Thermodynamic and Kinetic Characterization of Colloidal Polymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing
Abstract
:1. Introduction
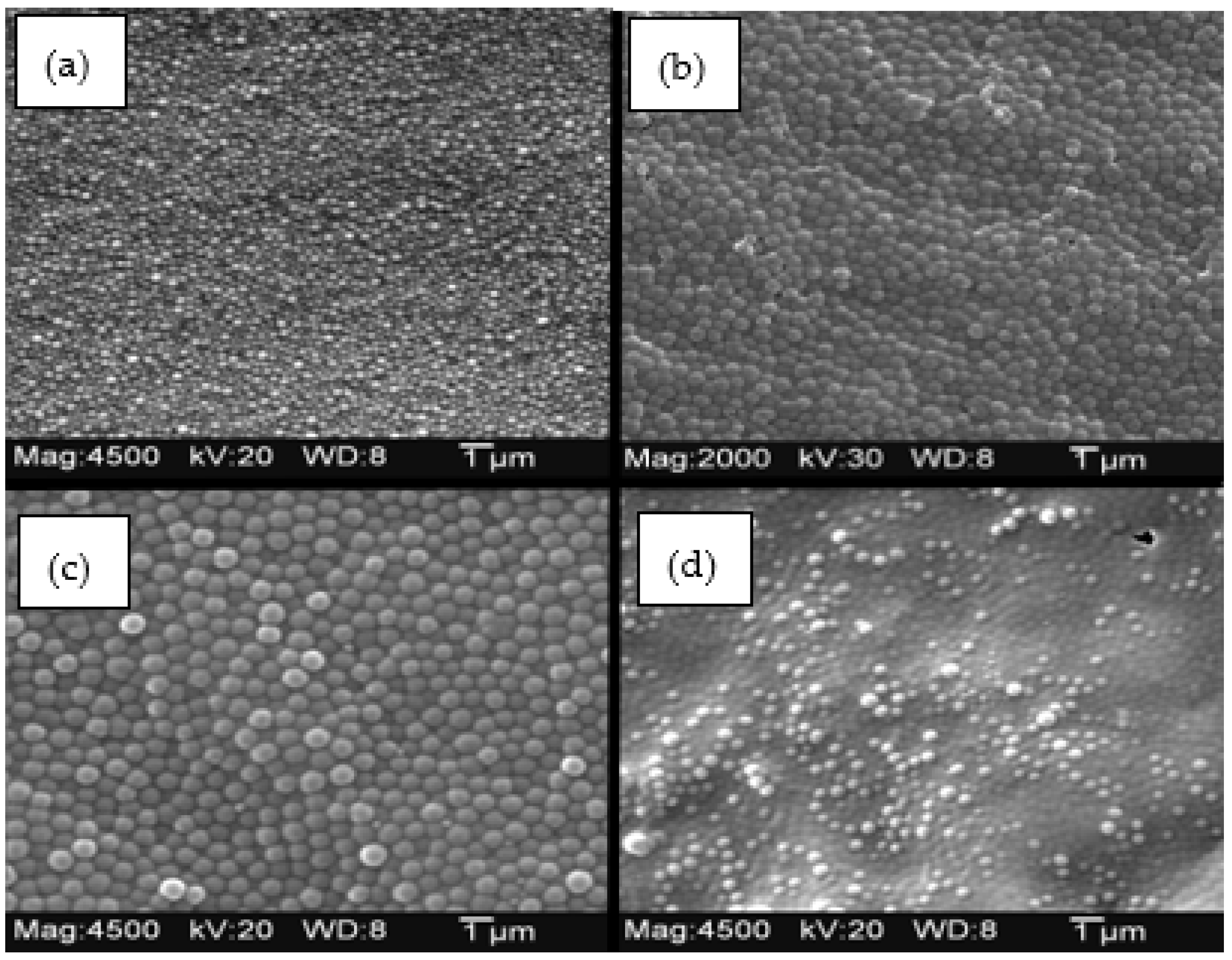
2. Results
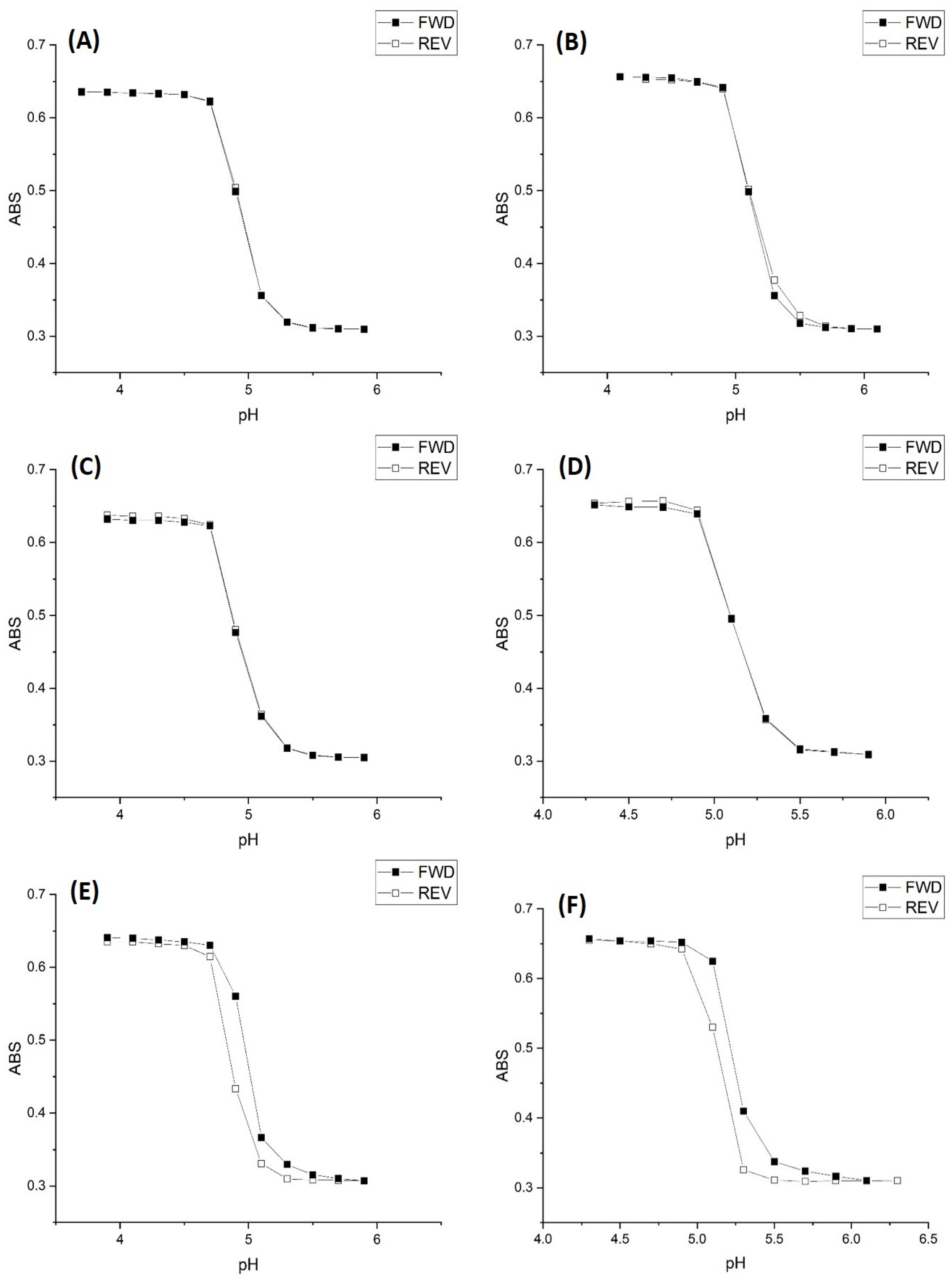
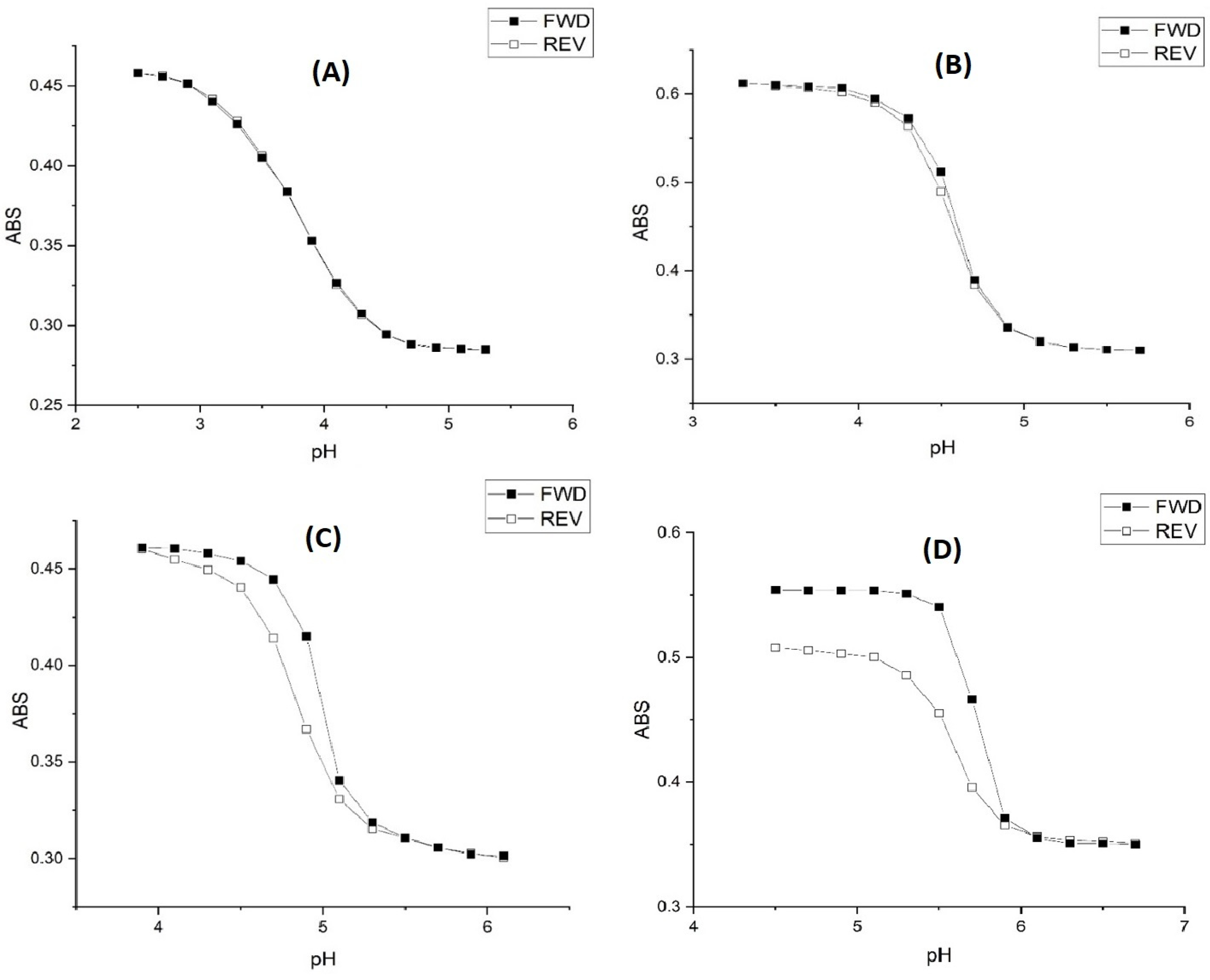
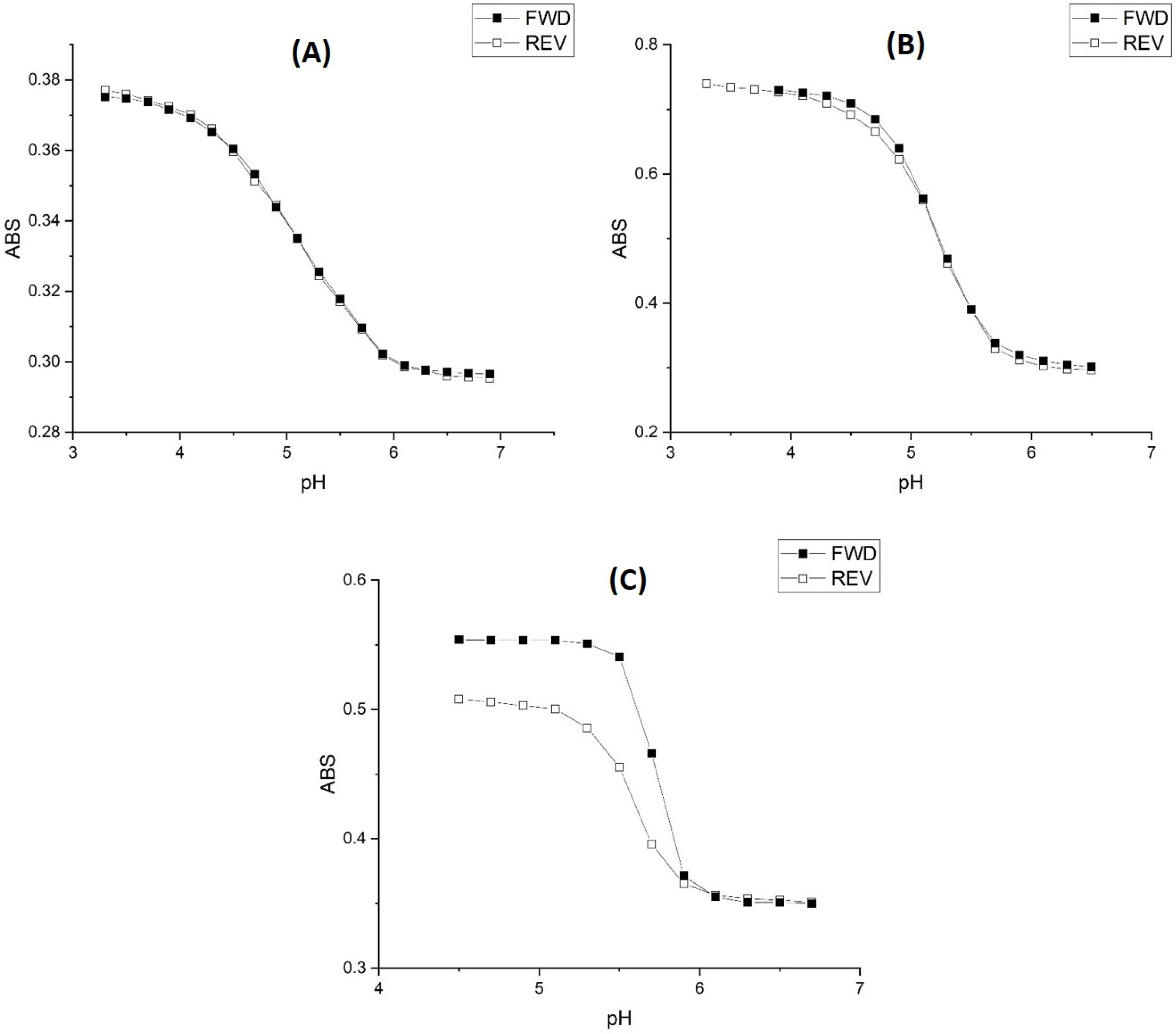
2.1. Copolymer of N-Isopropylacrylamide and Methacrylic Acid
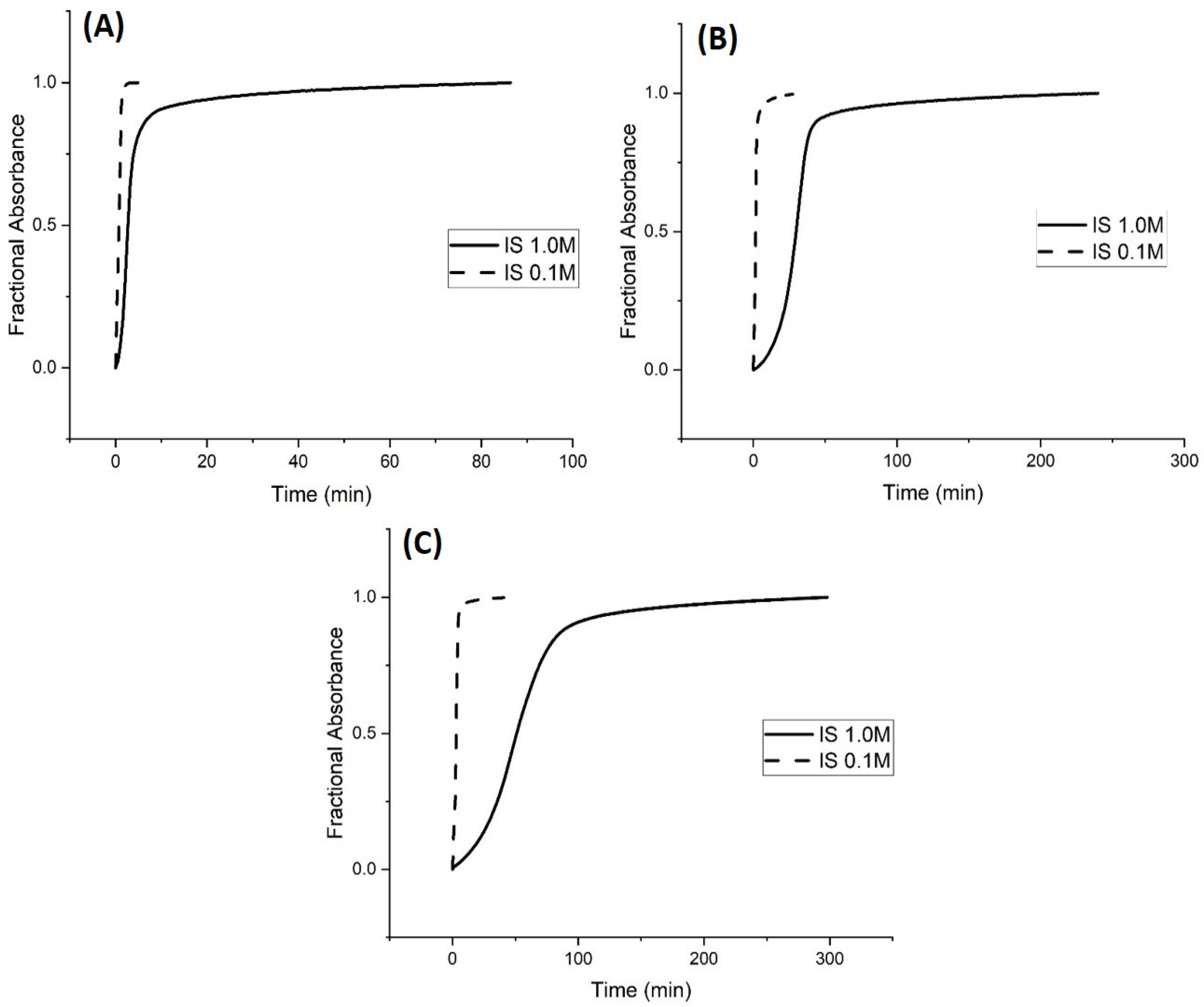
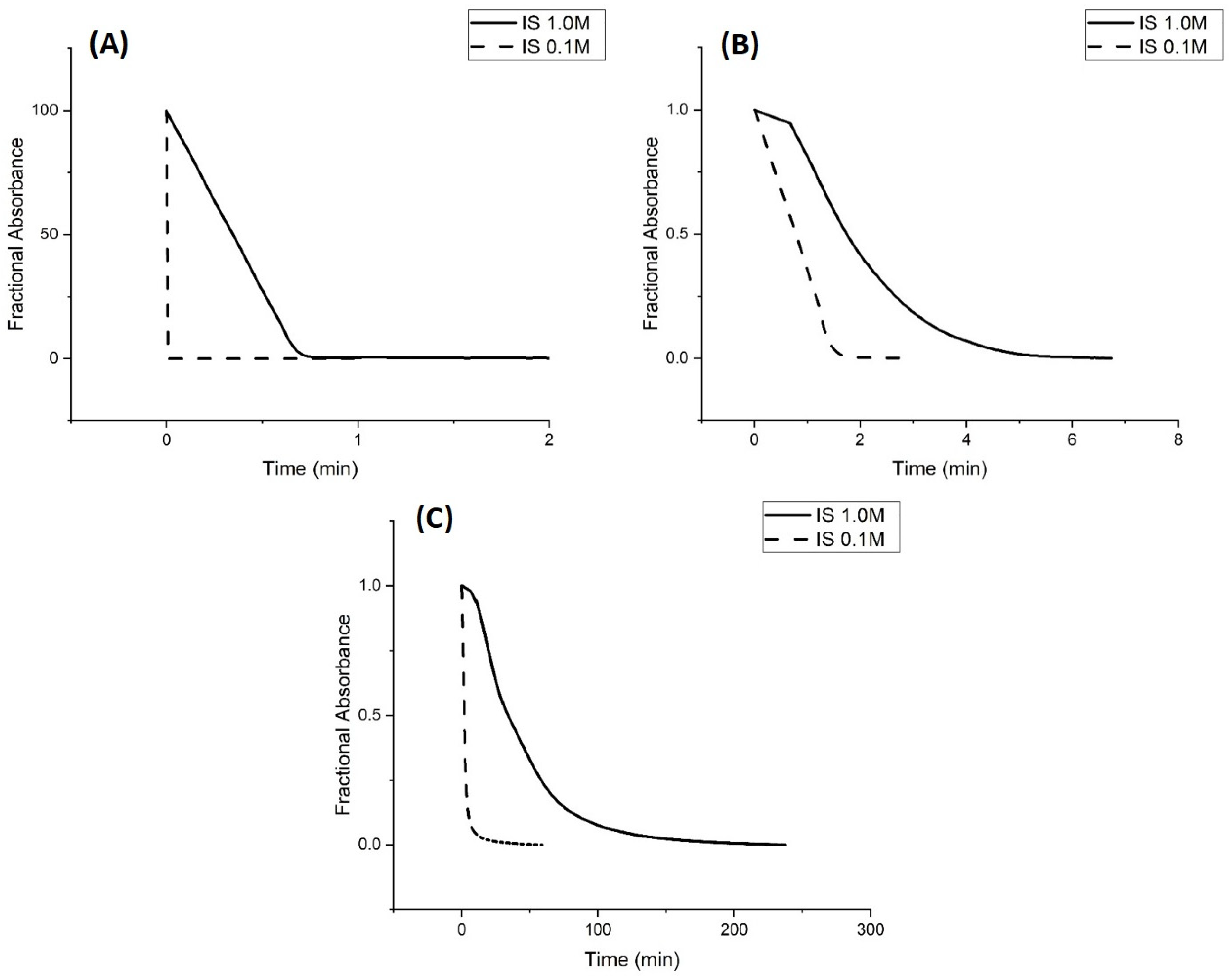
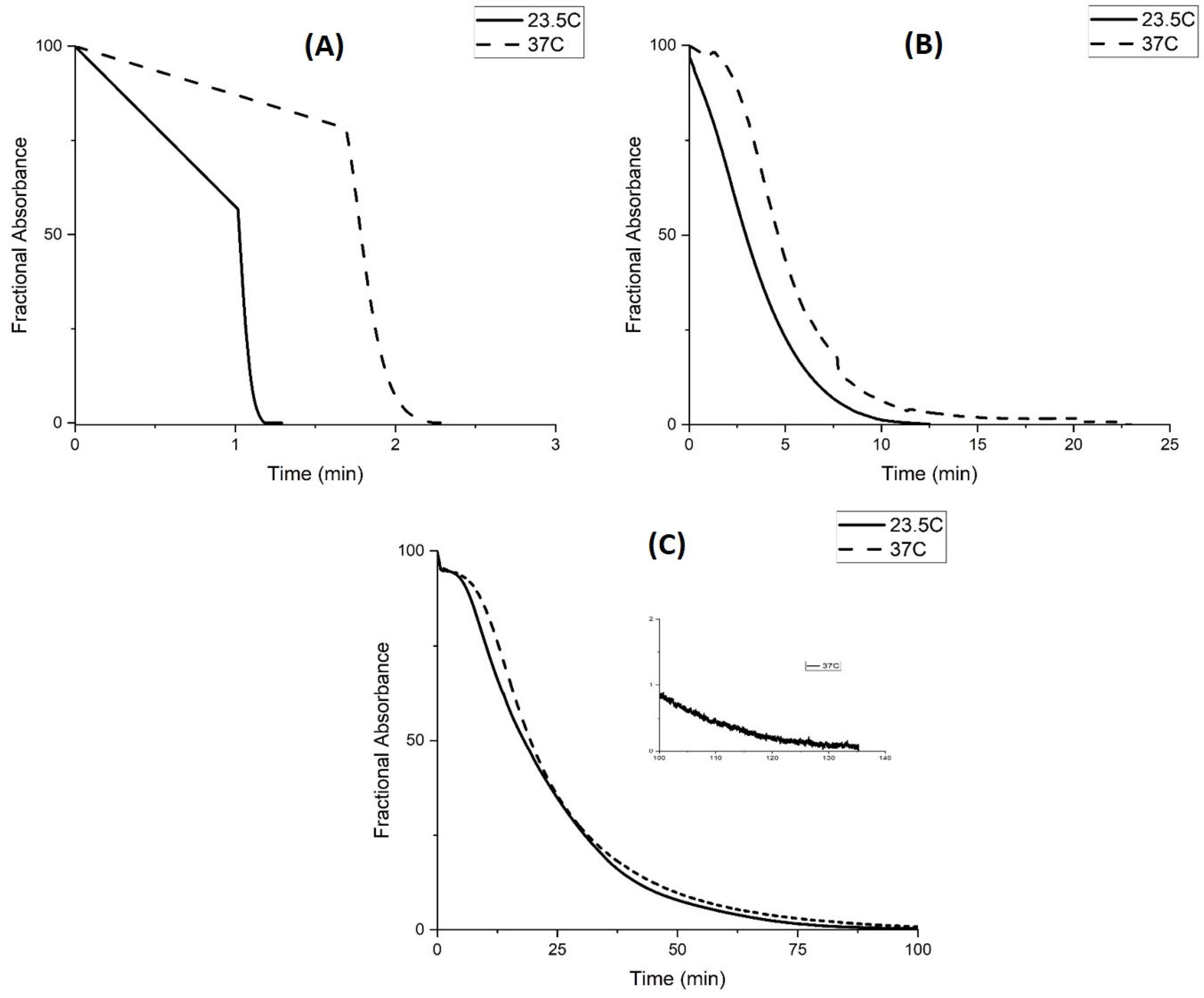
Kinetic Studies of pH-Induced Polymer Swelling and Shrinking
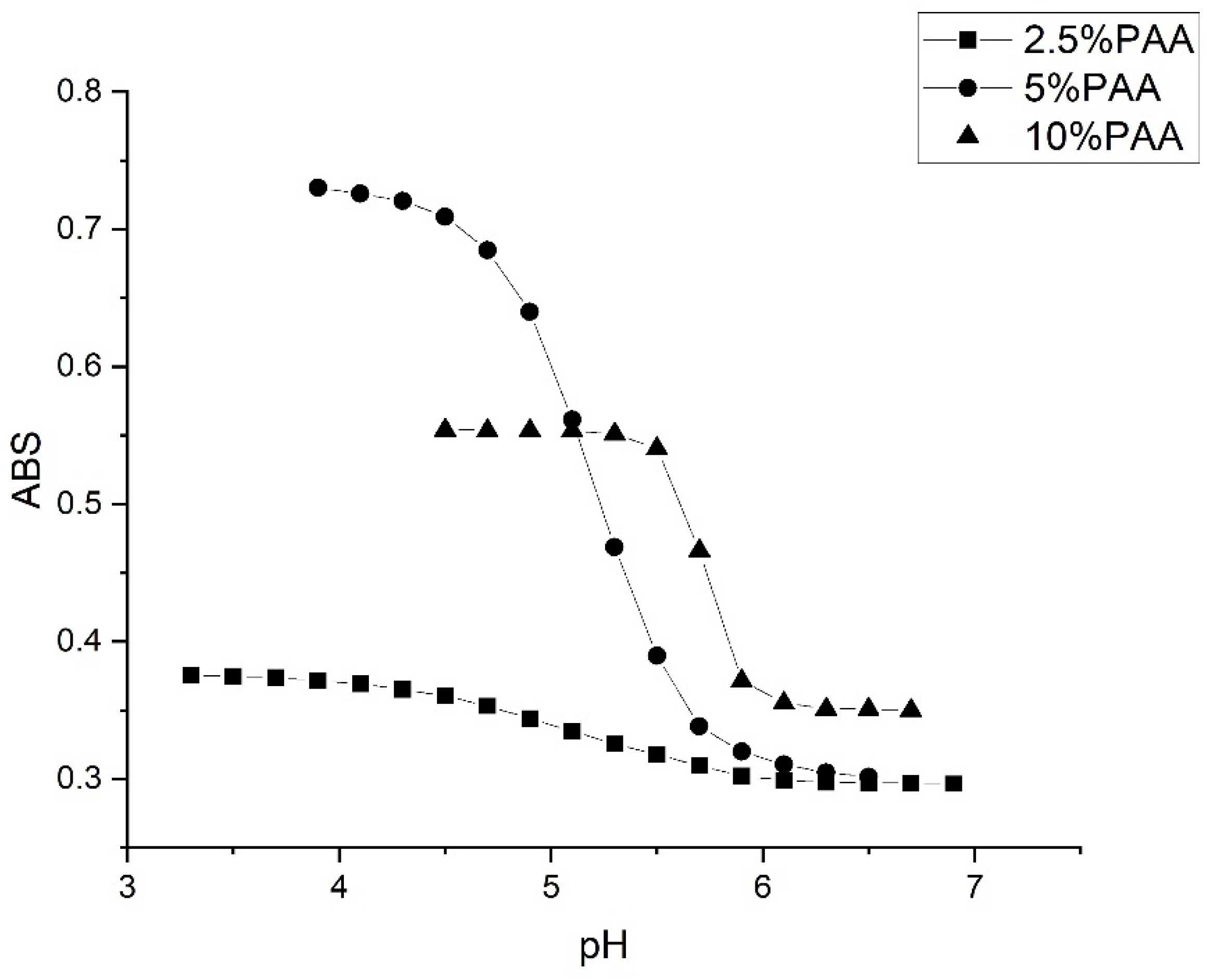
2.2. Alkyl Acrylic Acids
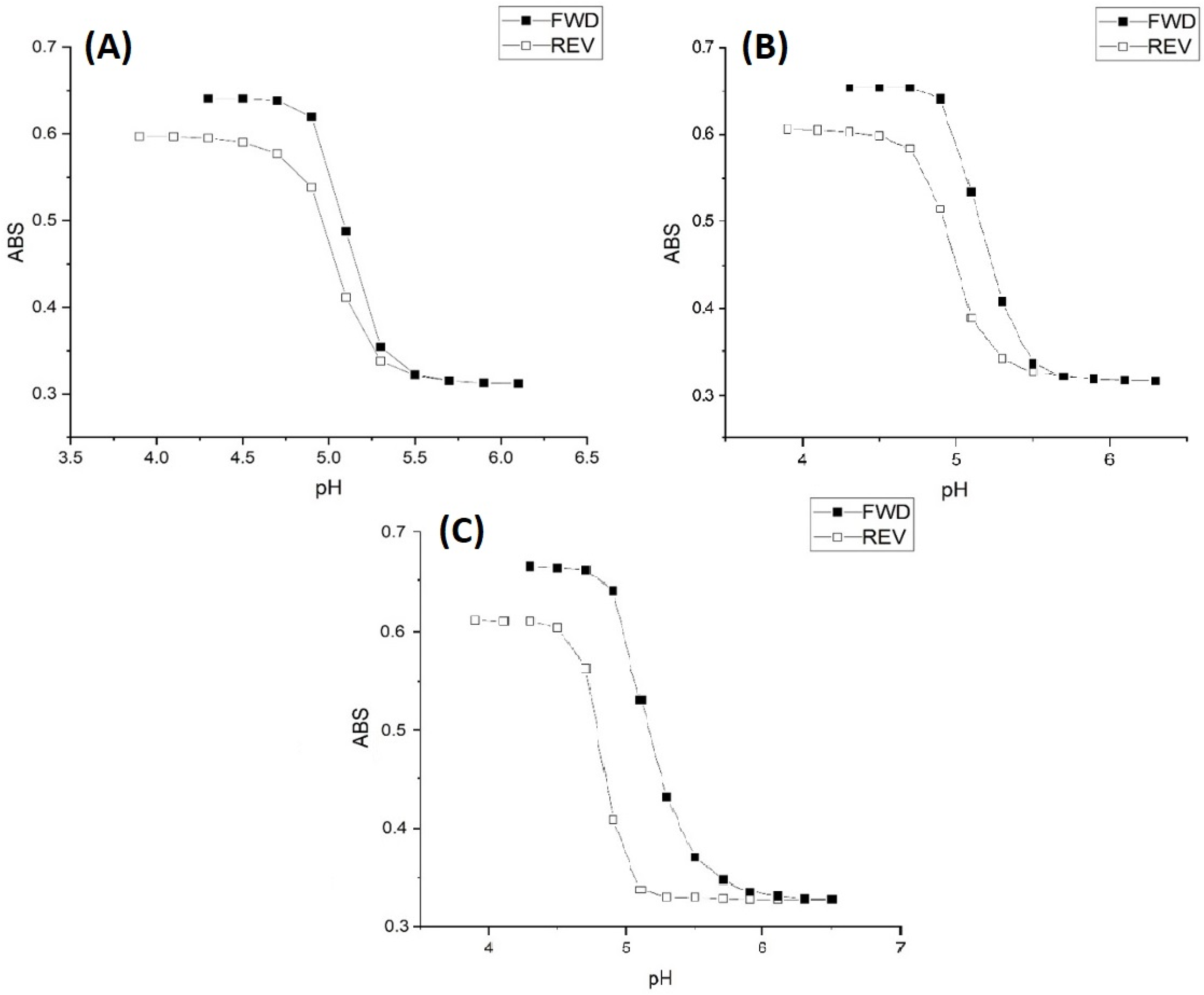
2.3. Copolymers of N-Isopropylacrylamide and Propacrylic Acid
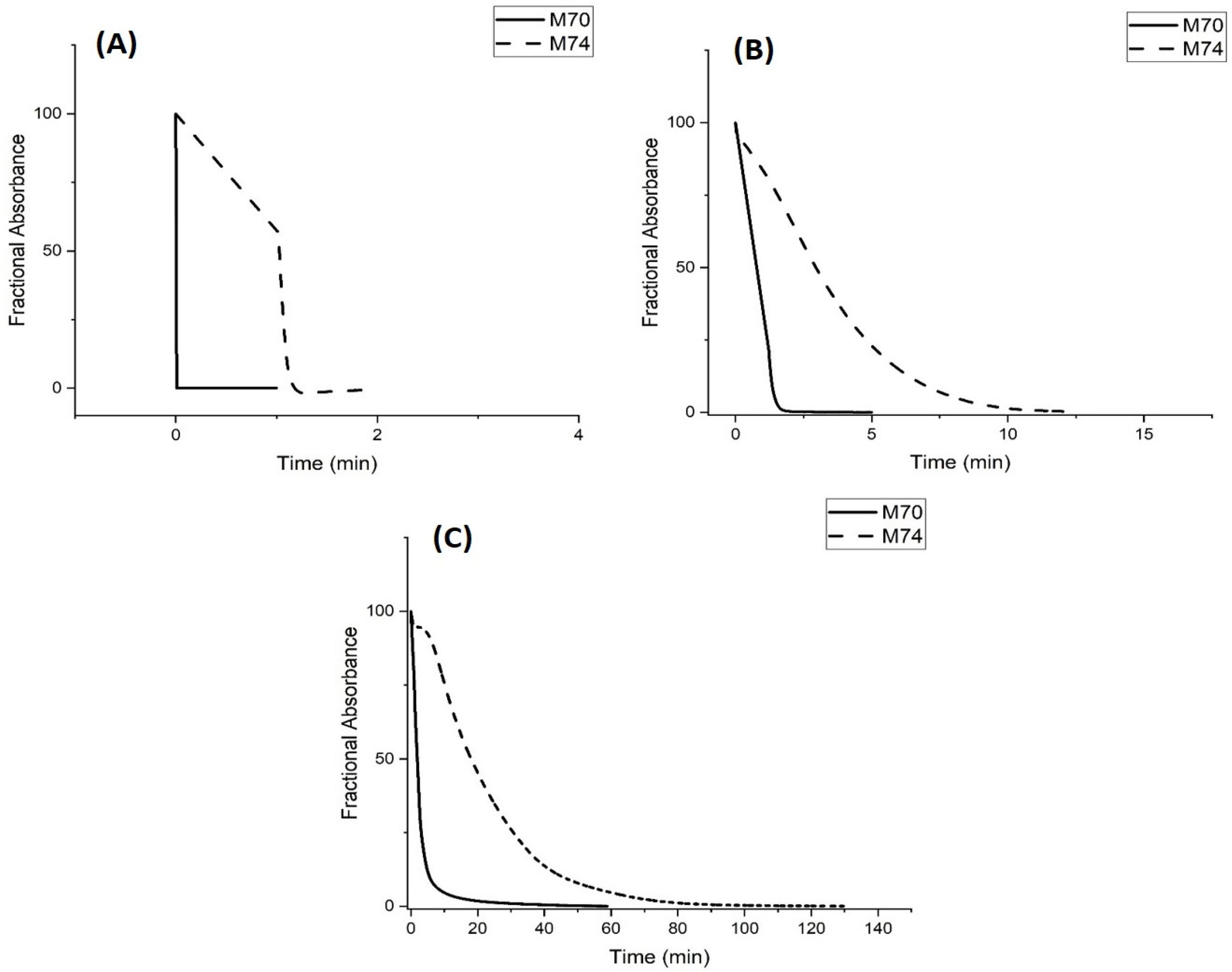
Kinetic Studies of pH-Induced Polymer Swelling and Shrinking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of pH-Sensitive Copolymers of N-Isoproplyacrylamide
4.3. Preparation of Polyvinyl Alcohol Membranes
4.4. Turbidity Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIPA | N-isopropylacrylamide |
| PVA | Polyvinyl alcohol |
| AA | Acrylic acid |
| MAA | Methacrylic acid |
| EAA | Ethacrylic acid |
| PAA | Propacrylic acid |
| NTBA | N-tert-butylacrylamide |
