Toward In Vivo Cancer Detection: X-Ray Scattering on Thick Phantom Samples
Abstract
1. Introduction
2. Results
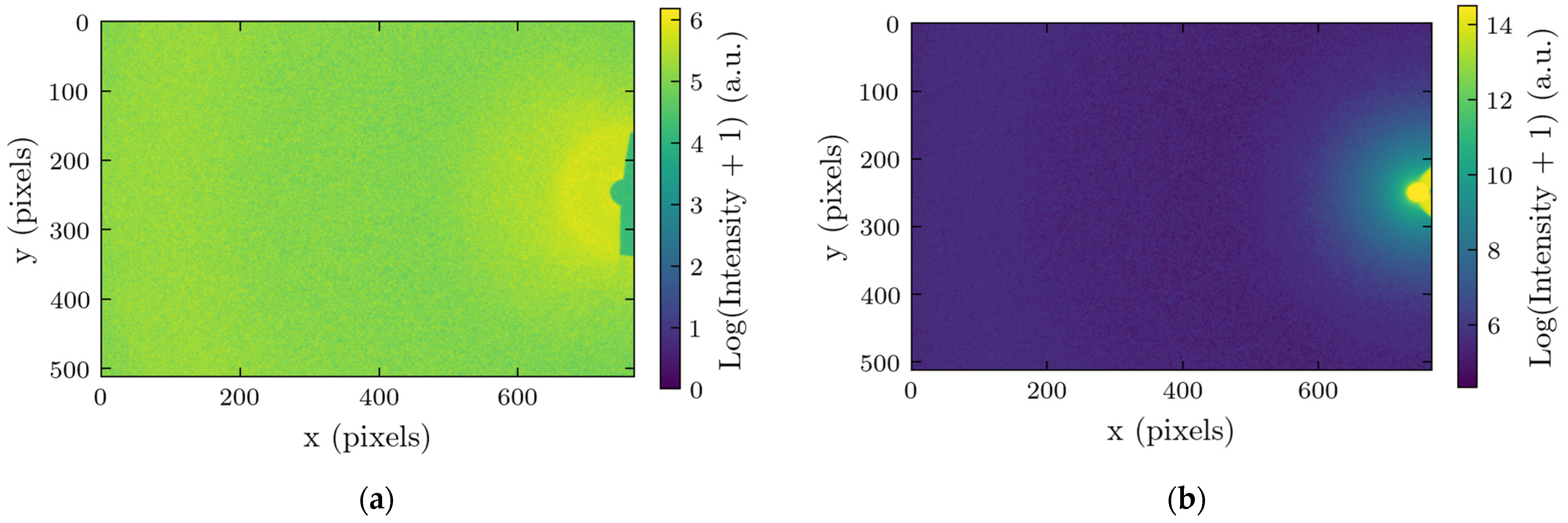
