An In Vitro Study of the Anti-Acne Effects of Scutellaria barbata
Abstract
:1. Introduction
2. Results
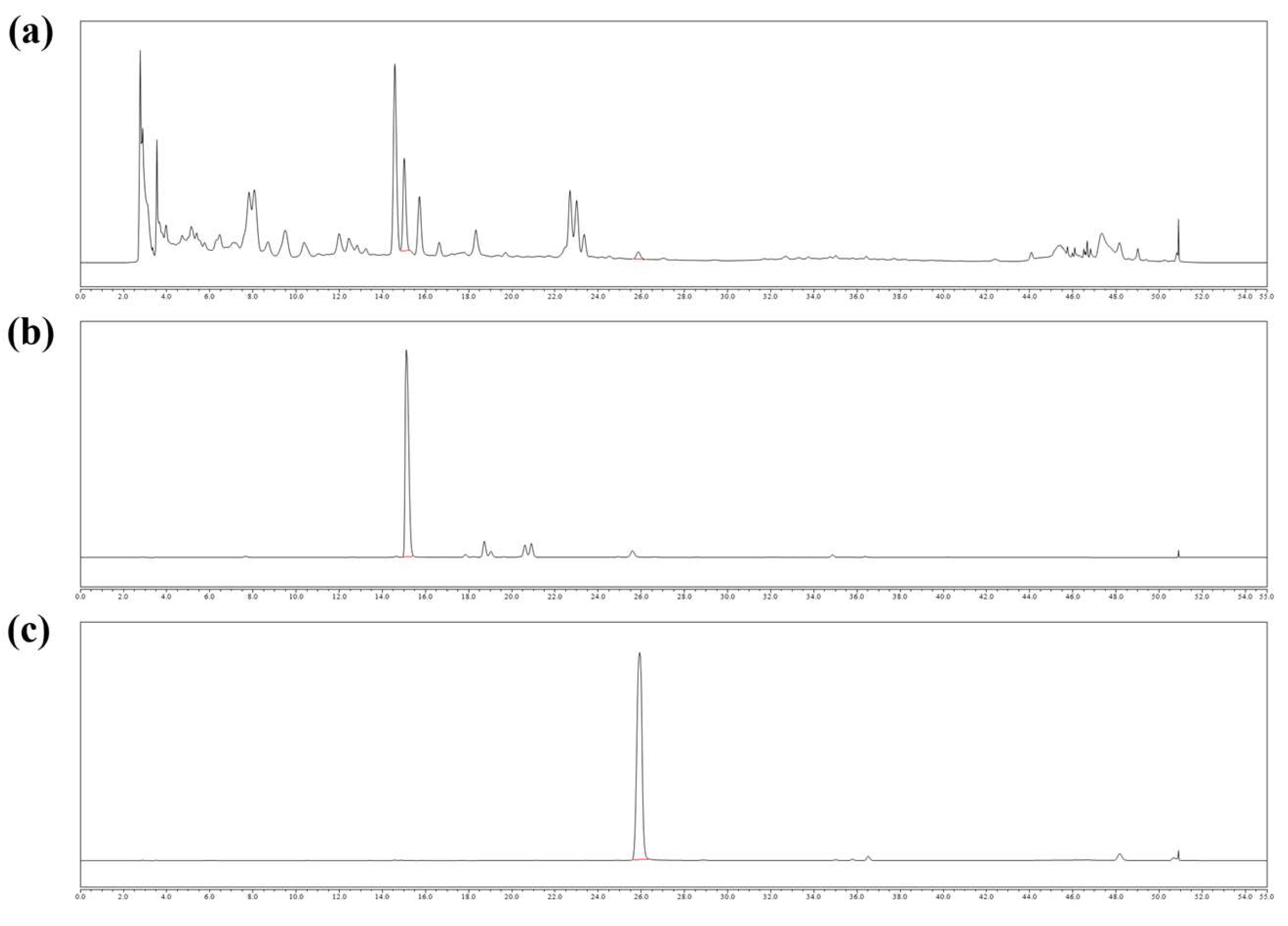
2.1. Chemical Contents of SLB
