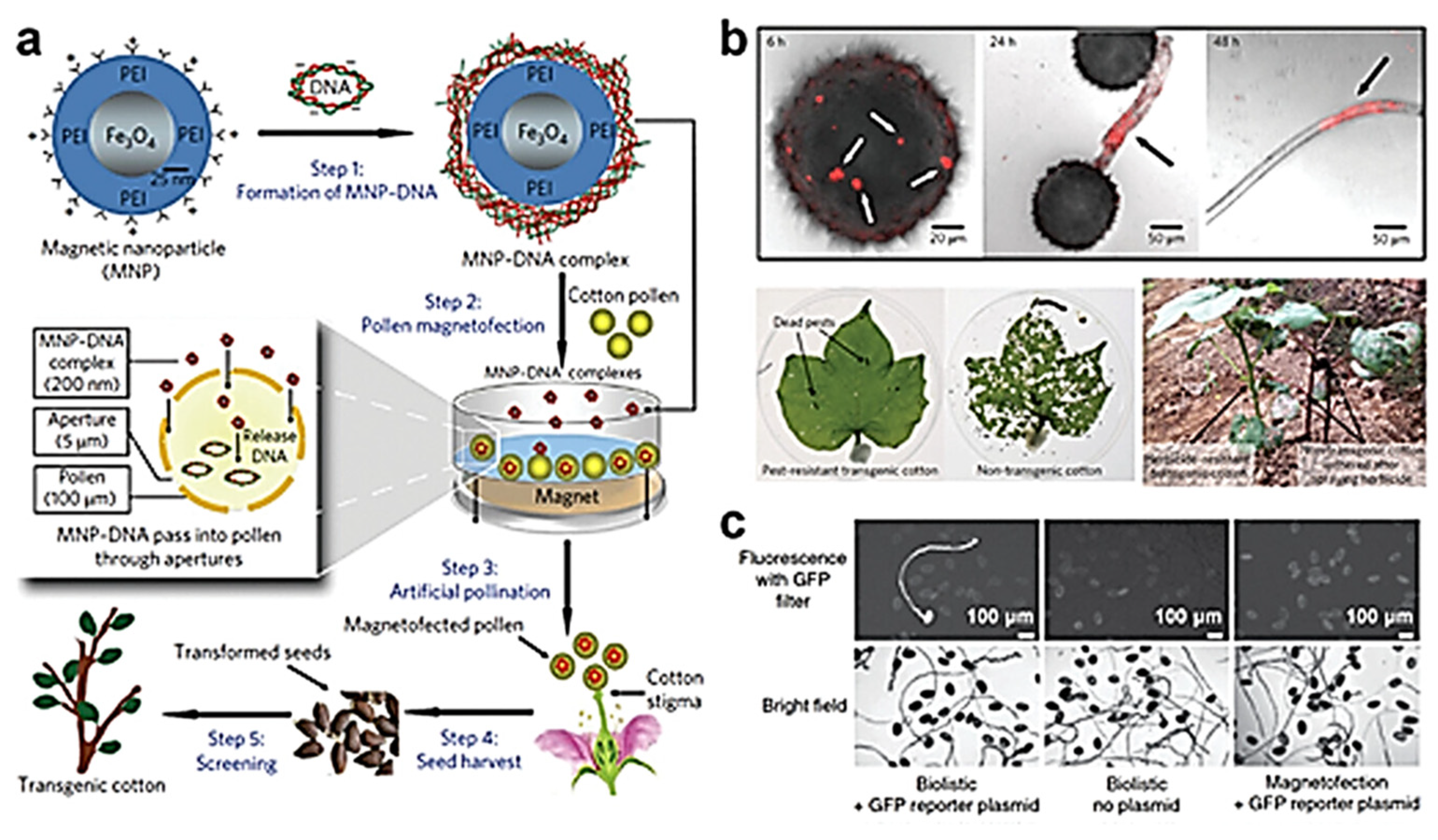
3.2. Nano-Fertilizers and Chemical Fertilizers
3.3. Nano-Sensors
3.4. Removal of Pollutants
3.5. Detection of Quality and Safety
3.6. Preservation of Fruits and Vegetables
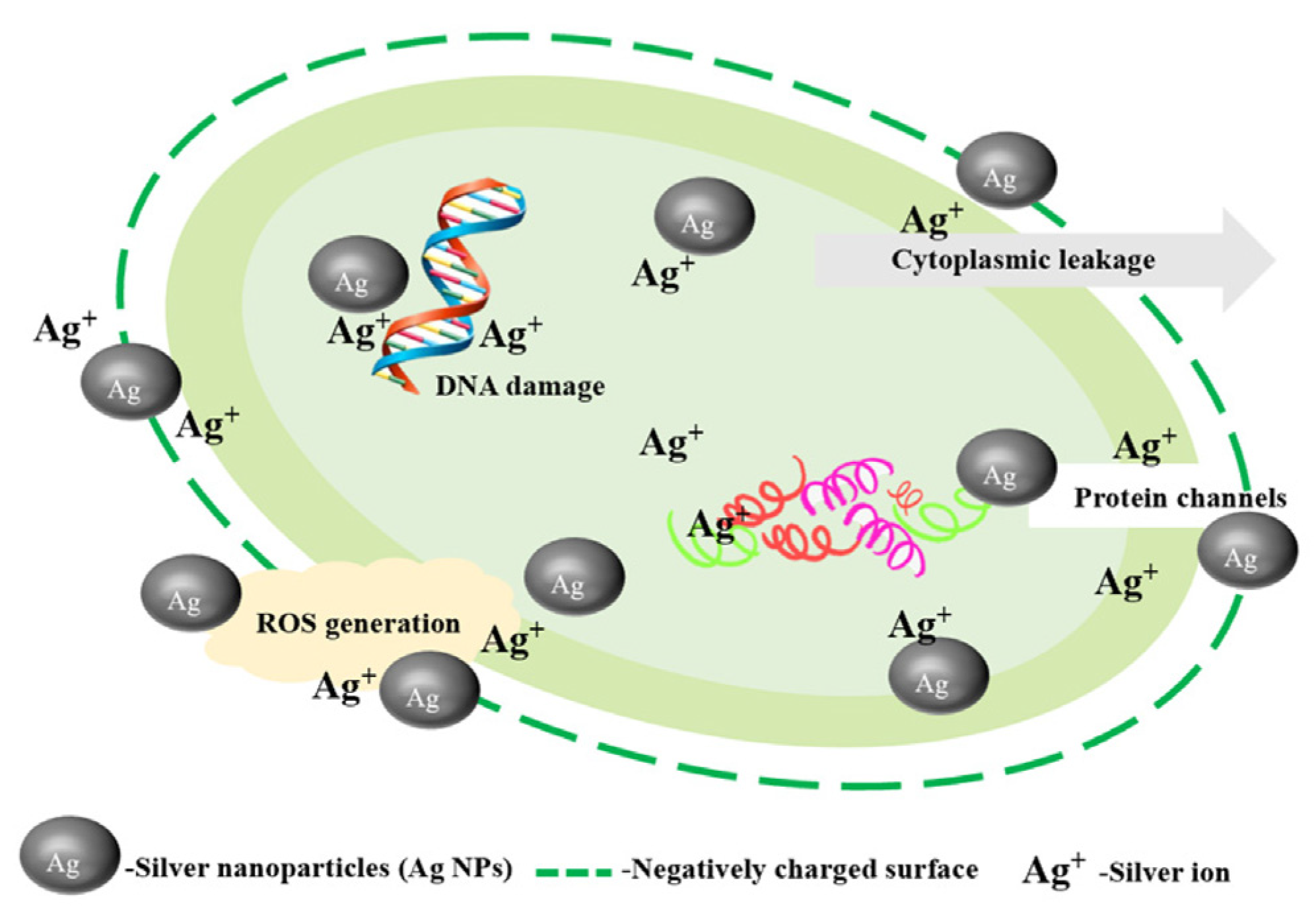
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Haris, M.; Hussain, T.; Mohamed, H.I.; Khan, A.; Ansari, M.S.; Tauseef, A.; Khan, A.A.; Akhtar, N. Nanotechnology—A new frontier of nano-farming in agricultural and food production and its development. Sci. Total Environ. 2023, 857, 159639. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, P.; Zhang, J.; Zhang, J.; Zhou, Y.; Peng, Y.; Zhang, S.; Cai, G.; Gao, G. Heavy metal accumulation, risk assessment and integrated biomarker responses of local vegetables: A case study along the Le’an river. Chemosphere 2018, 199, 361–371. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Mukherjee, A.; Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.-U.-R.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, 0246880. [Google Scholar]
- Kumari, M.; Pandey, S.; Mishra, S.K.; Giri, V.P.; Agarwal, L.; Dwivedi, S.; Pandey, A.K.; Nautiyal, C.S.; Mishra, A. Omics-Based Mechanistic Insight Into the Role of Bioengineered Nanoparticles for Biotic Stress Amelioration by Modulating Plant Metabolic Pathways. Front. Bioeng. Biotechnol. 2020, 8, 242. [Google Scholar] [CrossRef]
- Shahbaz, M.; Anwar, T.; Fatima, S.; Onursal, N.; Qureshi, H.; Qureshi, W.A.; Ullah, N.; Soufan, W.; Zaman, W. Mitigation of salinity stress in sunflower plants (Helianthus annuus L.) through topical application of salicylic acid and silver nanoparticles. Physiol. Mol. Biol. Plants 2024, 31, 27–40. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Li, M.; Guo, Z.; Ullah, S.; Rui, Y.; Lynch, I. Alleviation of nitrogen stress in rice (Oryza sativa) by ceria nanoparticles. Environ. Sci. Nano 2020, 7, 2930–2940. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Penuelas, J.; Sardans, J. Human-driven global nutrient imbalances increase risks to health. Eco-Environ. Health 2023, 2, 246–251. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Keswani, C.; Dilnashin, H.; Birla, H.; Roy, P.; Tyagi, R.K.; Singh, D.; Rajput, V.D.; Minkina, T.; Singh, S.P. Global footprints of organochlorine pesticides: A pan-global survey. Environ. Geochem. Health 2021, 44, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, G.-B.; Fawole, O.A.; Verboven, P.; Zhang, X.-R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K. Postharvest precooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Q.; Xiao, J.; You, L.; Zhu, S.; Li, C.; Fu, X. Physicochemical and functional properties of chitosan-based edible film incorporated with Sargassum pallidum polysaccharide nanoparticles. Food Hydrocoll. 2023, 138, 108476. [Google Scholar] [CrossRef]
- Cunningham, F.J.; Goh, N.S.; Demirer, G.S.; Matos, J.L.; Landry, M.P. Nanoparticle-Mediated Delivery towards Advancing Plant Genetic Engineering. Trends Biotechnol. 2018, 36, 882–897. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development Strategies and Prospects of Nano-based Smart Pesticide Formulation. J. Agric. Food Chem. 2017, 66, 6504–6512. [Google Scholar] [CrossRef]
- Ali, I.; Alyona, S.; Tatiana, K.K.; Anastasiya, G.; Albishri, H.M.; Alshitari, W.H. Facile adsorption-electroflotation method for the removal of heavy metal ions from water using carbon nanomaterials. Environ. Sci. Pollut. Res. 2023, 30, 38970–38981. [Google Scholar] [CrossRef]
