Recovery of Chitin from Agaricus bisporus Mushrooms: Influence of Extraction Parameters and Supercritical CO2 Treatment on Fresh Mushrooms and Production Residues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mushroom Characterisation
2.2. Influence of Deproteinisation Operating Conditions
2.2.1. Deproteinisation Time
2.2.2. Number of Successive Deproteinisation Steps
2.2.3. Sodium Hydroxide Concentration
2.3. Influence of Different Agaricus bisporus Samples
2.4. Chitin Characterisation
3. Materials and Methods
3.1. Raw Material
3.2. Standards and Reagents
3.3. Chitin Extraction
3.4. Characterisation Techniques
3.4.1. Laser Diffraction Analysis
3.4.2. Scanning Electron Microscopy
3.4.3. Fourier-Transform Infrared Spectroscopy
3.4.4. Thermogravimetry Analysis
3.4.5. X-Ray Powder Diffractometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIM | alkali-insoluble matter |
| ANOVA | analysis of variance |
| ATR | attenuated total reflectance |
| ChGC | chitin–glucan |
| CI | crystalline index |
| DA | degree of acetylation |
| DD | degree of deacetylation |
| DTG | derivative thermogravimetric |
| DVP | differential volume percentage |
| EFSA | European Food Safety Authority |
| ETD | Everhart–Thornley detector |
| EU | European Union |
| FDA | Food and Drug Administration |
| FTIR | Fourier-transform infrared spectroscopy |
| FWHM | full width at half maximum |
| HDS | honest significant difference |
| IPB | Instituto Politécnico de Bragança |
| MW | molecular weight |
| PD | polydispersity |
| scCO₂ | supercritical carbon dioxide |