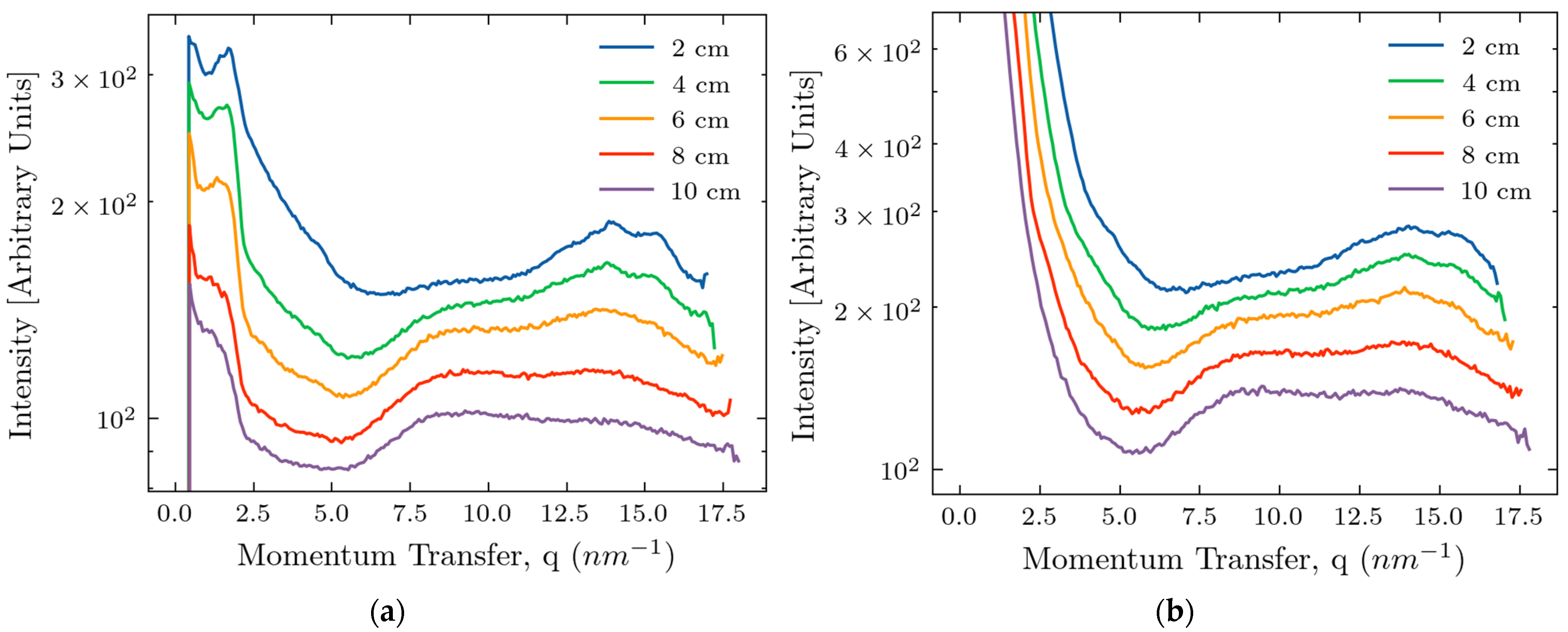
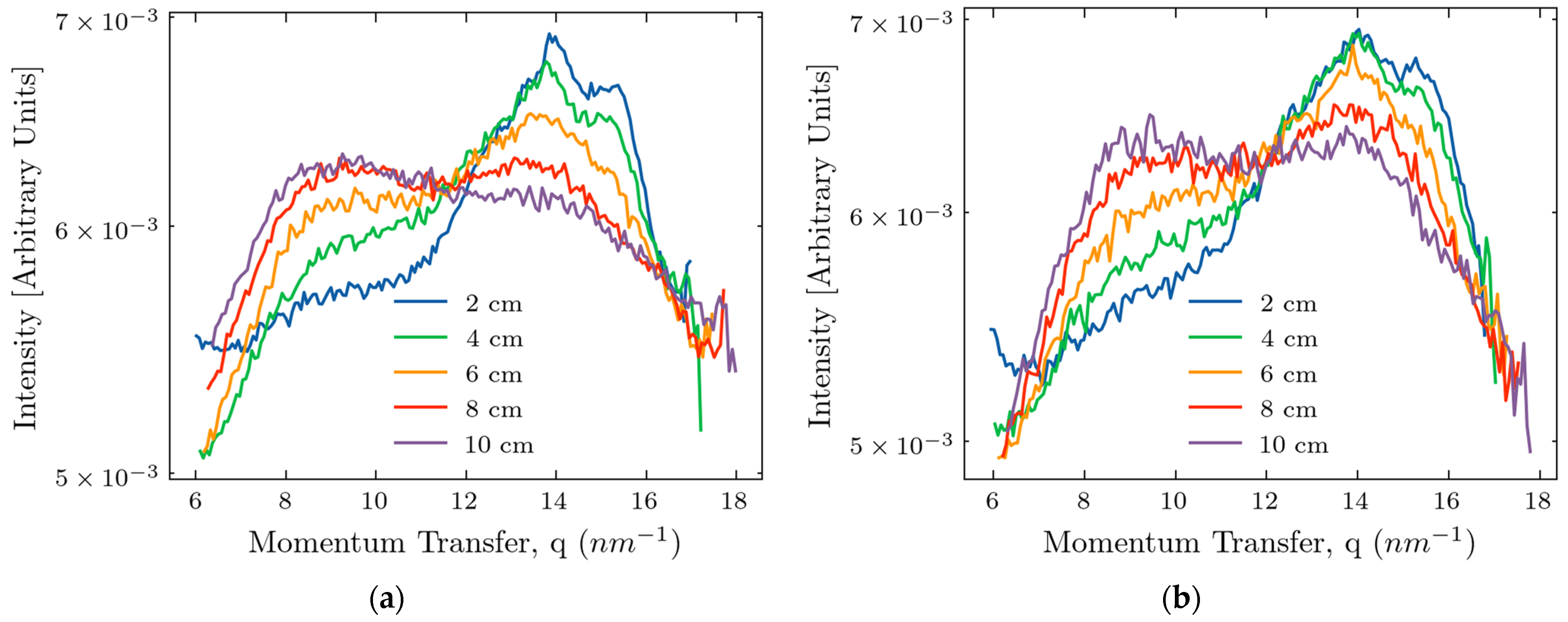
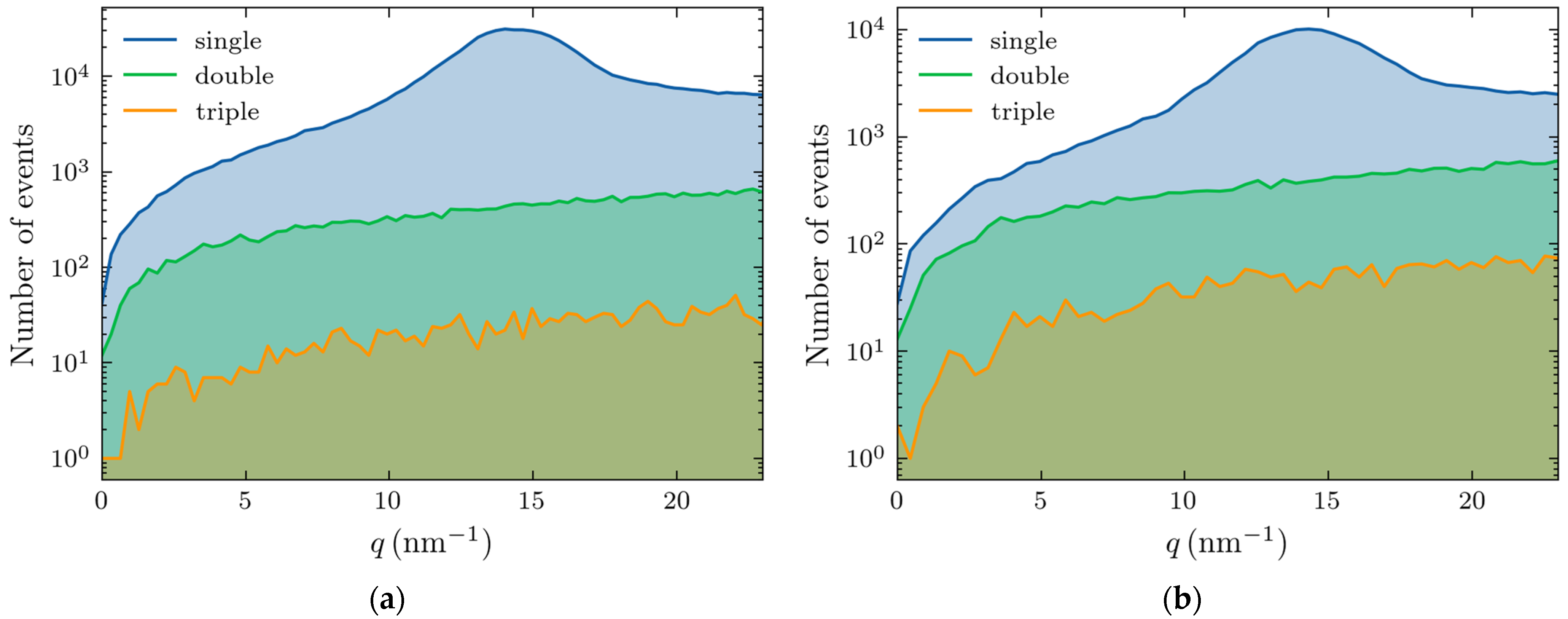