- Zhai, R.; Chen, G.; Liu, G.; Huang, X.; Xu, X.; Li, L.; Zhang, Y.; Wang, J.; Jin, M.; Xu, D.; et al. Enzyme inhibition methods based on Au nanomaterials for rapid detection of organophosphorus pesticides in agricultural and environmental samples: A review. J. Adv. Res. 2022, 37, 61–74. [Google Scholar] [CrossRef]
- Casonato, C.; García-Herrero, L.; Caldeira, C.; Sala, S. What a waste! Evidence of consumer food waste prevention and its effectiveness. Sustain. Prod. Consum. 2023, 41, 305–319. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P.; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Jat, S.K.; Bhattacharya, J.; Sharma, M.K. Nanomaterial based gene delivery: A promising method for plant genome engineering. J. Mater. Chem. B 2020, 8, 4165–4175. [Google Scholar] [CrossRef]
- Feregrino-Perez, A.A.; Magaña-López, E.; Guzmán, C.; Esquivel, K. A general overview of the benefits and possible negative effects of the nanotechnology in horticulture. Sci. Hortic. 2018, 238, 126–137. [Google Scholar] [CrossRef]
- Nordin, A.H.; Yusoff, A.H.; Husna, S.M.N.; Noor, S.F.M.; Norfarhana, A.S.; Paiman, S.H.; Ilyas, R.A.; Nordin, M.L.; Osman, M.S.; Abdullah, N. Recent advances in nanocellulose-based adsorbent for sustainable removal of pharmaceutical contaminants from water bodies: A review. Int. J. Biol. Macromol. 2024, 280, 135799. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.u.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, Y.; Cha, R.; Yang, J.; Jiang, X. Water-soluble nanocrystalline cellulose films with highly transparent and oxygen barrier properties. Nanoscale 2016, 8, 973–978. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo; Darmayanti, R.; Puspitasari, B.; Cheng, T.-M.; Kuo, T.-R. Gold-Based Nanostructures for Antibacterial Application. Int. J. Mol. Sci. 2023, 24, 10006. [Google Scholar] [CrossRef]
- De León Ramirez, J.I.; Reyes Villegas, V.A.; Cadena-Nava, R.D.; Loredo-Garcia, E.; Chávez-Rivas, F.; González-Torres, V.; Petranovskii, V. Antimicrobial activity of the LTA zeolite modified by zinc species. Microporous Mesoporous Mater. 2024, 380, 113295. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Rani, M.S.; Venkataswamy, P.; Mohan Reddy, B.J.; Vithal, M. Biosynthesis of CMC-Guar gum-Ag0 nanocomposites for inactivation of food pathogenic microbes and its effect on the shelf life of strawberries. Carbohydr. Polym. 2020, 236, 116053. [Google Scholar] [CrossRef]
- Wasilewska, A.; Bielicka, M.; Klekotka, U.; Kalska-Szostko, B. Nanoparticle applications in food—A review. Food Funct. 2023, 14, 2544–2567. [Google Scholar] [CrossRef]
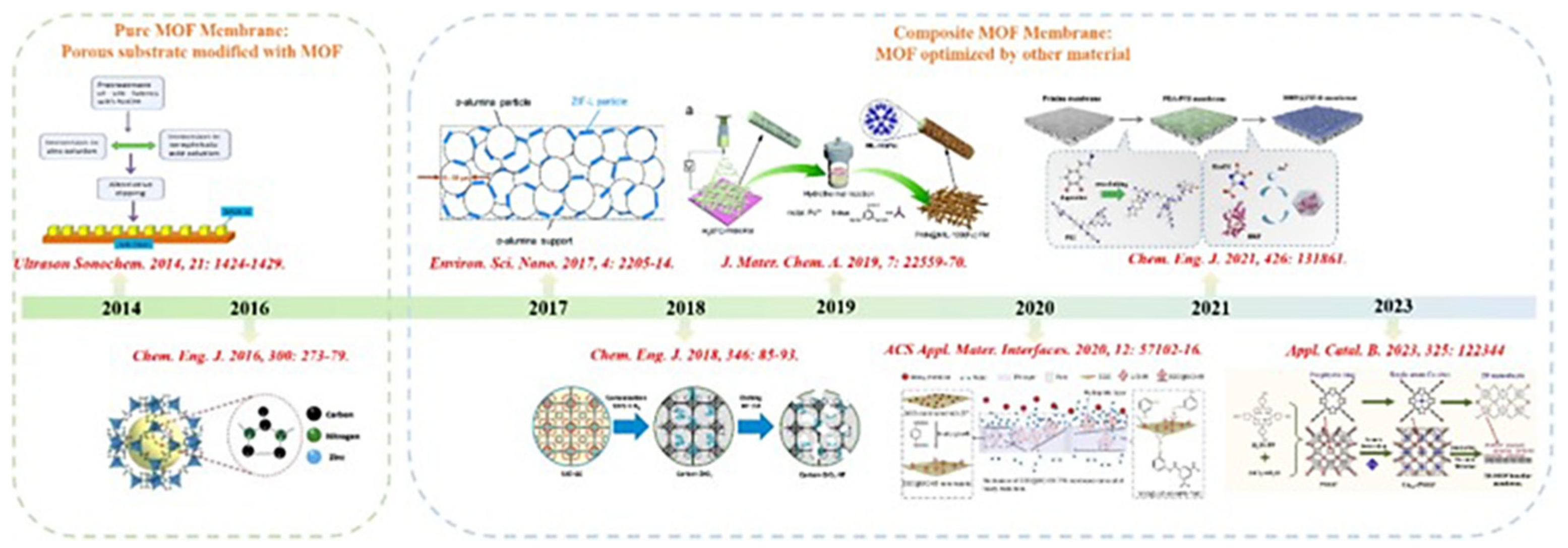
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically Conductive Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Han, J.; Xu, D.; Huang, Y.; Hua, Y.; Ding, X.; Lin, Z.; Zhou, J.; Lin, H.; Chen, G.; Wang, J.; et al. Developing fine-tuned MOF membranes for highly efficient separation and adsorption of chemical pollutant in water. Chem. Eng. J. 2024, 497, 154508. [Google Scholar] [CrossRef]
- Horita, K.; Kameda, T.; Suga, H.; Hirano, A. Molecular mechanism of the interactions between coffee polyphenols and milk proteins. Food Res. Int. 2025, 202, 115573. [Google Scholar] [CrossRef]
- Waqas, M.; Campbell, L.; Piyush, T. Polyaniline-Coated Surface-Modified Ag/PANI Nanostructures for Antibacterial and Colorimetric Melamine Sensing in Milk Samples. ACS Omega 2023, 8, 24010–24015. [Google Scholar] [CrossRef]
