Impact of Sulfurization Temperature on the Formation and Properties of Chalcogenide Perovskites
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of Chalcogenide Perovskite Powder Samples
3.2. Powder Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, I.; Sidhik, S.; Zhang, H.; Agrawal, A.; Persaud, J.; Hou, J.; Even, J.; Mohite, A.D. Synergy of 3D and 2D Perovskites for Durable, Efficient Solar Cells and Beyond. Chem. Rev. 2023, 123, 9565–9652. [Google Scholar] [CrossRef]
- Xie, H.B.; Lira-Cantu, M. Multi-Component Engineering to Enable Long-Term Operational Stability of Perovskite Solar Cells. J. Phys. Energy 2020, 2, 024008. [Google Scholar] [CrossRef]
- Ren, M.; Qian, X.; Chen, Y.; Wang, T.; Zhao, Y. Potential Lead Toxicity and Leakage Issues on Lead Halide Perovskite Photovoltaics. J. Hazard. Mater. 2022, 426, 127848. [Google Scholar] [CrossRef]
- Hossain, M.K.; Toki, G.F.I.; Samajdar, D.P.; Mushtaq, M.; Rubel, M.H.K.; Pandey, R.; Madan, J.; Mohammed, M.K.A.; Islam, M.R.; Rahman, M.F.; et al. Deep Insights into the Coupled Optoelectronic and Photovoltaic Analysis of Lead-Free CsSnI3 Perovskite-Based Solar Cell Using DFT Calculations and SCAPS-1D Simulations. ACS Omega 2023, 8, 22466–22485. [Google Scholar] [CrossRef] [PubMed]