| SE | secondary electrons |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| WD | working distance |
| XRD | X-ray powder diffractometry |
| ZnSe | zinc selenide |
References
- Kasongo, K.J.; Tubadi, D.J.; Bampole, L.D.; Kaniki, T.A.; Kanda, N.J.M.; Lukumu, M.E. Extraction and characterization of chitin and chitosan from Termitomyces titanicus. SN Appl. Sci. 2020, 2, 406. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Biopolymer-Based Materials from Polysaccharides: Properties, Processing, Characterization and Sorption Applications. In Advanced Sorption Process Applications; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef]
- Lv, S.H. High-performance superplasticizer based on chitosan. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 131–150. [Google Scholar] [CrossRef]
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. Evaluation of Biomass and Chitin Production of Morchella Mushrooms Grown on Starch-Based Substrates. Foods 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Kaya, M.; Akata, I. Chitin extraction and chitosan production from cell wall of two mushroom species (Lactarius vellereus and Phyllophora ribis). AIP Conf. Proc. 2017, 1809, 020012. [Google Scholar]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64–86. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Sams, C.E. Chitin and chitosan—Value-added products from mushroom waste. J. Agric. Food Chem. 2004, 52, 7905–7910. [Google Scholar] [CrossRef]
- Nitschke, J.; Altenbach, H.J.; Malolepszy, T.; Molleken, H. A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydr. Res. 2011, 346, 1307–1310. [Google Scholar] [CrossRef]