- Singha, S.K.; Hoque, S.M.; Das, H.; Alim, M.A. Evaluation of chitosan-Ag/TiO2 nanocomposite for the enhancement of shelf life of chili and banana fruits. Heliyon 2023, 9, e21752. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, L.; Wang, C.; Wang, C.; Yu, K.; Zhou, B. A Facile Grinding Method for the Synthesis of 3D Ag Metal–Organic Frameworks (MOFs) Containing Ag6Mo7O24 for High-Performance Supercapacitors. Chem.—A Eur. J. 2020, 26, 4613–4619. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Q.; Gao, Y.; Wan, S.; Meng, F.; Weng, W.; Zhang, Y. Characterization of silver nanoparticles loaded chitosan/polyvinyl alcohol antibacterial films for food packaging. Food Hydrocoll. 2023, 136, 108305. [Google Scholar] [CrossRef]
- Sionkowska, A.; Walczak, M.; Michalska-Sionkowska, M. Preparation and characterization of collagen/chitosan composites with silver nanoparticles. Polym. Compos. 2019, 41, 951–957. [Google Scholar] [CrossRef]
- Vejpravova, J.; Pacakova, B.; Kalbac, M. Magnetic impurities in single-walled carbon nanotubes and graphene: A review. Analyst 2016, 141, 2639–2656. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Wong, M.H.; Misra, R.P.; Giraldo, J.P.; Kwak, S.-Y.; Son, Y.; Landry, M.P.; Swan, J.W.; Blankschtein, D.; Strano, M.S. Lipid Exchange Envelope Penetration (LEEP) of Nanoparticles for Plant Engineering: A Universal Localization Mechanism. Nano Lett. 2016, 16, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Liu, Y.; Lin, Z.Y.; Liu, B.; Liu, J. Profiling Metal Oxides with Lipids: Magnetic Liposomal Nanoparticles Displaying DNA and Proteins. Angew. Chem. Int. Ed. 2016, 55, 12063–12067. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hao, Y.; Zhao, J.; Zuverza-Mena, N.; Meselhy, A.G.; Dhankher, O.P.; Rui, Y.; White, J.C.; Xing, B. Graphitic Carbon Nitride (C3N4) Reduces Cadmium and Arsenic Phytotoxicity and Accumulation in Rice (Oryza sativa L.). Nanomaterials 2021, 11, 839. [Google Scholar] [CrossRef]
- Qian, Y.; Qin, C.; Chen, M.; Lin, S. Nanotechnology in soil remediation—Applications vs. implications. Ecotoxicol. Environ. Saf. 2020, 201, 110815. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Tishkevich, D.I.; Podgornaya, S.V.; Kaniukov, E.; Darwish, M.A.; Zubar, T.I.; Timofeev, A.V.; Trukhanova, E.L.; Kostishin, V.G.; Trukhanov, S.V. Impact of the Nanocarbon on Magnetic and Electrodynamic Properties of the Ferrite/Polymer Composites. Nanomaterials 2022, 12, 868. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Surface Science of Nanozymes and Defining a Nanozyme Unit. Langmuir 2022, 38, 3617–3622. [Google Scholar] [CrossRef]
- Khairy, M.; Ayoub, H.A.; Banks, C.E. Non-enzymatic electrochemical platform for parathion pesticide sensing based on nanometer-sized nickel oxide modified screen-printed electrodes. Food Chem. 2018, 255, 104–111. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, J.; Wang, S. Nanozymes for Catalytic Cancer Immunotherapy. ACS Appl. Nano Mater. 2020, 3, 4925–4943. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Z.; Gao, Z.; Tammina, S.K.; Yang, Y. Nanozymes used for antimicrobials and their applications. Colloids Surf. B Biointerfaces 2020, 195, 111252. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar]
- Dong, H.; Du, W.; Dong, J.; Che, R.; Kong, F.; Cheng, W.; Ma, M.; Gu, N.; Zhang, Y. Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions. Nat. Commun. 2022, 13, 5365. [Google Scholar] [CrossRef]