- Konidakis, I.; Karagiannakia, A.; Stratakis, E. Advanced Composite Glasses with Metallic, Perovskite, and Two-Dimensional Nanocrystals for Optoelectronic and Photonic Applications. Nanoscale 2022, 14, 2966–2989. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Agiorgousis, M.L.; Zhang, P.; Zhang, S. Chalcogenide Perovskites for Photovoltaics. Nano Lett. 2015, 15, 581–585. [Google Scholar] [CrossRef]
- Mercy, E.N.V.; Srinivasan, D.; Marasamy, L. Emerging BaZrS3 and Ba(Zr,Ti)S3 Chalcogenide Perovskite Solar Cells: A Numerical Approach Toward Device Engineering and Unlocking Efficiency. ACS Omega 2024, 9, 4359–4376. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fang, X.; Shi, Z. Advances in Chalcogenide Perovskites: Fundamentals and Applications. Appl. Phys. Rev. 2024, 11, 021338. [Google Scholar] [CrossRef]
- Ivaniuk, K.; Cherpak, V.; Stakhira, P.; Baryshnikov, G.; Minaev, B.; Hotra, Z.; Turyk, P.; Zhydachevskii, Y.; Volyniuk, D.; Aksimentyeva, O.; et al. BaZrO3 Perovskite Nanoparticles as Emissive Material for Organic/Inorganic Hybrid Light-Emitting Diodes. Dyes Pigm. 2017, 145, 399–403. [Google Scholar] [CrossRef]
- Nishigaki, Y.; Nagai, T.; Nishiwaki, M.; Aizawa, T.; Kozawa, M.; Hanzawa, K.; Kato, Y.; Sai, H.; Hiramatsu, H.; Hosono, H.; et al. Extraordinary Strong Band-Edge Absorption in Distorted Chalcogenide Perovskites. Sol. RRL 2020, 4, 1900555. [Google Scholar] [CrossRef]
- Suhail, M.; Abbas, H.; Khan, M.B.; Khan, Z.H. Chalcogenide Perovskites for Photovoltaic Applications: A Review. J. Nanopart. Res. 2022, 24, 142. [Google Scholar] [CrossRef]