| MBA | N,N-methylenebisacrylamide |
| MES | 2-(N-Morpholino)ethanesulfonic acid |
| MOPS | 3-(N-Morpholino)propanesulfonic acid |
References
- Miao, Y.; Chen, J.; Fang, K. New Technology for the Detection of pH. J. Biochem. Biophys. Methods 2005, 63, 1–9. [Google Scholar] [CrossRef]
- Ferguson, J.A.; Healey, B.G.; Bronk, K.S.; Barnard, S.M.; Walt, D.R. Simultaneous monitoring of pH, CO, and O using an optical imaging fiber. Anal. Chim. Acta 1997, 340, 23–131. [Google Scholar] [CrossRef]
- Netto, E.; Peterson, J.; McShane, M.; Hampshire, V. A fiber optic broad-range pH sensor system for gastric measurements. Sens. Actuat. B 1995, 29, 157–163. [Google Scholar] [CrossRef]
- Zhang, S.; Soller, B.R.; Micheels, R.H. Partial least-squares modeling of near infrared reflectance data for noninvasive in vivo determination of deep tissue pH. Appl. Spect. 1998, 52, 393–399. [Google Scholar] [CrossRef]
- Jeevarajan, A.S.; Vani, S.; Taylor, T.D.; Anderson, M.M. Continuous pH monitoring in a perfused bioreactor system using an optical pH sensor. Biotechnol. Bioeng. 2002, 78, 467–472. [Google Scholar] [CrossRef]
- Miled, O.B.; Grasso, D.; Sanchez, C.; Livage, J. An optical fiber pH sensor based on dye Doped mesostructured silica. J. Phys. Chem. Solids 2004, 65, 1751–1755. [Google Scholar] [CrossRef]