- Guan, G.; Yang, L.; Mei, Q.; Zhang, K.; Zhang, Z.; Han, M.-Y. Chemiluminescence Switching on Peroxidase-Like Fe3O4 Nanoparticles for Selective Detection and Simultaneous Determination of Various Pesticides. Anal. Chem. 2012, 84, 9492–9497. [Google Scholar] [CrossRef]
- Guo, B.; Liu, G.; Ye, W.; Xu, Z.; Li, W.; Zhuang, J.; Zhang, X.; Wang, L.; Lei, B.; Hu, C.; et al. Multifunctional carbon dots reinforced gelatin-based coating film for strawberry preservation. Food Hydrocoll. 2024, 147, 109327. [Google Scholar] [CrossRef]
- Ding, J.; Liu, C.; Huang, P.; Li, H.; Liu, Y.; Sameen, D.E.; Zhang, Y.; Liu, Y.; Qin, W. Effects of konjac glucan-nan/low-acyl gellan edible coatings loaded thymol-β-cyclodextrin microcapsules on postharvest blueberry. Food Chem. 2024, 430, 137080. [Google Scholar] [CrossRef]
- Lin, H.; Xu, Y.; Guan, W.; Zhao, S.; Li, X.; Zhang, C.; Blecker, C.; Liu, J. The importance of supercooled stability for food during supercooling preservation: A review of mechanisms, influencing factors, and control methods. Crit. Rev. Food Sci. Nutr. 2023, 64, 12207–12221. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Fawole, O.A. Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review. Biomolecules 2023, 13, 1092. [Google Scholar] [CrossRef]
- Thanh Huong, Q.T.; Hoai Nam, N.T.; Duy, B.T.; An, H.; Hai, N.D.; Kim Ngan, H.T.; Ngan, L.T.; Le Hoai Nhi, T.; Yen Linh, D.T.; Khanh, T.N.; et al. Structurally natural chitosan films decorated with Andrographis paniculata extract and selenium nanoparticles: Properties and strawberry preservation. Food Biosci. 2023, 53, 102647. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Song, Y.; Li, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon dots promote the growth and photosynthesis of mung bean sprouts. Carbon 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Wang, B.; Huang, J.; Zhang, M.; Wang, Y.; Wang, H.; Ma, Y.; Zhao, X.; Wang, X.; Liu, C.; Huang, H.; et al. Carbon Dots Enable Efficient Delivery of Functional DNA in Plants. ACS Appl. Bio Mater. 2020, 3, 8857–8864. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection—Progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [PubMed]
- Zhao, X.; Meng, Z.; Wang, Y.; Chen, W.; Sun, C.; Cui, B.; Cui, J.; Yu, M.; Zeng, Z.; Guo, S.; et al. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants 2017, 3, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Vejlupkova, Z.; Warman, C.; Sharma, R.; Scheller, H.V.; Mortimer, J.C.; Fowler, J.E. No evidence for transient transformation via pollen magnetofection in several monocot species. Nat. Plants 2020, 6, 1323–1324. [Google Scholar] [CrossRef]
- Wasserman, L.A.; Krivandin, A.V.; Filatova, A.G.; Vasil’ev, V.G.; Kolachevskaya, O.O.; Tarasov, V.F.; Plashchina, I.G.; Romanov, G.A. Structural and Thermodynamic Characteristics of Potato Starches Depending on the Plant Genotype and Conditions of Their Cultivation. Russ. J. Phys. Chem. B 2020, 14, 525–532. [Google Scholar]
- Sun, C.; Wei, Z.; Xue, C.; Yang, L. Development, application and future trends of starch-based delivery systems for nutraceuticals: A review. Carbohydr. Polym. 2023, 308, 120675. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.-C.; Tseng, Y.-C.; Mozumdar, S.; Huang, L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control. Release 2010, 142, 416–421. [Google Scholar]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology Strategies for Plant Genetic Engineering. Adv. Mater. 2022, 34, 2106945. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Kundu, R.; Mukherjee, A. Nanocomposites for Delivering Agrochemicals: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 3691–3702. [Google Scholar]