- Surendran, M.; Chen, H.; Zhao, B.; Thind, A.S.; Singh, S.; Orvis, T.; Zhao, H.; Han, J.-K.; Htoon, H.; Kawasaki, M.; et al. Epitaxial Thin Films of a Chalcogenide Perovskite. Chem. Mater. 2021, 33, 7457–7464. [Google Scholar] [CrossRef]
- Hanzawa, K.; Iimura, S.; Hiramatsu, H.; Hosono, H. Material Design of Green-Light-Emitting Semiconductors: Perovskite-Type Sulfide SrHfS3. J. Am. Chem. Soc. 2019, 141, 5343–5349. [Google Scholar] [CrossRef]
- Perera, S.; Hui, H.; Zhao, C.; Xue, H.; Sun, F.; Deng, C.; Gross, N.; Milleville, C.; Xu, X.; Watson, D.F.; et al. Chalcogenide Perovskites-an Emerging Class of Ionic Semiconductors. Nano Energy 2016, 22, 129–135. [Google Scholar] [CrossRef]
- Meng, W.W.; Saparov, B.; Hong, F.; Wang, J.B.; Mitzi, D.B.; Yan, Y.F. Alloying and Defect Control within Chalcogenide Perovskites for Optimized Photovoltaic Application. Chem. Mater. 2016, 28, 821–829. [Google Scholar] [CrossRef]
- Yang, R.Q.; Jess, A.D.; Fai, C.; Hages, C.J. Low-Temperature, Solution-Based Synthesis of Luminescent Chalcogenide Perovskite BaZrS3 Nanoparticles. J. Am. Chem. Soc. 2022, 144, 15928–15931. [Google Scholar] [CrossRef]
- Yasmin, N.; Safdar, M.; Ali, G.; Khan, H.M.; Mirza, M. Solar Light Harvesting Nanomaterial (BaZrS3) for Photocatalytic Activity and OER Reaction. J. Phys. Chem. Solids 2023, 172, 111056. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, X.; Zheng, Y.; Hui, H.; Bian, M.; Dhole, S.; Seo, J.-H.; Sun, Y.-Y.; Jia, Q.; Zhang, S.; et al. Chalcogenide Perovskite BaZrS3 Thin-Film Electronic and Optoelectronic Devices by Low Temperature Processing. Nano Energy 2021, 85, 105959. [Google Scholar] [CrossRef]