- Korostynska, O.; Arshak, K.; Gill, E.; Arshak, A. Review on state-of-the-art in polymer-based pH sensors. Sensors 2007, 7, 3027–3042. [Google Scholar] [CrossRef]
- Leiner, M.J.P.; Hartmann, P. Theory and practice in optical pH sensing. Sens. Actuat. B 1993, 11, 281–289. [Google Scholar] [CrossRef]
- Salerno, M.; Ajimo, J.J.; Dudley, J.A.; Binzel, K.; Urayama, P. Characterization of dual Wavelength Seminapthofluorescein and Seminaphorhodaflor Dyes for pH Sensing Under High Hydrostatic Pressures. Anal. Biochem. 2007, 362, 258–267. [Google Scholar] [CrossRef]
- Schroder, C.R.; Weidgans, B.M.; Klimant, I. pH fluorescence for use in marine systems. Analyst 2005, 130, 907–916. [Google Scholar] [CrossRef]
- Shakhsher, Z.; Seitz, W.R.; Legg, K.D. Single fiber-optic pH sensor based on changes in reflection accompanying polymer swelling. Anal. Chem. 1994, 66, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shakhser, Z.; Seitz, W.R. Aminated polystyrene membranes for a fiber optic pH sensor based on reflectance changes accompanying polymer swelling. Mikro. Acta. 1995, 121, 41–50. [Google Scholar] [CrossRef]