- Abd El-Azeim, M.M.; Sherif, M.A.; Hussien, M.S.; Tantawy, I.A.A.; Bashandy, S.O. Impacts of nano- and non-nanofertilizers on potato quality and productivity. Acta Ecol. Sin. 2020, 40, 388–397. [Google Scholar] [CrossRef]
- Sadati Valojai, S.T.; Niknejad, Y.; Fallah Amoli, H.; Barari Tari, D. Response of rice yield and quality to nano-fertilizers in comparison with conventional fertilizers. J. Plant Nutr. 2021, 44, 1971–1981. [Google Scholar]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2020, 10, 2. [Google Scholar] [CrossRef]
- Toksha, B.; Sonawale, V.A.M.; Vanarase, A.; Bornare, D.; Tonde, S.; Hazra, C.; Kundu, D.; Satdive, A.; Tayde, S.; Chatterjee, A. Nanofertilizers: A review on synthesis and impact of their use on crop yield and environment. Environ. Technol. Innov. 2021, 24, 101986. [Google Scholar]
- Xu, T.; Ma, C.; Aytac, Z.; Hu, X.; Ng, K.W.; White, J.C.; Demokritou, P. Enhancing Agrichemical Delivery and Seedling Development with Biodegradable, Tunable, Biopolymer-Based Nanofiber Seed Coatings. ACS Sustain. Chem. Eng. 2020, 8, 9537–9548. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Sunoj, V.S.J.; Wen, Y.; Zhu, J.J.; Muralidharan, G.; Cao, K.F. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol. Biochem. 2020, 149, 50–60. [Google Scholar]
- Fischer, J.; Beckers, S.J.; Yiamsawas, D.; Thines, E.; Landfester, K.; Wurm, F.R. Targeted Drug Delivery in Plants: Enzyme-Responsive Lignin Nanocarriers for the Curative Treatment of the Worldwide Grapevine Trunk Disease Esca. Adv. Sci. 2019, 6, 1802315. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; An, S.; Yin, X. Nanochitin whisker enhances insecticidal activity of chemical pesticide for pest insect control and toxicity. J. Nanobiotechnol. 2021, 19, 49. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wu, L.; Zhang, L.; Si, Y. Impacts of silver nanoparticles on enzymatic activities, nitrifying bacteria, and nitrogen transformation in soil amended with ammonium and nitrate. Pedosphere 2021, 31, 934–943. [Google Scholar] [CrossRef]
- Kundu, M.; Krishnan, P.; Chobhe, K.A.; Manjaiah, K.M.; Pant, R.P.; Chawla, G. Fabrication of Electrochemical Nanosensor for Detection of Nitrate Content in Soil Extract. J. Soil Sci. Plant Nutr. 2022, 22, 2777–2792. [Google Scholar]
- Wang, X.; He, L.; Xu, L.; Liu, Z.; Xiong, Y.; Zhou, W.; Yao, H.; Wen, Y.; Geng, X.; Wu, R. Intelligent analysis of carbendazim in agricultural products based on a ZSHPC/MWCNT/SPE portable nanosensor combined with machine learning methods. Anal. Methods 2023, 15, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Martinazzo, J.; Moraes, M.C.B.; Steffens, J.; Steffens, C. Application of gas nanosensor for detection pheromone and its interferents compounds in vivo Euschistus heros (F.) stink bugs insects. Sens. Actuators A Phys. 2022, 345, 113804. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, B.; Miao, J.; Yu, Y.; Fu, J.; Jia, B.; Li, L. Superparamagnetic Fe3O4@Al-based metal-organic framework nanocomposites with high-performance removal of Congo red. J. Environ. Chem. Eng. 2023, 11, 109754. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Asadi, E. Synthesis of NENU metal-organic framework-graphene oxide nanocomposites and their pollutant removal ability from water using ultrasound. J. Clean. Prod. 2019, 211, 198–212. [Google Scholar] [CrossRef]