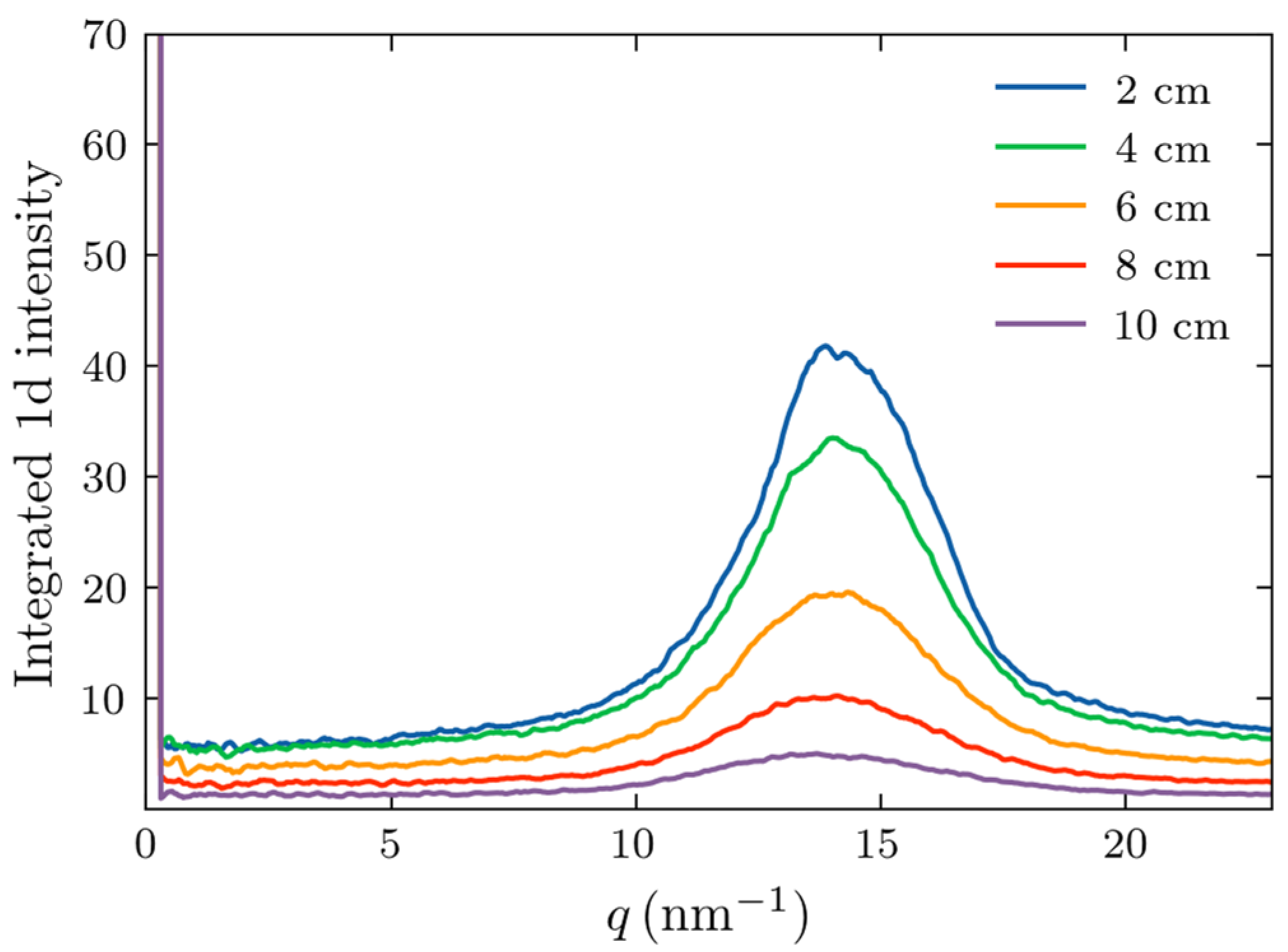
2.1. Measurements of X-Ray Scattering
2.2. Monte Carlo Simulations
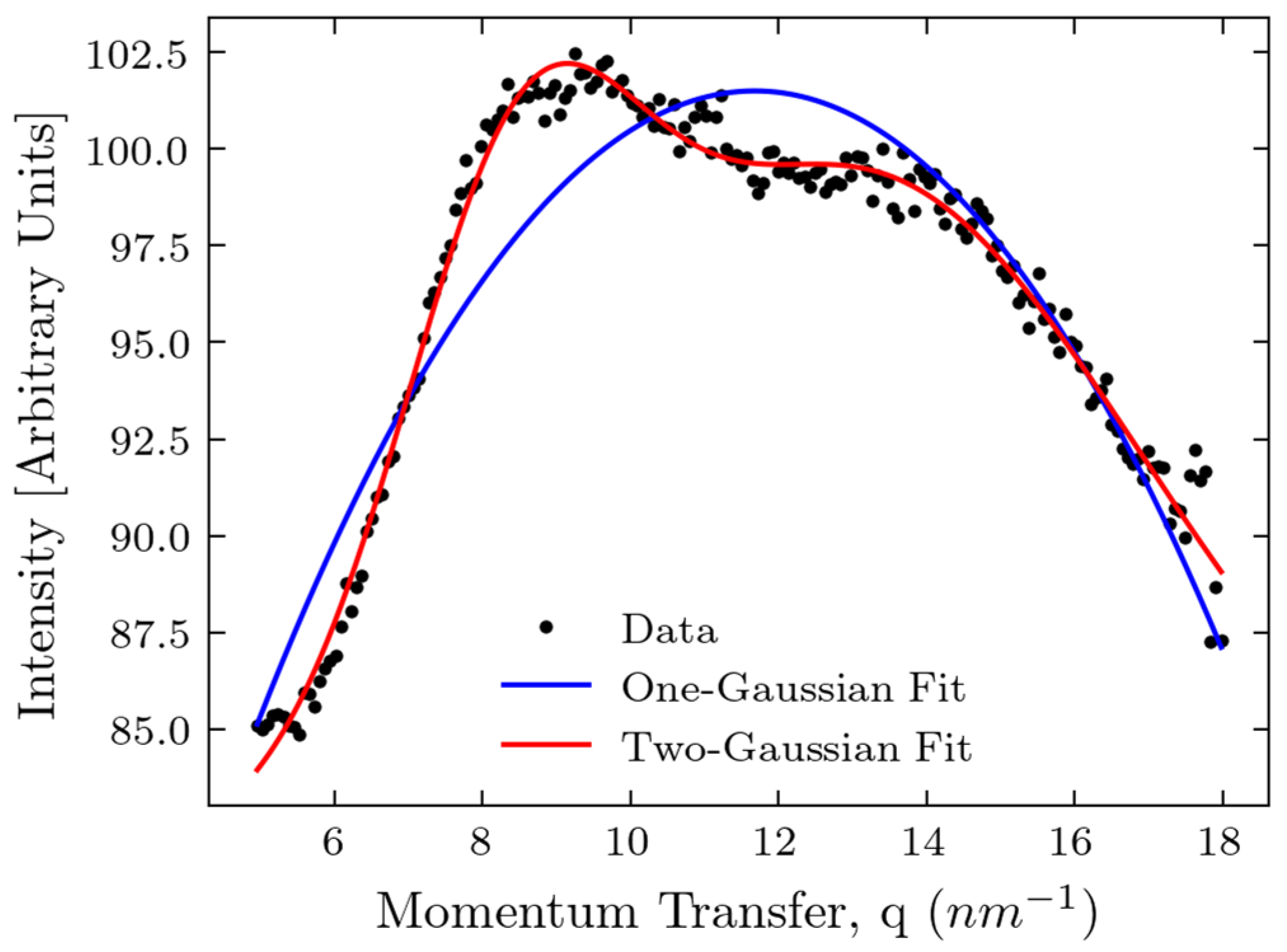