- Lavine, B.K.; Westover, D.J.; Kaval, N.; Oxenford, L. New approaches to chemical sensing: Sensors based on polymer swelling. Anal. Lett. 2006, 39, 1773–1783. [Google Scholar] [CrossRef]
- Seitz, W.R.; Rooney, M.T.V.; Miele, E.W.; Wang, H.; Kaval, N.; Zhang, L.; Doherty, S.; Milde, S.; Lenda, J. Derivatized, swellable polymer microspheres for chemical transduction. Anal. Chim. Acta 1999, 400, 55–64. [Google Scholar] [CrossRef]
- Rooney, M.T.V.; Seitz, W.R. An optically sensitive membrane for pH based on swellable polymer microspheres in a hydrogel. Anal. Commun. 1999, 36, 267–270. [Google Scholar] [CrossRef]
- Westover, D.; Seitz, W.R.; Lavine, B.K. Synthesis and evaluation of nitrated poly(4-hydroxystyrene microspheres for pH sensing. Microchem. J. 2003, 74, 121–129. [Google Scholar] [CrossRef]
- Benjamin, M. Swelling of pH Sensitive N-Isopropylacrylamide Polymer Particles. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2012. [Google Scholar]
- Fernando-Nieves, A.; Wyss, H.M.; Mattson, J.; Weitz, D.A. Swelling thermodynamics of microgel particles. In Microgel Suspensions Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2011; pp. 73–116. [Google Scholar]
- Lavine, B.K.; Pampati, S.R.; Dahal, K.S.; Kim, M.; Perera, U.D.N.; Benjamin, M.; Bunce, R.A. Swellable copolymers of N-isopropylacrylamide and alkyl acrylic acids for optical pH sensing. Molecules 2020, 25, 1408. [Google Scholar] [CrossRef]