- Mehmeti, V.; Halili, J.; Berisha, A. Which is better for Lindane pesticide adsorption, graphene or graphene oxide? An experimental and DFT study. J. Mol. Liq. 2022, 347, 118345. [Google Scholar] [CrossRef]
- Naseem, Z.; Naveed, M.; Nawaz, S.; Saeed, R.A.; Siddiqui, M.H.; Mustafa, A.; Núñez-Delgado, A. Addressing dye phytotoxicity and soil contamination: The dual action of chemically synthesized zinc nanoparticles in simultaneous adsorption and soil health improvement. J. Environ. Manag. 2025, 375, 124358. [Google Scholar] [CrossRef]
- Janani, R.; Gurunathan, B.; Sivakumar, K.; Varjani, S.; Ngo, H.H.; Gnansounou, E. Advancements in heavy metals removal from effluents employing nano-adsorbents: Way towards cleaner production. Environ. Res. 2022, 203, 111815. [Google Scholar]
- Tian, F.; Zhou, J.; Jiao, B.; He, Y. A nanozyme-based cascade colorimetric aptasensor for amplified detection of ochratoxin A. Nanoscale 2019, 11, 9547–9555. [Google Scholar] [CrossRef]
- Mohammed Ameen, S.S.; Alshatteri, A.H.; Latif, D.A.; Mohammad, Y.O.; Omer, K.M. Nanomineralzyme as a novel sustainable class of nanozyme: Chalcopyrite-based nanozyme for the visual detection of total antioxidant capacity in citrus fruit. Food Chem. 2025, 471, 142769. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Feng, S.; Xie, Y. Colorimetric zearalenone assay based on the use of an aptamer and of gold nanoparticles with peroxidase-like activity. Microchim. Acta 2018, 185, 535. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, L.; Wang, S.; Zhang, Y.; Li, Y. Sensitive colorimetric detection of K(I) using catalytically active gold nanoparticles triggered signal amplification. Biosens. Bioelectron. 2016, 79, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Rafflisman Zaidi, N.S.; Mah, S.-K. Poly(vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal oxide nanoparticles for safe active and intelligent food packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Duffy, B.; Pathania, S.; Jaiswal, A.K.; Jaiswal, S. An active biodegradable layer-by-layer film based on chitosan-alginate-TiO2 for the enhanced shelf life of tomatoes. Food Packag. Shelf Life 2022, 34, 100971. [Google Scholar] [CrossRef]
- Kang, S.; Xiao, Y.; Guo, X.; Huang, A.; Xu, H. Development of gum arabic-based nanocomposite films reinforced with cellulose nanocrystals for strawberry preservation. Food Chem. 2021, 350, 129199. [Google Scholar] [CrossRef]
- Efthymiou, M.-N.; Tsouko, E.; Papagiannopoulos, A.; Athanasoulia, I.-G.; Georgiadou, M.; Pispas, S.; Briassoulis, D.; Tsironi, T.; Koutinas, A. Development of biodegradable films using sunflower protein isolates and bacterial nanocellulose as innovative food packaging materials for fresh fruit preservation. Sci. Rep. 2022, 12, 6935. [Google Scholar] [CrossRef]
- Ding, X.; Lin, H.; Zhou, J.; Lin, Z.; Huang, Y.; Chen, G.; Zhang, Y.; Lv, J.; Chen, J.; Liu, G.; et al. Silver Nanocomposites with Enhanced Shelf-Life for Fruit and Vegetable Preservation: Mechanisms, Advances, and Prospects. Nanomaterials 2024, 14, 1244. [Google Scholar] [CrossRef]

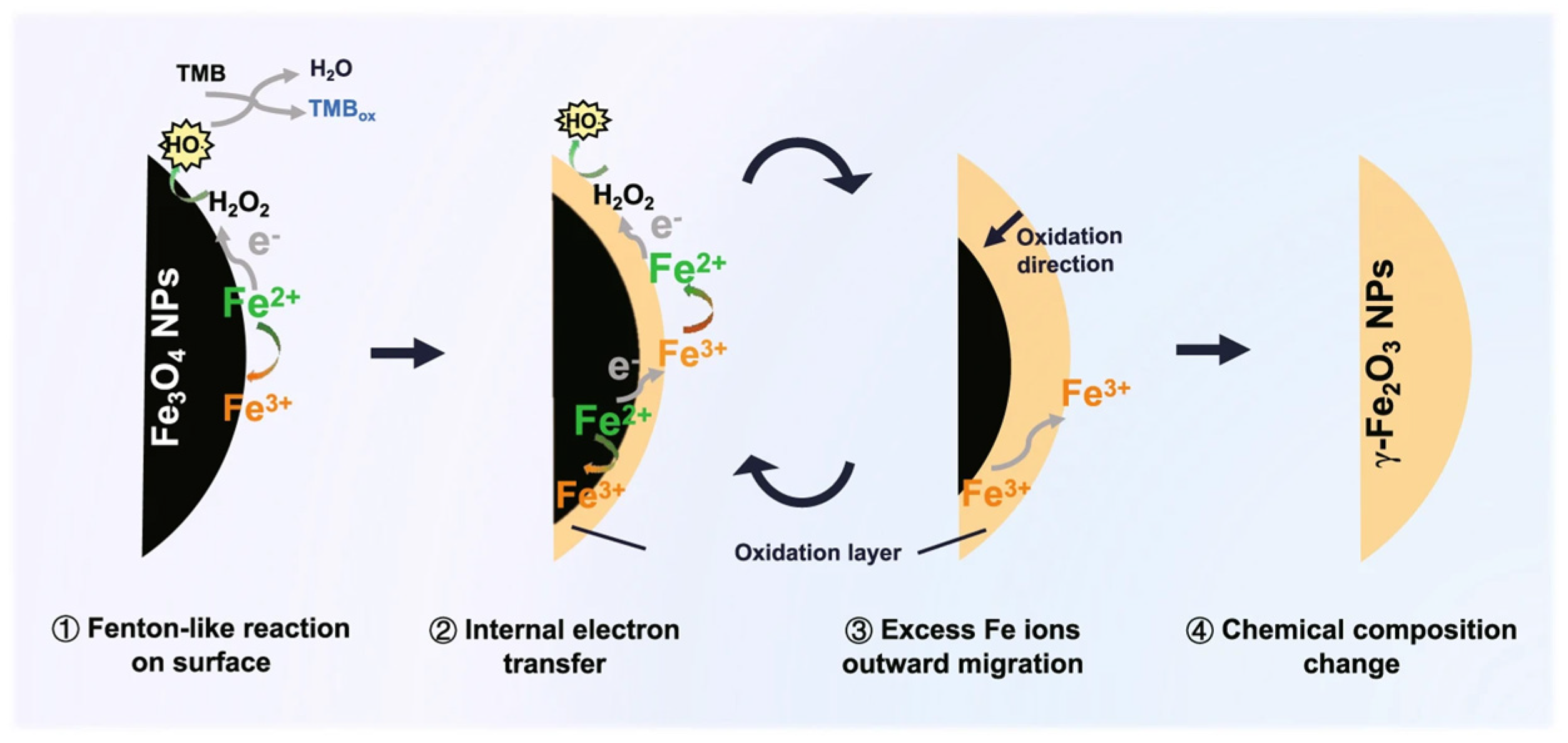
| Type | Conc. | Crop | Effect |
|---|---|---|---|
| Fe | 50, 500, 2000 mg/L | Cucumber | Dose-dependent effects on biomass and antioxidant enzymes |