- Lee, W.-K.; Tsai, M.-L.; Shieh, Y.-T. Fractionation of chitosan by supercritical carbon dioxide/acetic acid aqueous solution. J. Supercrit. Fluids 2012, 71, 86–91. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Sami, B. Two Routes to Produce Chitosan from Agaricus bisporus. J. Renew. Mater. 2020, 8, 101–111. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valorization 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Islam, N.; Hoque, M.; Taharat, S.F. Recent advances in extraction of chitin and chitosan. World J. Microbiol. Biotechnol. 2022, 39, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, F.; Tarzi, B.G.; Bassiri, A.R.; Hoseini, S.E.; Aminafshar, M. Comparative Study on the Main Chemical Composition of Button Mushroom’s (Agaricus bisporus) Cap and Stipe. J. Food Biosci. Technol. 2013, 3, 41–48. [Google Scholar]
- Diamantopoulou, P.A.; Philippoussis, A.N. Cultivated Mushrooms: Preservation and Processing. In Handbook of Vegetable Preservation and Processing; Hui, Y.H., Evranuz, E.Ö., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 495–526. [Google Scholar] [CrossRef]
- Chou, W.T.; Sheih, I.C.; Fang, T.J. The applications of polysaccharides from various mushroom wastes as prebiotics in different systems. J. Food Sci. 2013, 78, M1041–M1048. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Food Chem. 2007, 102, 6–9. [Google Scholar] [CrossRef]
- Singh, A.; Dutta, P.K. Extraction of Chitin-Glucan Complex from Agaricus bisporus: Characterization and Antibacterial Activity. J. Polym. Mater. 2017, 34, 677–687. [Google Scholar]