- Wei, X.; Hui, H.; Zhao, C.; Deng, C.; Han, M.; Yu, Z.; Sheng, A.; Roy, P.; Chen, A.; Lin, J.; et al. Realization of BaZrS3 Chalcogenide Perovskite Thin Films for Optoelectronics. Nano Energy 2020, 68, 104317. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Y.; Xu, J.; Ma, J.; Jiang, H.; Li, X.; Zhang, B.; Chen, X.; Tian, Y.; Han, Y.; et al. Parametric Study on Controllable Growth of SrZrS3 Thin Films with Good Conductivity for Photodetectors. Nano Res. 2023, 16, 7867–7873. [Google Scholar] [CrossRef]
- Niu, S.; Huyan, H.; Liu, Y.; Yeung, M.; Ye, K.; Blankemeier, L.; Orvis, T.; Sarkar, D.; Singh, D.J.; Kapadia, R.; et al. Bandgap Control via Structural and Chemical Tuning of Transition Metal Perovskite Chalcogenides. Adv. Mater. 2017, 29, 1604733. [Google Scholar] [CrossRef] [PubMed]
- Kassa, M.D.; Woldemariam, M.M.; Debelo, N.G.; Gebremeskel, D.K.; Habura, K.H. A Computational Exploration of Structural, Electronic, and Optical Properties of Hf- Substituted CaZr1−x HfxS3 (x = 0.25, 0.50, and 0.75) for Photovoltaic Applications. Phys. Scripta 2025, 100, 015974. [Google Scholar] [CrossRef]