- Lavine, B.K.; Kaval, N.; Oxenford, L.; Kim, M.; Sharma Dahal, K.; Perera, N.; Seitz, W.R.; Moulton, J.T.; Bunce, R.A. Synthesis and Characterization of N-Isopropylacrylamide Microspheres as pH Sensors. Sensors 2021, 21, 6493. [Google Scholar] [CrossRef]
- Service, R.F. Rising acidity levels bring an ocean of trouble. Science 2016, 337, 146–148. [Google Scholar] [CrossRef]
- Perrin, D.D. The Effect of Temperature on pK Values of Organic Bases. Aust. J. Chem. 1964, 17, 484–488. [Google Scholar] [CrossRef]
- Park, T.G.; Hoffman, A.S. Sodium chloride—Induced phase transition in nonionic poly(N-isopropylacrylamide gel. Macromolecules 1993, 26, 5045–5048. [Google Scholar] [CrossRef]
- Perera, U.D.N.T. Computer Enhanced Spectrochemical and Chromatographic Analysis Applied to Problems in Analytical and Forensic Chemistry. Ph.D. Dissertation, Oklahoma State University, Stillwater, OK, USA, 2015. [Google Scholar]
- Allcock, H.R.; Lampe, F.W.; Mark, J.E. Contemporary Polymer Chemistry, 3rd ed.; Pearson Education: Upper Saddle River, NJ, USA, 2003; pp. 363–365. [Google Scholar]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef]