- Nawawi, W.M.F.W.; Lee, K.Y.; Kontturi, E.; Bismarck, A.; Mautner, A. Surface properties of chitin-glucan nanopapers from Agaricus bisporus. Int. J. Biol. Macromol. 2020, 148, 677–687. [Google Scholar] [CrossRef]
- Zin, M.I.M.; Jimat, D.N.; Nawawi, W.M.F.W. Physicochemical properties of fungal chitin nanopaper from shiitake (L. edodes), enoki (F. velutipes) and oyster mushrooms (P. ostreatus). Carbohydr. Polym. 2022, 281, 119038–119044. [Google Scholar]
- Almeida, C.F.; Manrique, Y.A.; Lopes, J.C.B.; Martins, F.G.; Dias, M.M. Recovery of ergosterol from Agaricus bisporus mushrooms via supercritical fluid extraction: A response surface methodology optimisation. Heliyon 2024, 10, e21943–e21955. [Google Scholar] [CrossRef]
- Villares, A.; García-Lafuente, A.; Guillamón, E.; Ramos, Á. Identification and quantification of ergosterol and phenolic compounds occurring in Tuber spp. truffles. J. Food Compos. Anal. 2012, 26, 177–182. [Google Scholar] [CrossRef]
- He, W.-S.; Cui, D.; Li, L.; Rui, J.; Tong, L.-T. Plasma triacylglycerol-reducing activity of ergosterol linolenate is associated with inhibition of intestinal lipid absorption. J. Funct. Foods 2020, 64, 103686. [Google Scholar] [CrossRef]
- Gil-Ramirez, A.; Ruiz-Rodriguez, A.; Marin, F.R.; Reglero, G.; Soler-Rivas, C. Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. J. Funct. Foods 2014, 11, 589–597. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, J.E.; Mishra, S.K.; Lee, H.J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the beta-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef]