- Song, X.; Shai, X.; Deng, S.; Wang, J.; Li, J.; Ma, X.; Li, X.; Wei, T.; Ren, W.; Gao, L.; et al. Anisotropic Chalcogenide Perovskite CaZrS3: A Promising Thermoelectric Material. J. Phys. Chem. C 2022, 126, 11751–11760. [Google Scholar] [CrossRef]
- Sopiha, K.V.; Comparotto, C.; Márquez, J.A.; Scragg, J.J.S. Chalcogenide Perovskites: Tantalizing Prospects, Challenging Materials. Adv. Opt. Mater. 2021, 10, 2101704. [Google Scholar] [CrossRef]
- Niu, S.; Joe, G.; Zhao, H.; Zhou, Y.; Orvis, T.; Huyan, H.; Salman, J.; Mahalingam, K.; Urwin, B.; Wu, J.; et al. Giant Optical Anisotropy in a Quasi-One-Dimensional Crystal. Nat. Photonics 2018, 12, 392–396. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Guo, G.; Gao, S.; Li, X.; Meng, J.; Yin, Z.; Liu, H.; Gao, M.; Cheng, L.; et al. Large-Area Synthesis of Layered HfS2(1−x)Se2x Alloys with Fully Tunable Chemical Compositions and Bandgaps. Adv. Mater. 2018, 30, 1803285. [Google Scholar] [CrossRef]
- Wang, D.; Meng, J.; Zhang, X.; Guo, G.; Yin, Z.; Liu, H.; Cheng, L.; Gao, M.; You, J.; Wang, R. Selective Direct Growth of Atomic Layered HfS2 on Hexagonal Boron Nitride for High Performance Photodetectors. Chem. Mater. 2018, 30, 3819–3826. [Google Scholar] [CrossRef]
- Kong, S.; Dong, H.; Yu, Z.; Guo, J.; Cao, K.; Chen, K.; Ke, X.; Zhou, C.; Deng, J.; Yang, S.; et al. Ti-S Antibonding Coupling Enables Enhanced Bandgap Tuning in Ti-Substituted BaHfS3 Perovskite. Ceram. Int. 2024, 50, 10889–10896. [Google Scholar] [CrossRef]

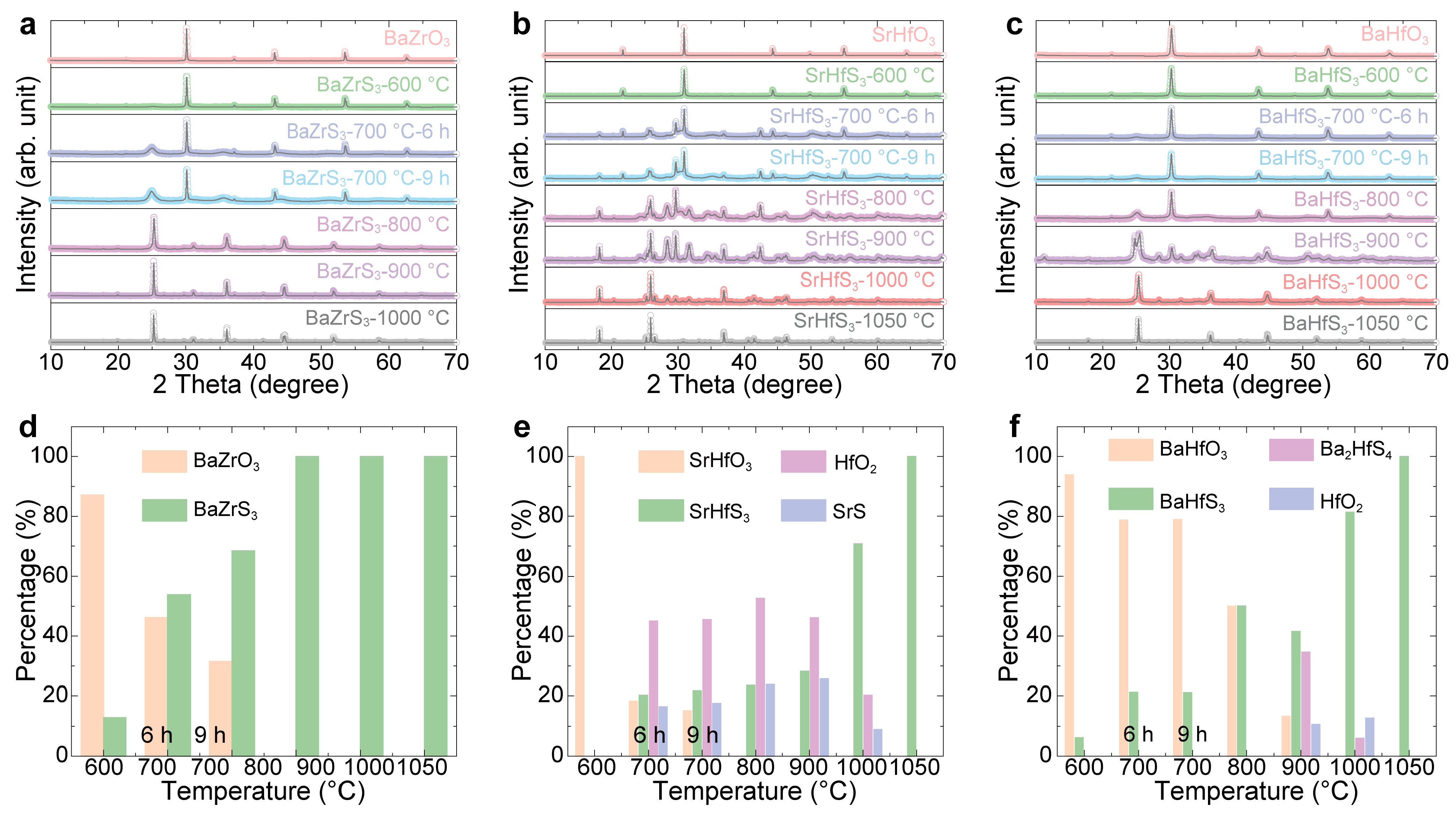