- Gan, D.; Lyon, L.A. Interfacial nonradiative energy transfer in response core-shell hydrogel nanoparticles. J. Am. Chem. Soc. 2001, 123, 8203–8209. [Google Scholar] [CrossRef] [PubMed]
- Madhu, C.R.; Patel, B.H. pH-Sensitive Polymers with their Important Applications (A Review). Curr. Phys. Chem. 2024, 14, 93–115. [Google Scholar] [CrossRef]
- Xiao, L.; Ling, L.; Kun, L.; Liu, B.; Tu, J.; Li, T.; Li, Y.-T.; Zhang, G.-J. A pH-Sensitive Field-Effect Transistor for Monitoring of Cancer Cell External Acid Environment. Talanta 2023, 252, 123764. [Google Scholar] [CrossRef]
- Zhou, S.; Chu, N. Synthesis and volume phase transition of poly(methacrylic acid-co-N-isopropylacrylamide) microgel particles in water. J. Phys. Chem. 1998, 102, 1364–1371. [Google Scholar] [CrossRef]
- Velada, J.L.; Liu, Y.; Hughes, M.B. Effect of pH on the swelling behavior of hydrogels based on N-isopropylacrylamide with acidic comonomers. Macromol. Chem. 1998, 199, 1127–1134. [Google Scholar] [CrossRef]
- Ferritto, M.; Tirrell, D.A. Poly(2-ethacrylic acid). Macro. Synth. 1997, 11, 59–62. [Google Scholar]
- Buffer Calculator, Centre for Proteomic Research, Liverpool. Available online: www.liv.ac.uk/buffers/buffercalc.html (accessed on 3 March 2022).