- Al-Hmoud, L.; Fara, D.A.; Rashid, I.; Chowdhry, B.Z.; Badwan, A.A. Influence of Chitin Source and Polymorphism on Powder Compression and Compaction: Application in Drug Delivery. Molecules 2020, 25, 5269–5291. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Wong, D.G.; Ramírez-Cardona, M.; Sánchez-Leija, R.J.; Rugerio, A.; Mauricio-Sánchez, R.A.; Hernández-Landaverde, M.A.; Carranza, A.; Pojman, J.A.; Garay-Tapia, A.M.; Prokhorov, E.; et al. Sustainable-solvent-induced polymorphism in chitin films. Green Chem. 2016, 18, 4303–4311. [Google Scholar] [CrossRef]
- Majtan, J.; Bilikova, K.; Markovic, O.; Grof, J.; Kogan, G.; Simuth, J. Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 2007, 40, 237–241. [Google Scholar] [CrossRef]
- Photometry & Reflectometry: IR Spectrum Table & Chart. Sigma Aldrich. 2023. Available online: https://www.sigmaaldrich.com/PT/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 21 March 2023).
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613–6629. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Taylor, K.D.A.; Roberts, G.A.F. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Hajizadeh, Z.; Taheri-Ledari, R.; Asl, F.R. Identification and analytical methods. In Heterogeneous Micro and Nanoscale Composites for the Catalysis of Organic Reactions; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–51. [Google Scholar] [CrossRef]
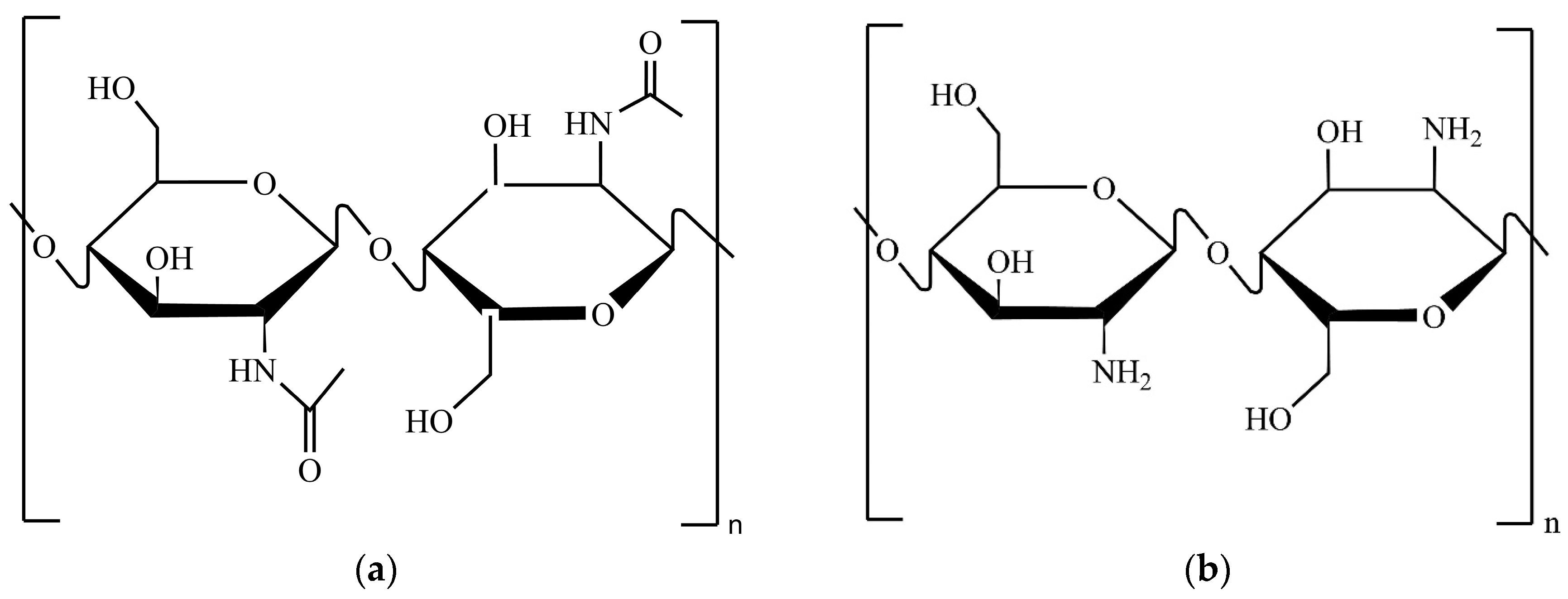
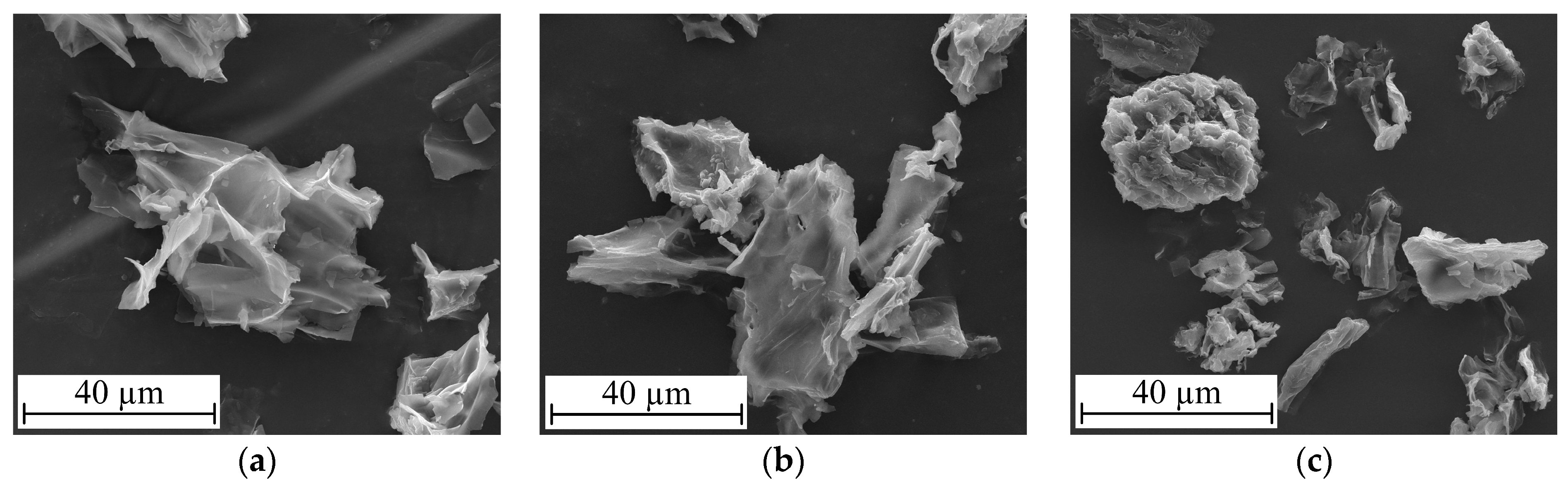
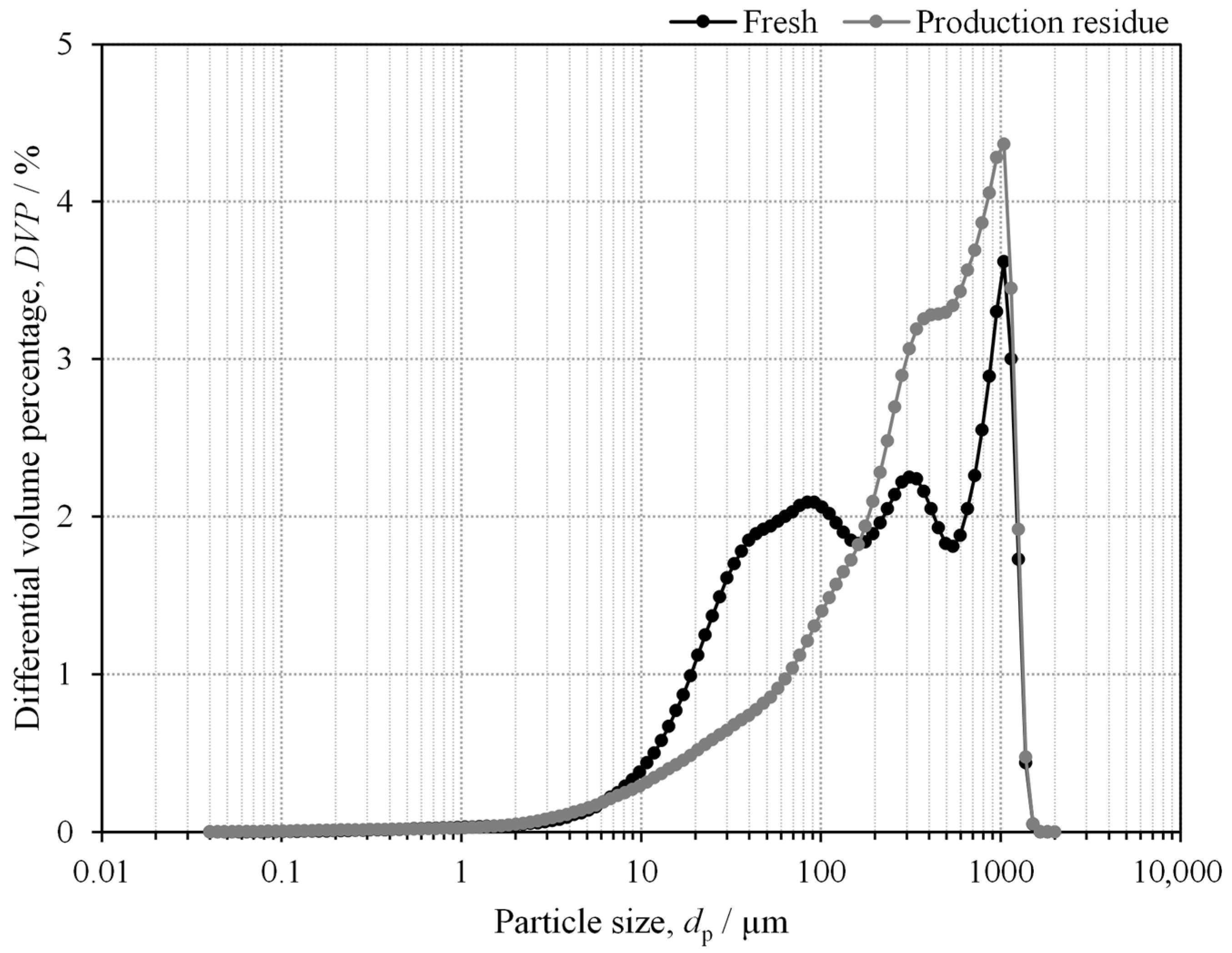
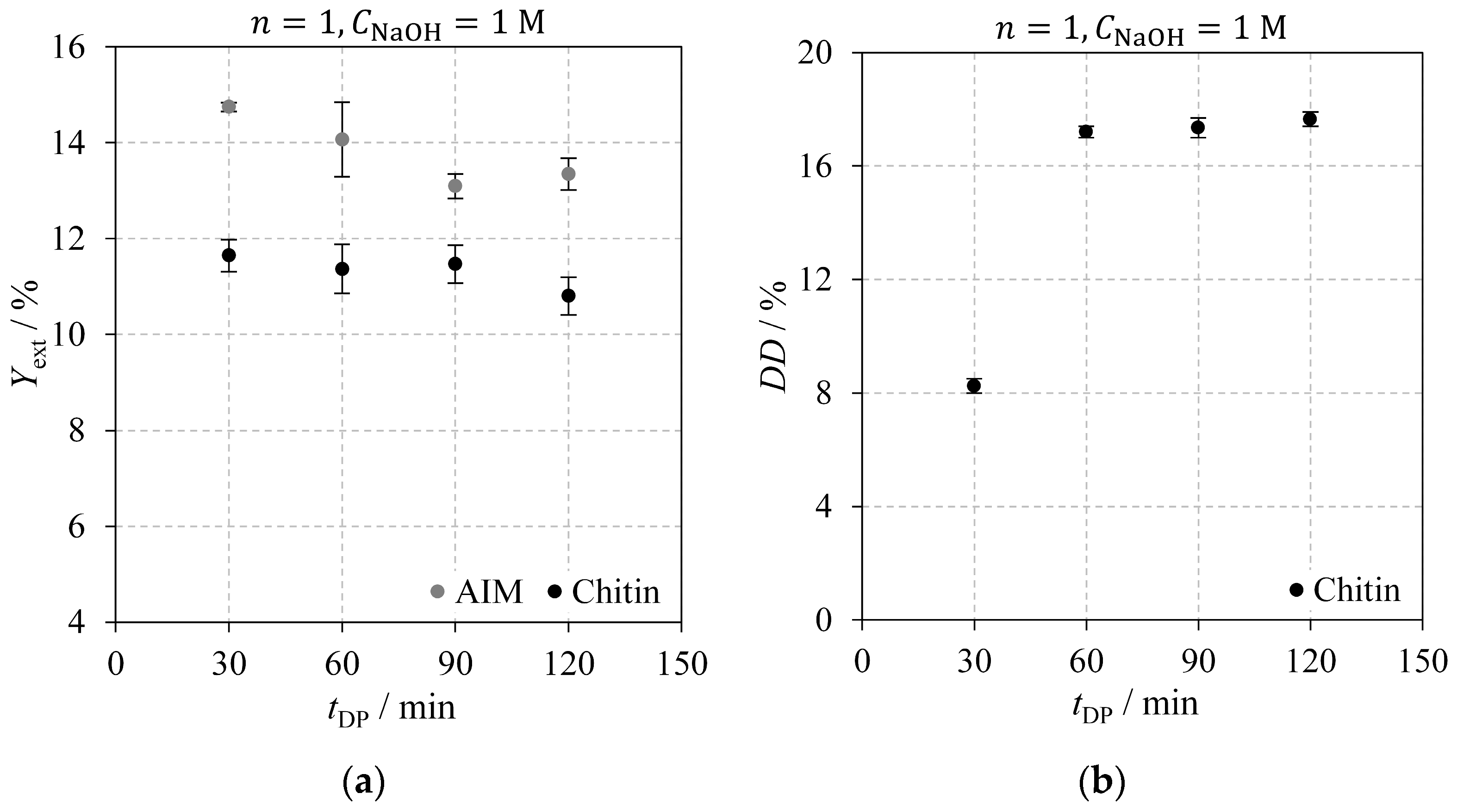

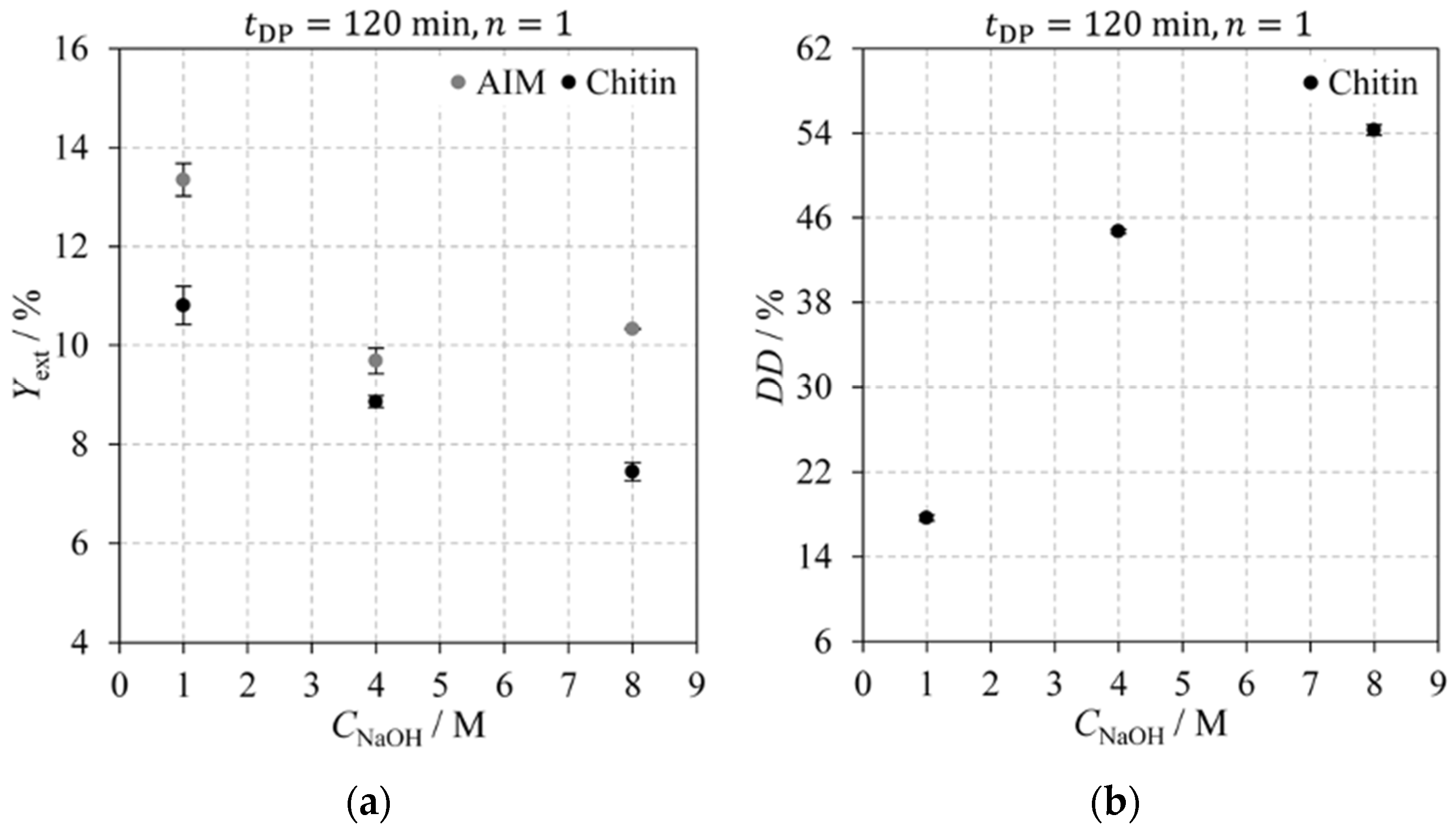