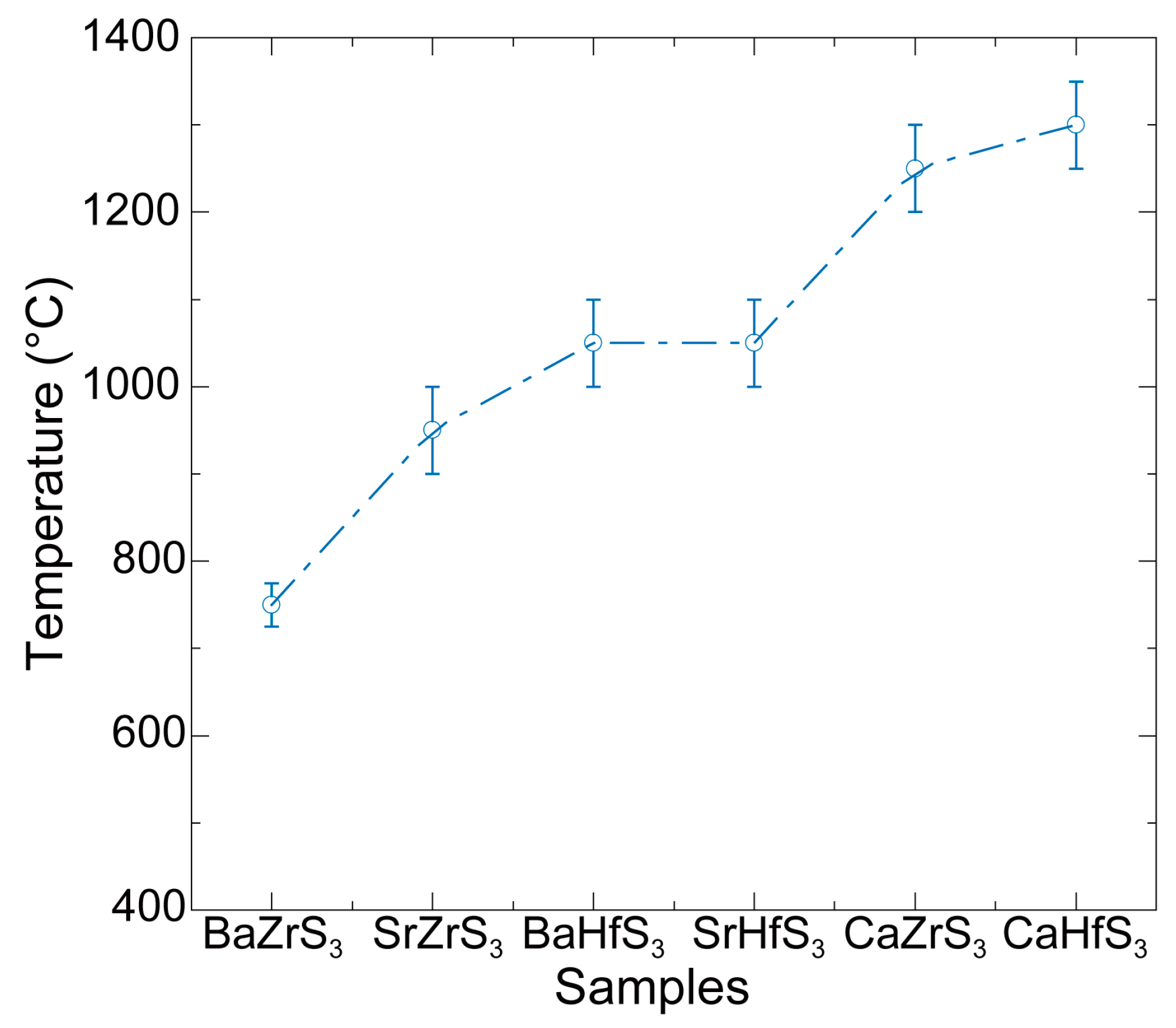
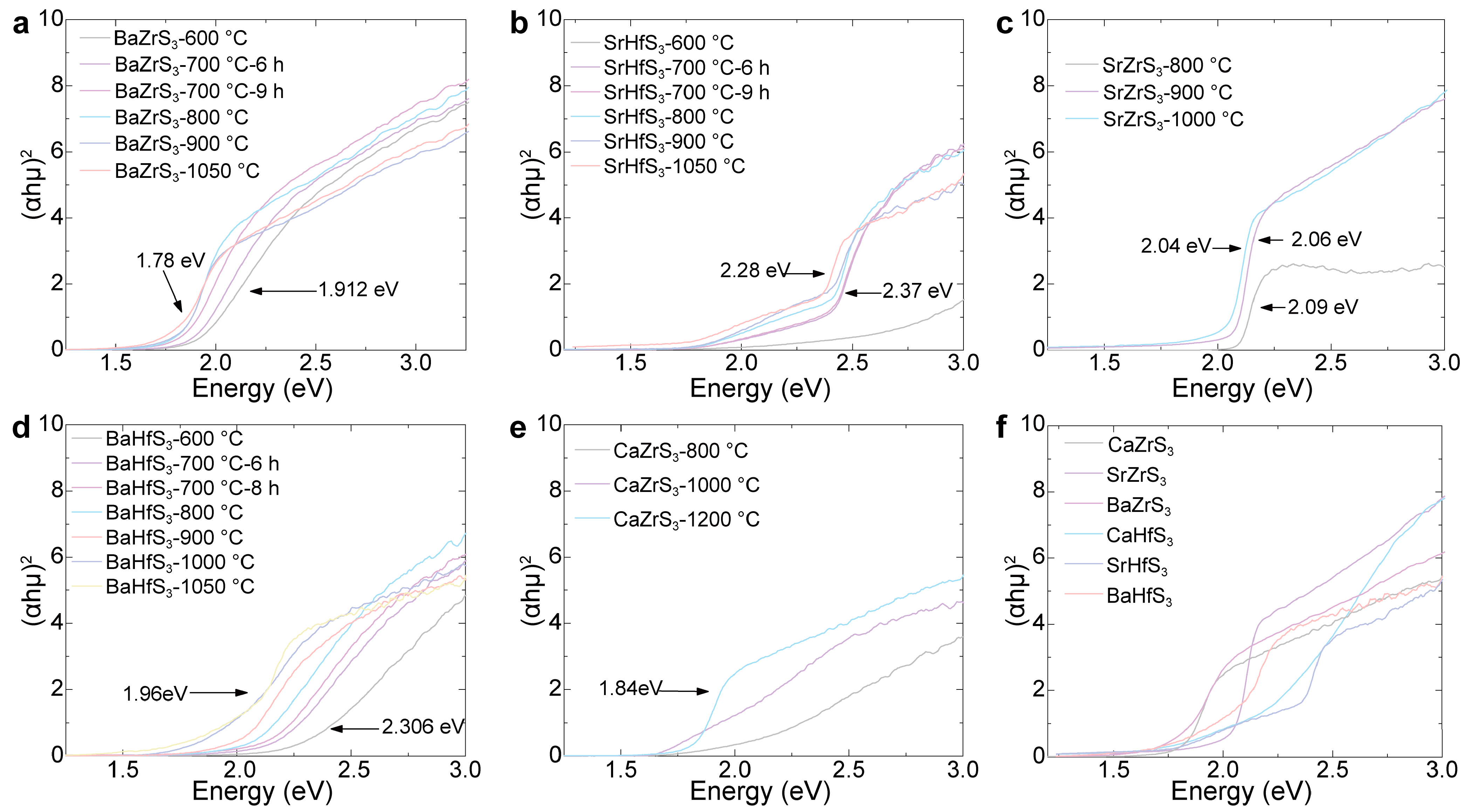
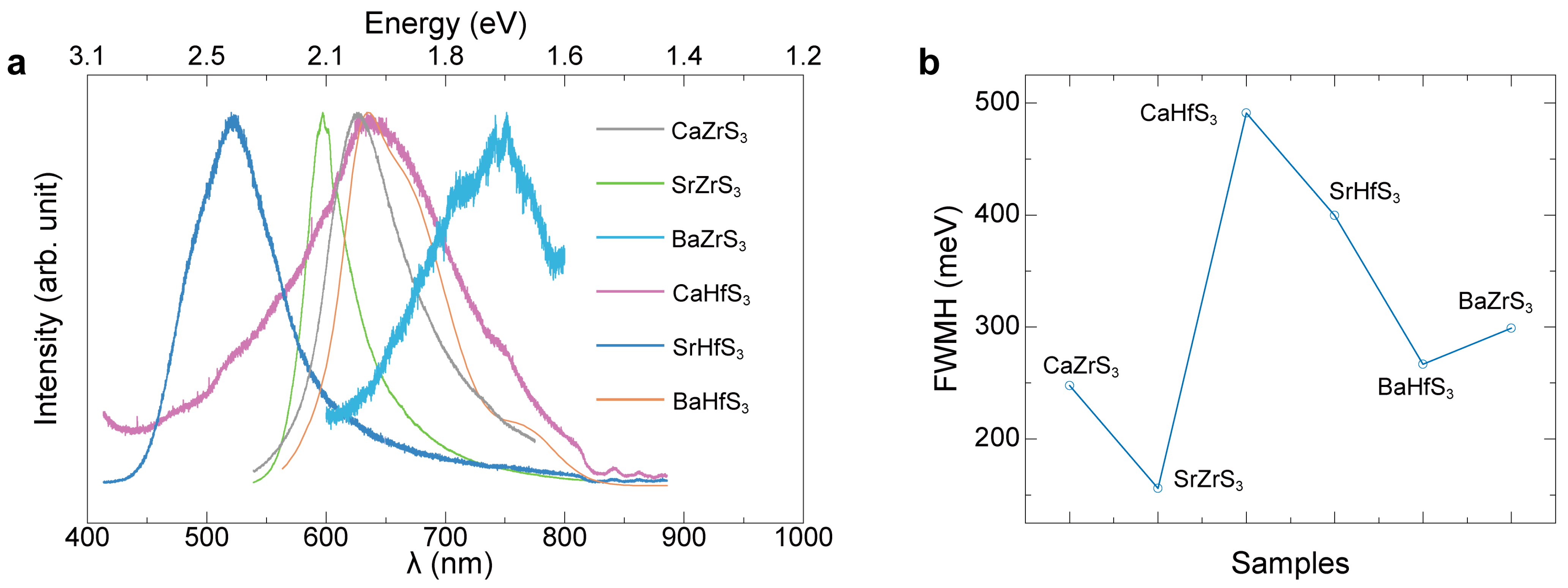
| Samples | Bandgap (eV) | PL Peak (eV) |
|---|---|---|
| BaZrS3 | 1.78 | 1.79 |
| CaZrS3 | 1.84 | 1.98 |
| BaHfS3 | 1.96 | 1.95 |
| SrZrS3 | 2.04 | 2.08 |
| CaHfS3 | 2.13 | 1.93 |
| SrHfS3 | 2.28 | 2.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Yang, L.; Kong, S.; Hui, H.; Samson, L.; Guo, K.; Bian, B.; Chen, K.; Yu, Z. Impact of Sulfurization Temperature on the Formation and Properties of Chalcogenide Perovskites. Molecules 2025, 30, 1198. https://doi.org/10.3390/molecules30061198
Zhao P, Yang L, Kong S, Hui H, Samson L, Guo K, Bian B, Chen K, Yu Z. Impact of Sulfurization Temperature on the Formation and Properties of Chalcogenide Perovskites. Molecules. 2025; 30(6):1198. https://doi.org/10.3390/molecules30061198
Chicago/Turabian StyleZhao, Pengnan, Lihuan Yang, Sen Kong, Haolei Hui, Lauren Samson, Kaiwei Guo, Bingyue Bian, Kaiyun Chen, and Zhonghai Yu. 2025. "Impact of Sulfurization Temperature on the Formation and Properties of Chalcogenide Perovskites" Molecules 30, no. 6: 1198. https://doi.org/10.3390/molecules30061198
APA StyleZhao, P., Yang, L., Kong, S., Hui, H., Samson, L., Guo, K., Bian, B., Chen, K., & Yu, Z. (2025). Impact of Sulfurization Temperature on the Formation and Properties of Chalcogenide Perovskites. Molecules, 30(6), 1198. https://doi.org/10.3390/molecules30061198