- Lavine, B.K.; Oxenford, L.; Kim, M.; Kaval, N.; Benjamin, M.; Seitz, W.R. Novel Turbidimetric Method to Study Polymer Swelling. Microchem. J. 2012, 103, 97–104. [Google Scholar] [CrossRef]
- Moulton, J.T. Thermodynamic and Chemical Characterization of Colloidal Polymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2023. [Google Scholar]
- Stoian, R.-I.; Lavine, B.K.; Rosenberger, A.T. pH Sensing using whispering gallery modes of a silica hollow bottle resonator. Talanta 2019, 194, 585–590. [Google Scholar] [CrossRef]
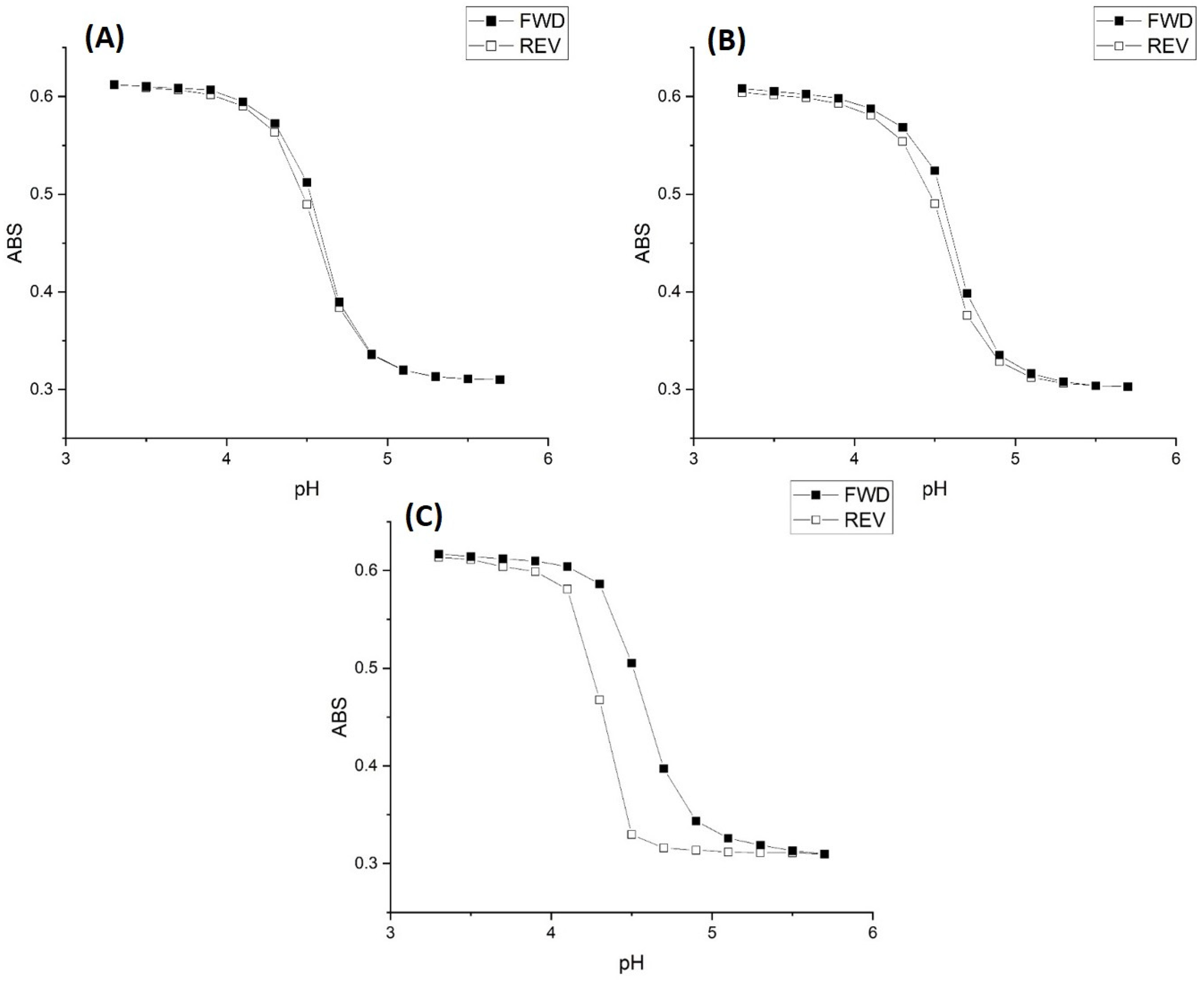
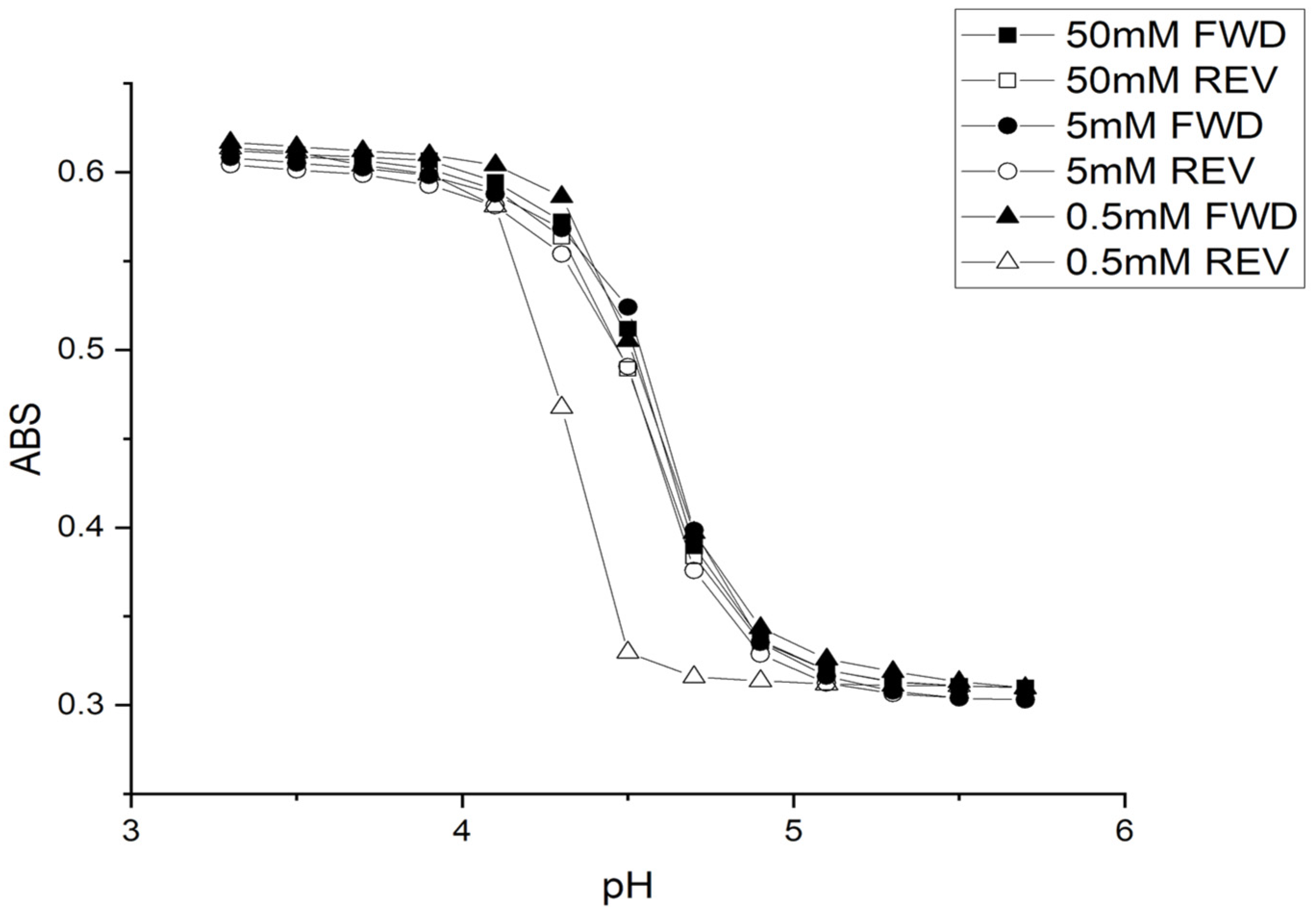


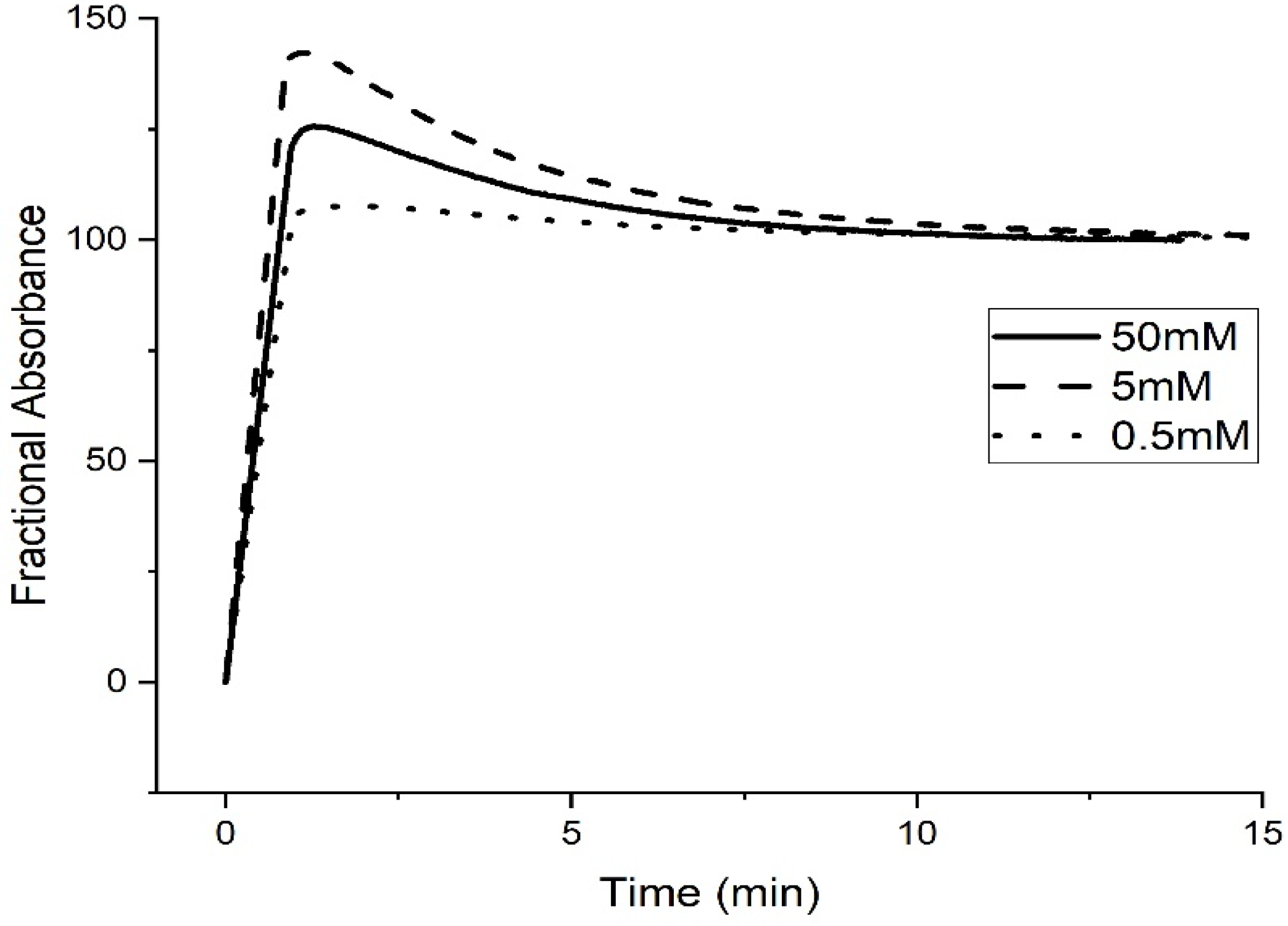


| Buffer Concentration | Temp | Ionic Strength | FWD | REV |
|---|---|---|---|---|
| 50 mM | 23.5 °C | 0.1 M | 4.59 | 4.57 |
| 5 mM | 23.5 °C | 0.1 M | 4.62 | 4.59 |
| 0.5 mM | 23.5 °C | 0.1 M | 4.57 | 4.33 |
| Temp | I.S. | FWD (Swelling) | REV (Shrinking) |
|---|---|---|---|
| 23.5 °C | 0.1 M | 4.59 | 4.57 |
| 31 °C | 0.1 M | 4.93 | 4.94 |
| 37 °C | 0.1 M | 5.10 | 5.08 |
| Temp | I.S. | FWD (Swelling) | REV (Shrinking) |
|---|---|---|---|
| 23.5 °C | 0.1 M | 4.62 | 4.59 |
| 31 °C | 0.1 M | 4.86 | 4.87 |
| 37 °C | 0.1 M | 5.09 | 5.09 |
| Temp | I.S. | FWD (Swelling) | REV (Shrinking) |
|---|---|---|---|
| 23.5 °C | 0.1 M | 4.57 | 4.33 |
| 31 °C | 0.1 M | 4.99 | 4.84 |
| 37 °C | 0.1 M | 5.21 | 5.17 |
| Buffer Concentration | 50 mM | 5 mM | 0.5 mM |
|---|---|---|---|
| Deprotonation | 5.10 | 5.15 | 5.15 |
| Protonation | 5.02 | 4.98 | 4.82 |
| NIPA Polymer | Apparent pKa Swelling | Apparent pKa Shrinking | NIPA | Functional Comonomer | NTBA | MBA |
|---|---|---|---|---|---|---|
| M-79 | 3.84 | 3.85 | 14.8 mmoles | 2 mmoles of acrylic acid 1 | 2 mmoles | 1.2 mmoles |
| M-70 | 4.59 | 4.57 | 15 mmoles | 2 mmoles of methacrylic acid 2 | 2 mmoles | 1 mmole |
| M-80 | 4.99 | 4.82 | 17 mmoles | 2 mmoles of ethacrylic acid 3 | 0 mmoles | 1 mmole |
| M-74 | 5.74 | 5.60 | 16.8 mmoles | 2 mmoles of propacrylic acid 4 | 0 mmoles | 1.2 mmoles |
| Polymer | pH Ascending (Swelling) 50 mM Buffer | ||
|---|---|---|---|
| M-Series | DH (J/mol) | DS (J/mol) | R2 |