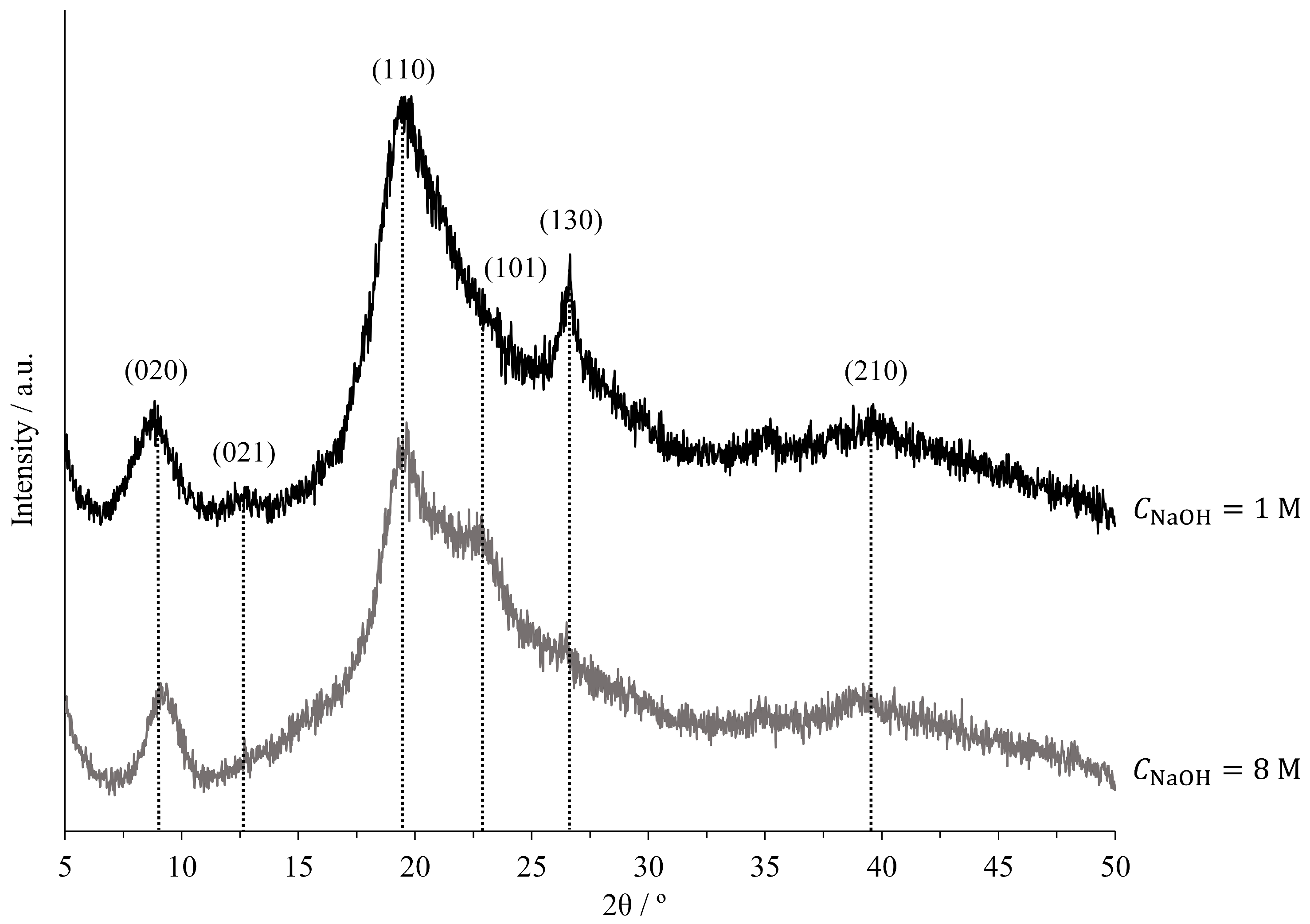
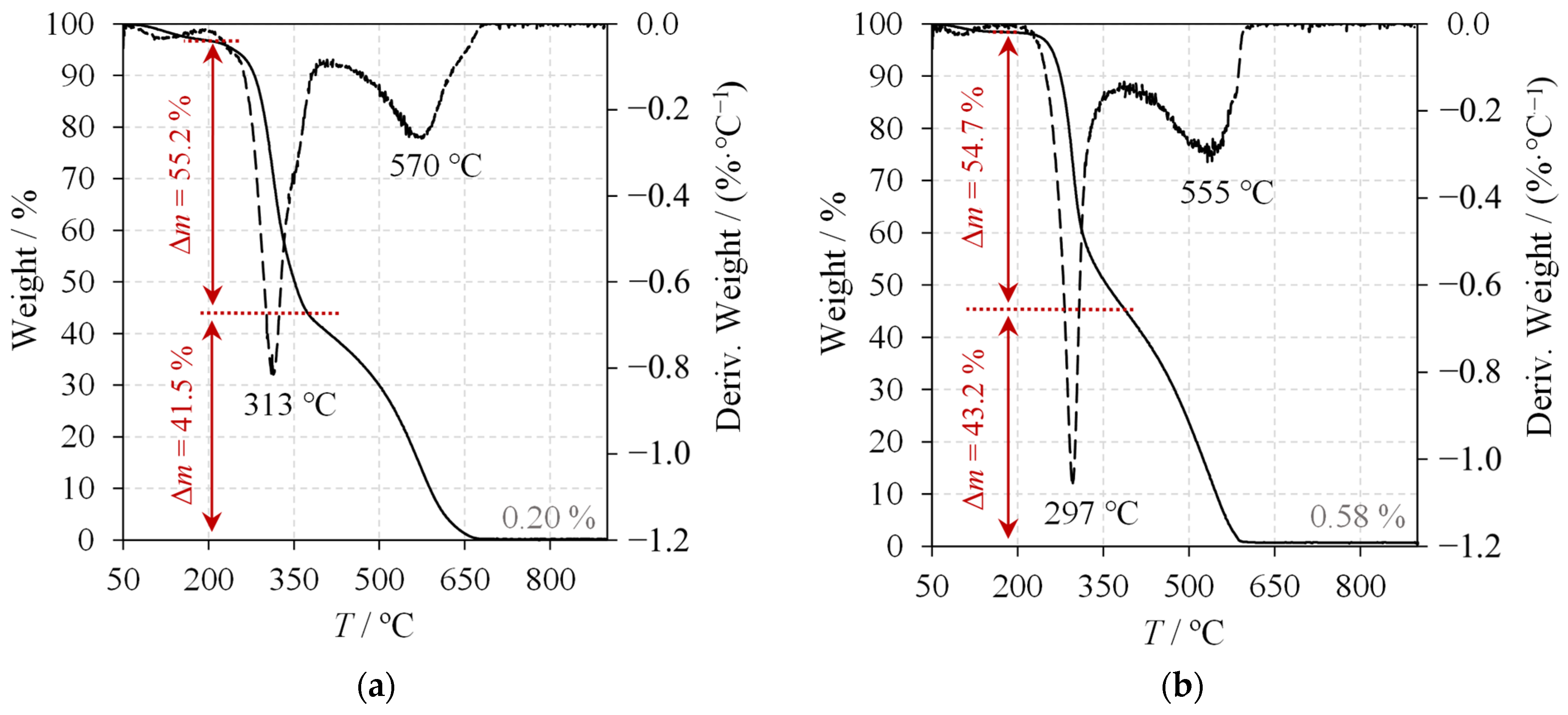


| ID | Material Sample | Deproteinisation | /% | /% | /% | ||
|---|---|---|---|---|---|---|---|
| E01 | Fresh | 1 | 1 | 30 | 14.7 ± 0.1 a | 11.7 ± 0.3 bc | 8.3 ± 0.3 g |
| E02 | Fresh | 1 | 1 | 60 | 14.1 ± 0.8 a | 11.4 ± 0.5 c | 17.2 ± 0.2 f |
| E03 | Fresh | 1 | 1 | 90 | 13.1 ± 0.3 ab | 11.5 ± 0.4 bc | 17.4 ± 0.4 f |
| E04 | Fresh | 1 | 1 | 120 | 13.4 ± 0.3 ab | 10.8 ± 0.4 c | 17.7 ± 0.3 f |
| E05 | Fresh | 2 | 1, and 2 * | 120 | 11.1 ± 0.1 cd | 9.1 ± 0.3 d | 23.1 ± 0.7 e |
| E06 | Fresh | 3 | 1, 2, and 3 * | 120 | 10.0 ± 0.5 cde | 8.0 ± 0.2 def | 34.1 ± 0.5 d |
| E07 | Fresh | 4 | 1, 2, 3, and 4 * | 120 | 7.5 ± 0.1 f | 5.2 ± 0.1 g | 39.9 ± 0.5 c |
| E08 | Fresh | 1 | 4 | 120 | 9.7 ± 0.3 de | 8.9 ± 0.1 de | 44.7 ± 0.2 b |
| E09 | Fresh | 1 | 8 | 120 | 10.3 ± 0.01 cde | 7.5 ± 0.2 ef | 54.3 ± 0.5 a |
| Peak | /cm−1 | Bond and Vibration Mode | Remarks |
|---|---|---|---|
| 1 | 3500−3200 | O-H and N-H stretching | Broad peak |
| 2 | 3000−2800 | C-H stretching | Symmetrical and asymmetrical vibrations of polysaccharides |
| 3 | 2349 | O=C=O stretching | Atmospheric carbon dioxide |
| 4 | 1650 | C=O stretching | Amide I |
| 5 | 1622 | C=O stretching | Amide I |
| 6 | 1554 | N-H bending and C-N stretching | Amide II |
| 7 | 1429−1410 | CH2 bending | - |
| 8 | 1371 | C-H and C-CH3 bending | - |
| 9 | 1323−1304 | C-N stretching and N-H bending | Amide III |
| 10 | 1159 | C-O-C stretching | Asymmetrical bridge of C-O-C (ring) |
| 11 | 1014 | C-O stretching | - |
| 12 | 899 | C-H | -glycosidic bonds |