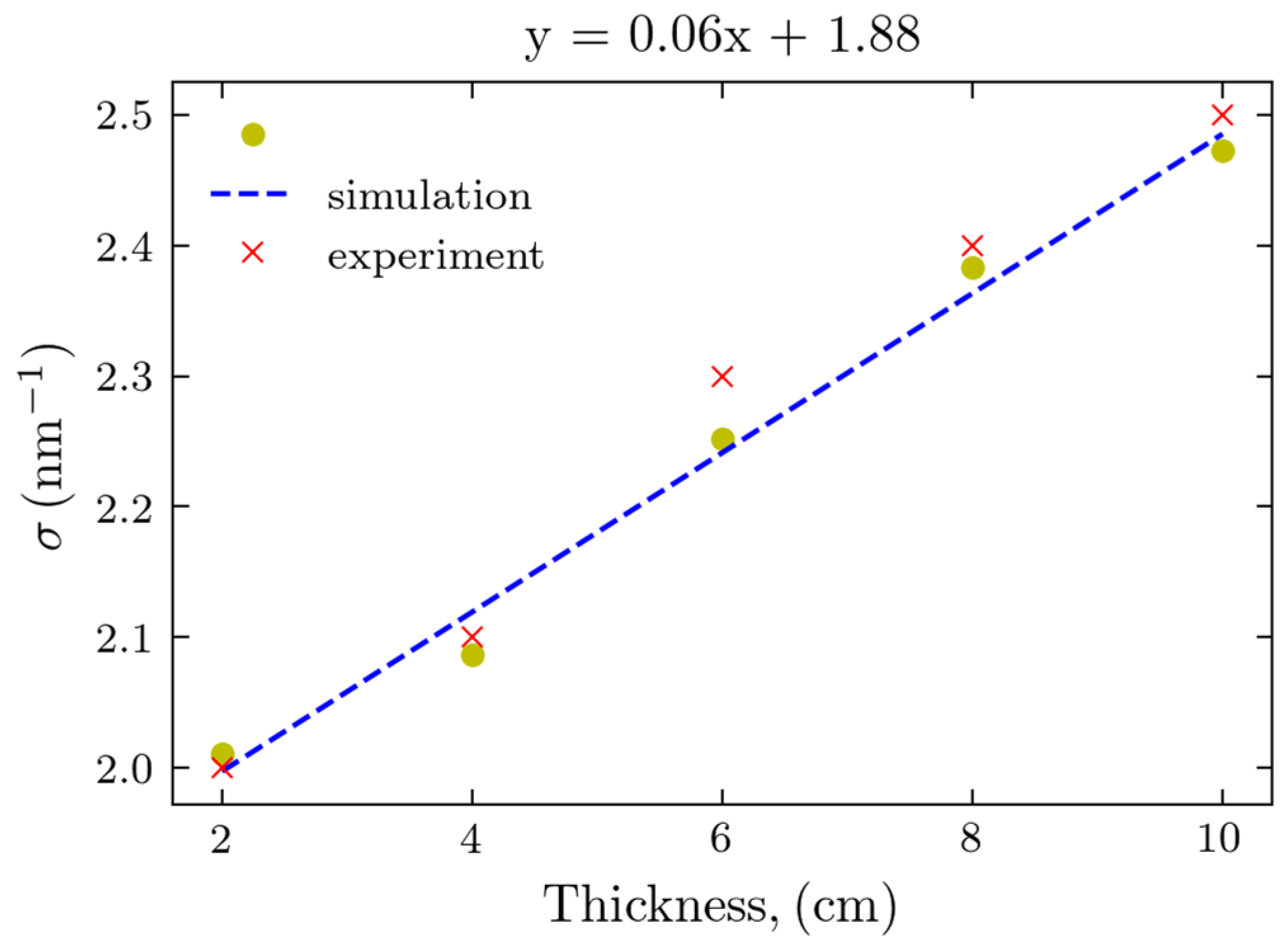
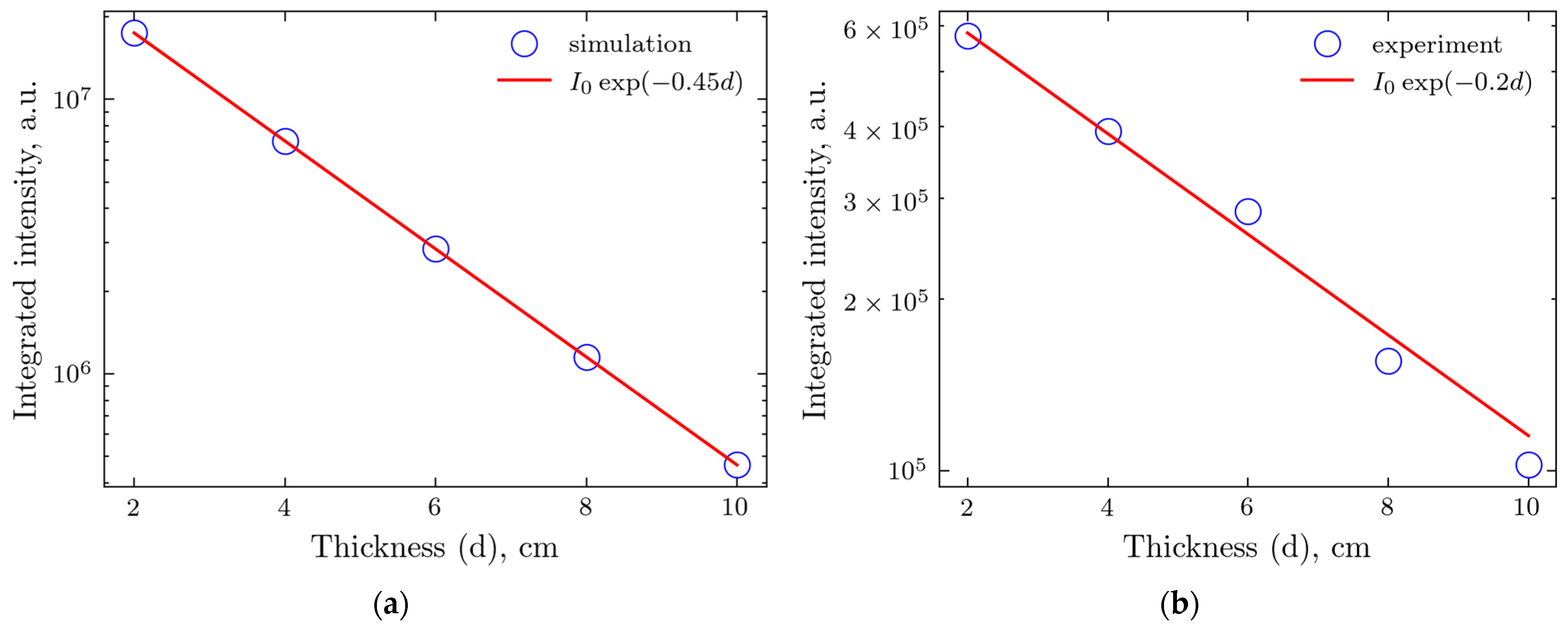
2.3. Comparison of Experiments and Simulations
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. XRD Measurements
4.3. Image Processing
4.4. Data Processing
4.5. Monte Carlo Simulations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society, Breast Cancer Facts & Figures 2024–2025; American Cancer Society: Atlanta, GA, USA, 2024.