| M-79 (AA) | −94,884 ± 12,297 | −393.9 ± 40.5 | 0.9835 |
| M-70 (MAA) | −67,131 ± 7649 | −314.5 ± 25.2 | 0.9872 |
| M-80 (EAA) | −56,297 ± 2452 | −284.6 ± 8.1 | 0.9981 |
| M-74 (PAA) | −59,548 ± 6077 | −310.4 ± 20.0 | 0.9897 |
| Polymer | pH Descending (Shrinking) 50 mM Buffer | ||
|---|---|---|---|
| DH (J/mol) | DS (J/mol) | R2 | |
| M-79 (AA) | −91,949 ± 8007 | −384.0 ± 26.4 | 0.9925 |
| M-70 (MAA) | −67,472 ± 12,147 | −315.4 ± 25.2 | 0.9686 |
| M-80 (EAA) | −79,096 ± 10,501 | −359.4 ± 34.6 | 0.9827 |
| M-74 (PAA) | −77,740 ± 9853 | −369.7 ± 32.4 | 0.9842 |
| Polymer | Apparent pKa Swelling | Apparent pKa Shrinking | NIPA | PAA | MBA |
|---|---|---|---|---|---|
| M-103 | 5.15 | 5.16 | 18.5 mmoles | 0.5 mmoles | 1 mmole |
| M-101 | 5.20 | 5.21 | 18 mmoles | 1 mmole | 1 mmole |
| M-74 | 5.74 | 5.60 | 16.8 mmoles | 2 mmoles | 1.2 mmoles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulton, J.T.; Bruce, D.; Bunce, R.A.; Kim, M.; Snyder, L.O.; Seitz, W.R.; Lavine, B.K. Thermodynamic and Kinetic Characterization of Colloidal Polymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing. Molecules 2025, 30, 1416. https://doi.org/10.3390/molecules30071416
Moulton JT, Bruce D, Bunce RA, Kim M, Snyder LO, Seitz WR, Lavine BK. Thermodynamic and Kinetic Characterization of Colloidal Polymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing. Molecules. 2025; 30(7):1416. https://doi.org/10.3390/molecules30071416
Chicago/Turabian StyleMoulton, James T., David Bruce, Richard A. Bunce, Mariya Kim, Leah Oxenford Snyder, W. Rudolf Seitz, and Barry K. Lavine. 2025. "Thermodynamic and Kinetic Characterization of Colloidal Polymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing" Molecules 30, no. 7: 1416. https://doi.org/10.3390/molecules30071416
APA StyleMoulton, J. T., Bruce, D., Bunce, R. A., Kim, M., Snyder, L. O., Seitz, W. R., & Lavine, B. K. (2025). Thermodynamic and Kinetic Characterization of Colloidal Polymers of N-Isopropylacrylamide and Alkyl Acrylic Acids for Optical pH Sensing. Molecules, 30(7), 1416. https://doi.org/10.3390/molecules30071416