| ID | Material Sample | Deproteinisation | /% | /% | /% | ||
|---|---|---|---|---|---|---|---|
| E04 | Fresh | 1 | 1 | 120 | 13.4 ± 0.3 ab | 10.8 ± 0.4 c | 17.7 ± 0.3 f |
| E10 | Fresh after scCO2 extraction | 1 | 1 | 120 | 11.6 ± 0.2 bc | 11.1 ± 0.2 c | 16.9 ± 0.4 f |
| E11 | Fresh after scCO2 extraction | 1 | 8 | 120 | 8.7 ± 0.3 ef | 7.1 ± 0.03 f | 54.1 ± 0.2 a |
| E12 | Production residue | 1 | 1 | 120 | 14.1 ± 0.3 a | 12.9 ± 0.2 ab | 17.6 ± 0.2 f |
| E13 | Production residue after scCO2 extraction | 1 | 1 | 120 | 14.4 ± 0.1 a | 13.8 ± 0.1 a | 18.3 ± 0.1 f |
| ID | Plane | Lhkl/Å | ||
|---|---|---|---|---|
| E04 | 020 | 8.98 | 36.8 | 88.4 |
| 021 | 12.84 | 28.3 | ||
| 110 | 19.42 | 14.9 | ||
| 130 | 26.64 | 19.3 | ||
| 210 | 39.63 | 23.9 | ||
| E09 | 020 | 9.34 | 33.8 | 78.4 |
| 110 | 19.64 | 24.9 | ||
| 101 | 22.83 | 9.2 | ||
| 210 | 39.46 | 6.5 |
| ID | Raw Material Sample | Deproteinisation Parameter | ||
|---|---|---|---|---|
| E01 | Agaricus bisporus fresh mushroom | 1 | 1 | 30 |
| E02 | Agaricus bisporus fresh mushroom | 1 | 1 | 60 |
| E03 | Agaricus bisporus fresh mushroom | 1 | 1 | 90 |
| E04 | Agaricus bisporus fresh mushroom | 1 | 1 | 120 |
| E05 | Agaricus bisporus fresh mushroom | 2 | 1, and 2 * | 120 |
| E06 | Agaricus bisporus fresh mushroom | 3 | 1, 2, and 3 * | 120 |
| E07 | Agaricus bisporus fresh mushroom | 4 | 1, 2, 3, and 4 * | 120 |
| E08 | Agaricus bisporus fresh mushroom | 1 | 4 | 120 |
| E09 | Agaricus bisporus fresh mushroom | 1 | 8 | 120 |
| E10 | Agaricus bisporus fresh mushroom after scCO2 extraction | 1 | 1 | 120 |