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Migowski, A. Early detection of breast cancer and the interpretation of results of survival studies. Cienc. Saude Coletiva 2015, 20, 1309. [Google Scholar]
- Taylor, C.; McGale, P.; Probert, J.; Broggio, J.; Charman, J.; Darby, S.C.; Kerr, A.J.; Whelan, T.; Cutter, D.J.; Mannu, G.; et al. Breast cancer mortality in 500 000 women with early invasive breast cancer diagnosed in England, 1993–2015: Population based observational cohort study. BMJ 2023, 381, e074684. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef]
- Majid, A.S.; de Paredes, E.S.; Doherty, R.D.; Sharma, N.R.; Salvador, X. Missed breast carcinoma: Pitfalls and pearls. Radiographics 2003, 23, 881–895. [Google Scholar] [CrossRef]
- Ong, M.S.; Mandl, K.D. National expenditure for false-positive mammograms and breast cancer overdiagnoses estimated at $4 billion a year. Health Aff. 2015, 34, 576–583. [Google Scholar]
- Vlahiotis, A.; Griffin, B.; Stavros, A.T.; Margolis, J. Analysis of utilization patterns and associated costs of the breast imaging and diagnostic procedures after screening mammography. Clin. Outcomes Res. 2018, 10, 157–167. [Google Scholar] [CrossRef]
- Keemers-Gels, M.E.; Groenendijk, R.P.; van den Heuvel, J.H.; Boetes, C.; Peer, P.G.; Wobbes, T.H. Pain experienced by women attending breast cancer screening. Breast Cancer Res. Treat. 2000, 60, 235–240. [Google Scholar] [CrossRef]
- Kirchner, J.T. Accuracy of Fine Needle Aspiration Breast Biopsy. Am. Fam. Physician 1998, 57, 2835–2836. [Google Scholar]
- Boca Bene, I.; Ciurea, A.I.; Ciortea, C.A.; Dudea, S.M. Pros and Cons for Automated Breast Ultrasound (ABUS): A Narrative Review. J. Pers. Med. 2021, 11, 703. [Google Scholar] [CrossRef]
- Hulka, B.S. Overview of biological markers. In Biological Markers in Epidemiology; Hulka, B.S., Griffith, J.D., Wilcosky, T.C., Eds.; Oxford University Press: New York, NY, USA, 1990; pp. 3–15. [Google Scholar]
- Naylor, S. Biomarkers: Current perspectives and future prospects. Expert Rev. Mol. Diagn. 2003, 3, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Al Bakir, M.; Hamilton, E.G.; Diehn, M.; André, F.; Roy-Chowdhuri, S.; Mountzios, G.; Wistuba, I.I.; Swanton, C.; Peters, S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell 2024, 187, 1617–1635. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Hassan, M.; Ullah, S.; Abbas, H.; Tawakkal, F.; Khan, M.A. Breast Cancer; Discovery of Novel Diagnostic Biomarkers, Drug Resistance, and Therapeutic Implications. Front. Mol. Biosci. 2022, 9, 783450. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Lewis, R.A.; Rogers, K.D.; Hall, C.J.; Towns-Andrews, E.; Slawson, S.; Evans, A.; Pinder, S.E.; Ellis, I.O.; Boggis, C.R.M.; Hufton, A.P.; et al. Breast cancer diagnosis using scattered X-rays. J. Synchrotron Radiat. 2000, 7, 348–352. [Google Scholar] [CrossRef]
- Fernandez, M.; Keyrilainen, J.; Serimaa, R.; Torkkeli, M.; Karjalainen-Lindsberg, M.-L.; Tenhunen, M.; Thomlinson, W.; Urban, V.; Suortti, P. Small-angle x-ray scattering studies of human breast tissue samples. Phys. Med. Biol. 2002, 47, 577–592. [Google Scholar] [CrossRef]
- Sidhu, S.; Siu, K.K.W.; Falzon, G.; Nazaretian, S.; Hart, S.A.; Fox, J.G.; Susil, B.J.; Lewis, R.A. X-ray scattering for classifying tissue types associated with breast disease. Med. Phys. 2008, 35, 4660–4670. [Google Scholar]
- Conceição, A.L.; Antoniassi, M.; Poletti, M.E. Analysis of breast cancer by small angle X-ray scattering. Analyst 2009, 134, 1077–1082. [Google Scholar] [CrossRef]
- Sidhu, S.; Siu, K.K.W.; Falzon, G.; Hart, S.A.; Fox, J.G.; Lewis, R.A. Mapping structural changes in breast tissue disease using x-ray scattering. Med. Phys. 2009, 36, 3211–3217. [Google Scholar]
- Conceiçao, A.L.C.; Antoniassi, M.; Geraldelli, W.; Poletti, M.E. Mapping transitions between healthy and pathological lesions in human breast tissues by diffraction enhanced imaging computed tomography (DEI-CT) and small angle x-ray scattering (SAXS). Radiat. Phys. Chem. 2014, 95, 313–316. [Google Scholar]
- Kidane, G.; Speller, R.D.; Royle, G.J.; Hanby, A.M. X-ray scatter signatures for normal and neoplastic breast tissues. Phys. Med. Biol. 1999, 44, 1791. [Google Scholar]
- Poletti, M.E.; Gonçalves, O.D.; Mazzaro, I. X-ray scattering from human breast tissues and breast-equivalent materials. Phys. Med. Biol. 2002, 47, 47–63. [Google Scholar] [CrossRef]
- Oliveira, O.R.; Conceiçao, A.L.C.; Cunha, D.M.; Poletti, M.E.; Pela, C.A. Identification of neoplasias of breast tissues using a powder diffractometer. J. Radiat. Res. 2008, 49, 527–532. [Google Scholar]