| Fe | 10, 20 mg/L | Lettuce | Reduced growth and chlorophyll contents, and increased antioxidant enzyme activities |
| Fe | 30–60 ppm | Garden pea | Improved seed mass and chlorophyll content |
| Cu | 0, 100, 500 mg/L | Squash | Higher ionic Cu found in media amended with bulk Cu than with nCu |
| Cu | 130, 660 mg/Kg | Lettuce | Increased shoot/root length ratio |
| Cu | 0, 10, 20 mg/L | Lettuce | Negative effects on nutrient content, dry biomass, water content and seedling growth |
| Cu | 0–1000 mg/L | Cucumber | Reduced growth and increased antioxidant enzymes |
| Cu | 10–1000 mg/L | Radish, grasses | DNA damage, growth inhibition |
| Cu | 50–500 mg/L | Tomato | Improved fruit firmness and antioxidant content |
| Cu | 0, 20, 80 mg/Kg | Cilantro | Reduced germination and shoot elongation |
| Cu | 100, 250, 500 ppm | Bean | Growth inhibition and nutrition imbalance |
| Cu | 100–500 mg/L | Garden pea | Reduced plant growth and enhanced ROS production and lipid peroxidation |
| Zn | 1000 mg/Kg | Cucumber | Root tip deformation and growth inhibition |
| Zn | 500 mg/Kg | Garden pea | Decreased chlorophyll and H2O2 contents |
| Zn | 1000 mg/L | Spinach | Growth reduction |
| Zn | 1 mg/mL | Tomato, eggplant | Reduced fungal disease |
| Zn | 100, 200, 500 ppm | Chili pepper | Improved germination |
| Zn | 0–400 mg/Kg | Coriander | Improved pigment contents and defense responses |
| Zn | 5, 10, 20 mg/L | Onion | Inhibition of root growth |
| Application Method | Type | Concentration | Plant Species | Type of Stress | Effects |
|---|---|---|---|---|---|
| Pre-sowing | MWCNT | 10, 20, 40, 60 mg/L | Cabbage | Salinity | Increased growth, water uptake and net assimilation of CO2. Induced alterations in lipid rigidity, composition and permeability in the root plasma membranes |
| Pre-sowing | SiO2 | 0, 1.5, 3.0, 4.5, 6.0, 7.5 g/L | Pumpkin | Salinity | Enhanced seed germination, growth, photosynthetic parameters and antioxidant enzyme activity. Reduced MDA, H2O2, chlorophyll degradation and oxidative damage |
| Pre-sowing | Nano-silicon | 10 mg/L | Hollyhock | Salinity | Increased germination, plant height, relative water content, fresh and dry weights, relative growth rate, total soluble sugars and membrane stability |
| Pre-sowing | Ag | 0, 40, 80, 120 mg/L | Saffron | Flooding | Increased root growth, dry leaf weight and root length |
| Pre-sowing | Nano-selenium | 1, 4, 8, 12 μM | Ajwain | High and low temperature | Improved plant growth, chlorophyll and leaf relative water content |
| Post-sowing | TiO2 | 0.05, 0.1, 0.2 g/L | Tomato | Heat stress | Enhanced photosynthesis by regulating energy dissipation and caused cooling of leaves through inducing stomatal opening |
| Pre-sowing | CuO, Al2O3, TiO2 | 0, 20, 200, 2000 μg/mL | Onion | Oxidative stress | Induced chromosomal aberration effect SOD and POD activities as well |
| Pre-sowing | SiO2 | 0.05, 0.5, 1.5, 2, 2.5 mg/L | Tomato | Salinity | Up-regulated the expression profile of salt stress genes (AREB, TAS14, NCED3 and CRK1). |
| Pre-transplanting | Ag | 10, 20, 40 mg/L | Tomato | Salinity | Negatively affected the plant height, number of branches and fruit traits (diameter, weight and number of fruits). |
| Post-transplanting | Si | 1, 2, 4, 5 cm3/L | Chili pepper | Salinity | Significantly regulated the plant to endure salt stress. |
| Post-transplanting | Nano-Ca | 0.5, 1, 2, 3 g/L | Tomato | Salinity | Lower level significantly reduced the negative effects of salinity |