| E11 | Agaricus bisporus fresh mushroom after scCO2 extraction | 1 | 8 | 120 |
| E12 | Agaricus bisporus production residue mushroom | 1 | 1 | 120 |
| E13 | Agaricus bisporus production residue mushroom after scCO2 extraction | 1 | 1 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.F.; Amorim, I.; Silva, C.G.; Lopes, J.C.B.; Manrique, Y.A.; Dias, M.M. Recovery of Chitin from Agaricus bisporus Mushrooms: Influence of Extraction Parameters and Supercritical CO2 Treatment on Fresh Mushrooms and Production Residues. Molecules 2025, 30, 1479. https://doi.org/10.3390/molecules30071479
Almeida CF, Amorim I, Silva CG, Lopes JCB, Manrique YA, Dias MM. Recovery of Chitin from Agaricus bisporus Mushrooms: Influence of Extraction Parameters and Supercritical CO2 Treatment on Fresh Mushrooms and Production Residues. Molecules. 2025; 30(7):1479. https://doi.org/10.3390/molecules30071479
Chicago/Turabian StyleAlmeida, Cláudia F., Ivan Amorim, Cláudia G. Silva, José Carlos B. Lopes, Yaidelin A. Manrique, and Madalena M. Dias. 2025. "Recovery of Chitin from Agaricus bisporus Mushrooms: Influence of Extraction Parameters and Supercritical CO2 Treatment on Fresh Mushrooms and Production Residues" Molecules 30, no. 7: 1479. https://doi.org/10.3390/molecules30071479
APA StyleAlmeida, C. F., Amorim, I., Silva, C. G., Lopes, J. C. B., Manrique, Y. A., & Dias, M. M. (2025). Recovery of Chitin from Agaricus bisporus Mushrooms: Influence of Extraction Parameters and Supercritical CO2 Treatment on Fresh Mushrooms and Production Residues. Molecules, 30(7), 1479. https://doi.org/10.3390/molecules30071479