- Conceicao, A.L.C.; Antoniassi, M.; Poletti, M.E. Assessment of the differential linear coherent scattering coefficient of biological samples. Nucl. Instrum. Methods Phys. Res. Sect. A 2010, 619, 67–70. [Google Scholar]
- Griffiths, J.A.; Royle, G.J.; Hanby, A.M.; Horrocks, J.A.; Bohndiek, S.E.; Speller, R.D. Correlation of energy dispersive diffraction signatures and microCT of small breast tissue samples with pathological analysis. Phys. Med. Biol. 2007, 52, 6151–6164. [Google Scholar] [CrossRef]
- Cunha, D.M.; Oliveira, O.R.; Pérez, C.A.; Poletti, M.E. X-ray scattering profiles of some normal and malignant human breast tissues. X-Ray Spectrom. 2006, 35, 370–374. [Google Scholar] [CrossRef]
- Conceicao, A.L.C.; Antoniassi, M.; Cunha, D.M.; Ribeiro-Silva, A.; Poletti, M.E. Multivariate analysis of the scattering profiles of healthy and pathological human breast tissues. Nucl. Instrum. Methods Phys. Res. Sect. A 2011, 652, 870–873. [Google Scholar]
- Conceicao, A.L.C.; Meehan, K.; Antoniassi, M.; Piacenti-Silva, M.; Poletti, M.E. The influence of hydration on the architectural rearrangement of normal and neoplastic human breast tissues. Heliyon 2019, 5, e01219. [Google Scholar]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-R.; Wang, J.; Wang, Z.-J.; Xi, M.-J.; Xia, B.-H.; Deng, K.; Yang, J.-L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Zhang, Q.; Celik, C.E.; Reich, E.M.; Yilmaz, O.H. Dietary fat and lipid metabolism in the tumor microenvironment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188984. [Google Scholar]
- Friedman, J.; Blinchevsky, B.; Slight, M.; Tanaka, A.; Lazarev, A.; Zhang, W.; Aram, B.; Ghadimi, M.; Lomis, T.; Mourokh, L.; et al. Structural Biomarkers for Breast Cancer Determined by X-Ray Diffraction. In Quantum Effects and Measurement Techniques in Biology and Biophotonics; Aiello, C., Polyakov, S.V., Derr, P., Eds.; SPIE: Bellingham, WA, USA, 2024; Volume 12863, p. 1286302. [Google Scholar]
- Denisov, S.; Blinchevsky, B.; Friedman, J.; Gerbelli, B.; Ajeer, A.; Adams, L.; Greenwood, C.; Rogers, K.; Mourokh, L.; Lazarev, P. Vitacrystallography: Structural Biomarkers of Breast Cancer Obtained by X-ray Scattering. Cancers 2024, 16, 2499. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Polymorphic phase transitions in triglycerides and their mixtures studied by SAXS/WAXS techniques: In bulk and in emulsions. Adv. Colloid Interface Sci. 2024, 323, 103071. [Google Scholar] [CrossRef]
- Penagos, I.A.; De Witte, F.; Rimaux, T.; Chèvremont, W.; Pintelon, I.; Dewettinck, K.; Van Bockstaele, F. Multiscale analysis of triglycerides using X-ray scattering: Implementing a shape-dependent model for CNP characterization. Soft Matter 2024, 20, 5071–5085. [Google Scholar]
- Paternò, G.; Cardarelli, P.; Contillo, A.; Gambaccini, M.; Taibi, A. Geant4 implementation of inter-atomic interference effect in small-angle coherent X-ray scattering for materials of medical interest. Phys. Med. 2018, 51, 64–70. [Google Scholar]
- Oshina, I.; Spigulis, J. Beer-Lambert law for optical tissue diagnostics: Current state of the art and the main limitations. J. Biomed. Opt. 2021, 26, 100901. [Google Scholar]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A 2003, 506, 250–303. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubytskyi, V.; Khonkhodzhaev, M.; Tanaka, A.; Nguyen, A.; Lazarev, A.; Aram, B.; Rogers, K.; Mourokh, L.; Lazarev, P. Toward In Vivo Cancer Detection: X-Ray Scattering on Thick Phantom Samples. Molecules 2025, 30, 1655. https://doi.org/10.3390/molecules30081655
Kubytskyi V, Khonkhodzhaev M, Tanaka A, Nguyen A, Lazarev A, Aram B, Rogers K, Mourokh L, Lazarev P. Toward In Vivo Cancer Detection: X-Ray Scattering on Thick Phantom Samples. Molecules. 2025; 30(8):1655. https://doi.org/10.3390/molecules30081655
Chicago/Turabian StyleKubytskyi, Viacheslav, Masroor Khonkhodzhaev, Aika Tanaka, Audrey Nguyen, Alexander Lazarev, Byron Aram, Keith Rogers, Lev Mourokh, and Pavel Lazarev. 2025. "Toward In Vivo Cancer Detection: X-Ray Scattering on Thick Phantom Samples" Molecules 30, no. 8: 1655. https://doi.org/10.3390/molecules30081655
APA StyleKubytskyi, V., Khonkhodzhaev, M., Tanaka, A., Nguyen, A., Lazarev, A., Aram, B., Rogers, K., Mourokh, L., & Lazarev, P. (2025). Toward In Vivo Cancer Detection: X-Ray Scattering on Thick Phantom Samples. Molecules, 30(8), 1655. https://doi.org/10.3390/molecules30081655