| Post-transplanting | Monopotassium phosphate, nano-calcium | 0.5, 0.75, 1 g/L | Tomato | Salinity | Medium concentration of NMs improved stem diameter and number of flowers. |
| Post-transplanting | Nano-silicon | 0, 1, 2 mM | Tomato | Salinity | Improve fresh weight, chlorophyll concentration, photosynthetic rate and leaf water content |
| Foliar application | Nano-silicon | 1, 2 mM | Peregrina | Salinity | Enhanced vegetative parameters and chemical composition, decreased accumulation of Na, Cl and total phenolics and flavonoids in leaves |
| Foliar application | Ag | 0.4, 40 mg/plant | Cucumber | Oxidative stress (nanotoxicity) | Enhanced respiration, inhibited photorespiration and reduced inorganic nitrogen fixation. |
| Nanoadsorbent | Target Heavy Metal | pH | Temp °C | Time min | Dose | Adsorption Capacity mg/g |
|---|---|---|---|---|---|---|
| DMSA@Fe3O4 MNRs | Pb2+ | 5 | 28 | 60 | 0.1 g/L | 46.18 |
| Activated carbon prepared from apple peels (ACAP) | Cr (VI) | 2 | 28 | 4 h | 0.05 g/50 mL | 36.01 |
| Titanium dioxide nanoparticles with nano-zero-valent iron (nZVI) | Nitrate | 4.185 | – | 150.09 | 10 | 0.982 g/L |
| Magnetic activated carbon (MAC) | Pb2+ and Cd2+ | 6 and 5 | – | 30 | 0.2 g/L | 49.8, 86.2 |
| Polypyrrole–iron oxide–seaweed nanocomposite | Pb2+ | 5 | 40 | 20 | 0.5 mg/g | 97.25 |
| Magnetic-activated carbon nanocomposite (mFe3O4@ACCs) | Pb (II) | 11 | – | 15 | 40 mg | 239 |
| Activated carbon/nanoclay/thiolated graphene oxide nanocomposite (AC/NC/TGO) | Pb (II) | 5 | – | 40 | 0.5 g/L | 208 |
| Magnetite-polyrhodanine core–shell nanoparticles | Hg2+ | 6.5 | 25 | 5 h | 10 g/L | 29.14 |
| Chitosan–iron oxide (CS–Fe2O3) nanocomposite | Pb2+, Cd2+ | 6 | 50 | 180 | 0.01 g | 214.923, 204.318 |
| Nickel ferrite/titanium oxide magnetic nanocomposite(FeNi3/TiO2 nanocomposite) | Cr6+ | 3 | – | 15 | 500 mg/L | 0.998 |
| Thiol-lignocellulose sodium bentonite (TLSB) nanocomposites | Zn2+, Cd2+, Hg2+ | 4.84 | 40 | 40 | 0.05 | 357.29, 458.32, 208.12 |
| Chitosan/multiwalled carbon nanotube (MWCNT)/PVA membrane composites | Pb2+ | 7 | RT | 300 | 0.5 | 35.148 |
| Polyacrylamide/Sodium Montmorillonite (PAM/Na-MMT) Nanocomposites | Co2+ and Ni2+ | 6 | – | – | 0.1 | 98.67, 99.30 |
| Magnetic Mesoporous Calcium Carbonate-Based Nanocomposite | Pb2+, Cd2+ | 5.5 | – | 12 h | 0.2 g/L | 1179.821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Lin, Z.; Sheng, B.; Ye, X.; Zhao, Y.; Liu, G.; Chen, G.; Qin, L.; Liu, X.; Xu, D. Research Status of Agricultural Nanotechnology and Its Application in Horticultural Crops. Nanomaterials 2025, 15, 765. https://doi.org/10.3390/nano15100765
Wen X, Lin Z, Sheng B, Ye X, Zhao Y, Liu G, Chen G, Qin L, Liu X, Xu D. Research Status of Agricultural Nanotechnology and Its Application in Horticultural Crops. Nanomaterials. 2025; 15(10):765. https://doi.org/10.3390/nano15100765
Chicago/Turabian StyleWen, Xiaobin, Zhihao Lin, Bin Sheng, Xueling Ye, Yiming Zhao, Guangyang Liu, Ge Chen, Lin Qin, Xinyan Liu, and Donghui Xu. 2025. "Research Status of Agricultural Nanotechnology and Its Application in Horticultural Crops" Nanomaterials 15, no. 10: 765. https://doi.org/10.3390/nano15100765
APA StyleWen, X., Lin, Z., Sheng, B., Ye, X., Zhao, Y., Liu, G., Chen, G., Qin, L., Liu, X., & Xu, D. (2025). Research Status of Agricultural Nanotechnology and Its Application in Horticultural Crops. Nanomaterials, 15(10), 765. https://doi.org/10.3390/nano15100765