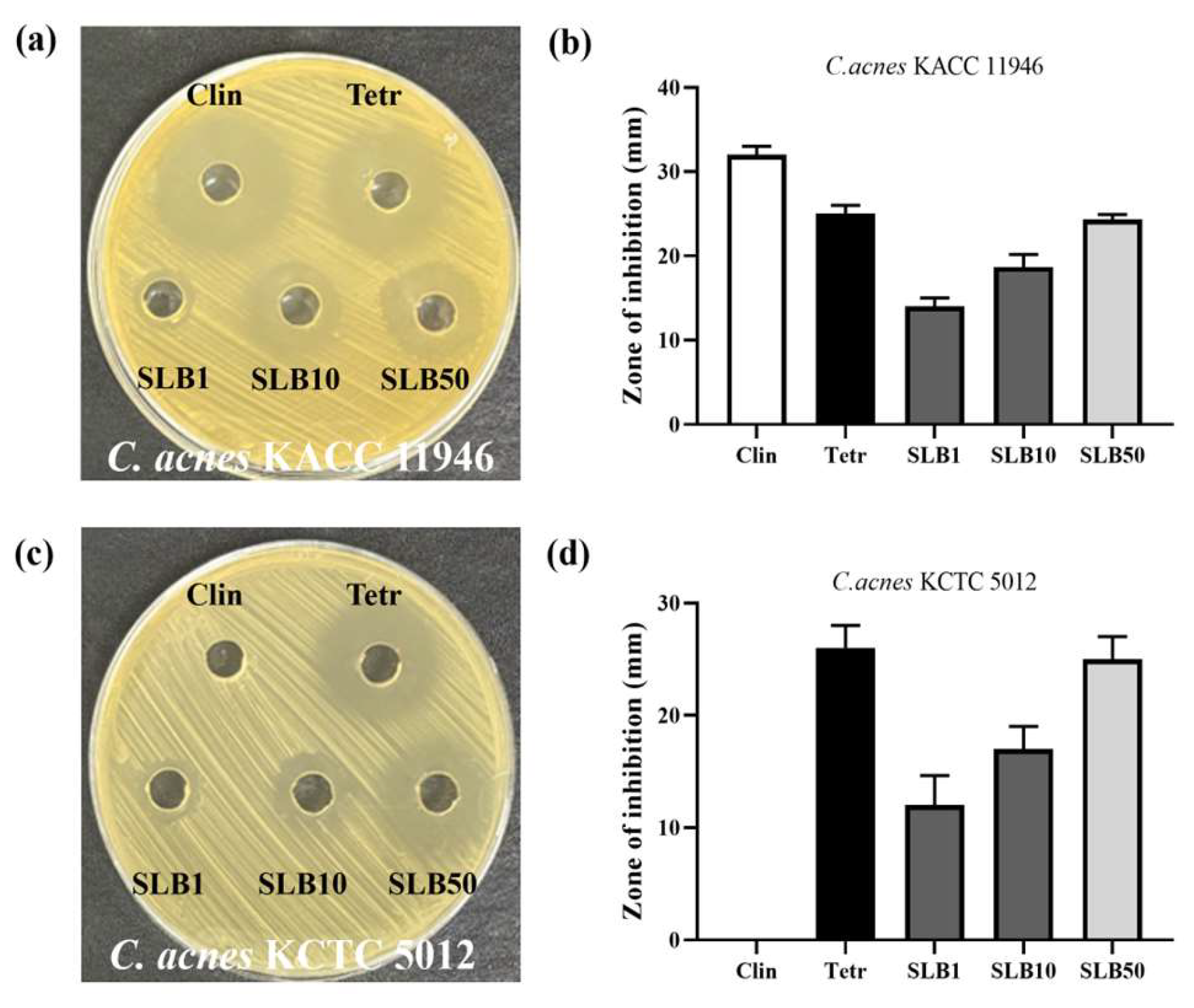
2.2. Antimicrobial Activity
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
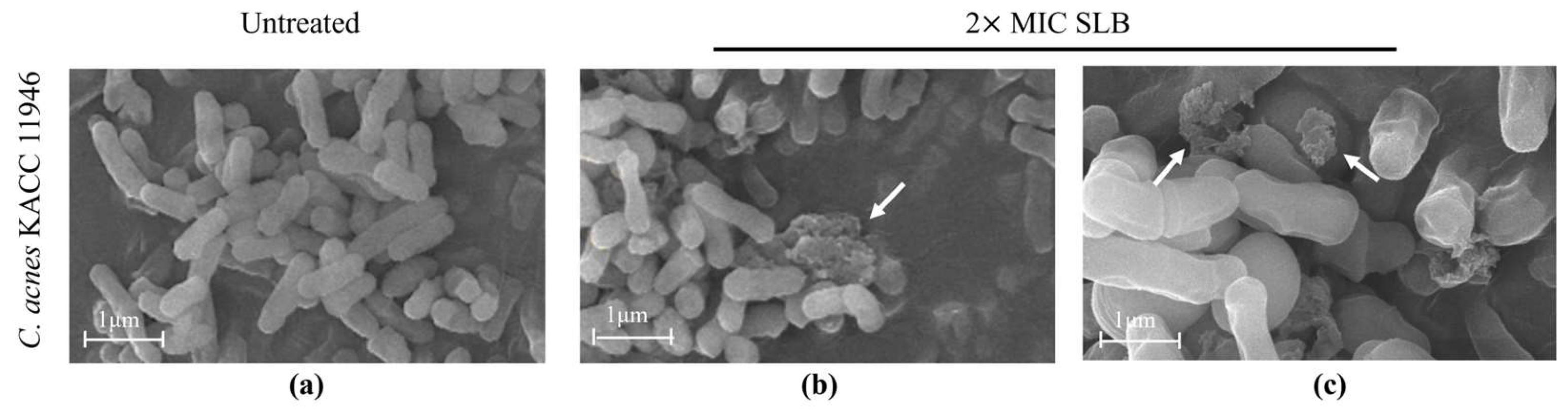
2.4. Morphological Alterations in C. acnes
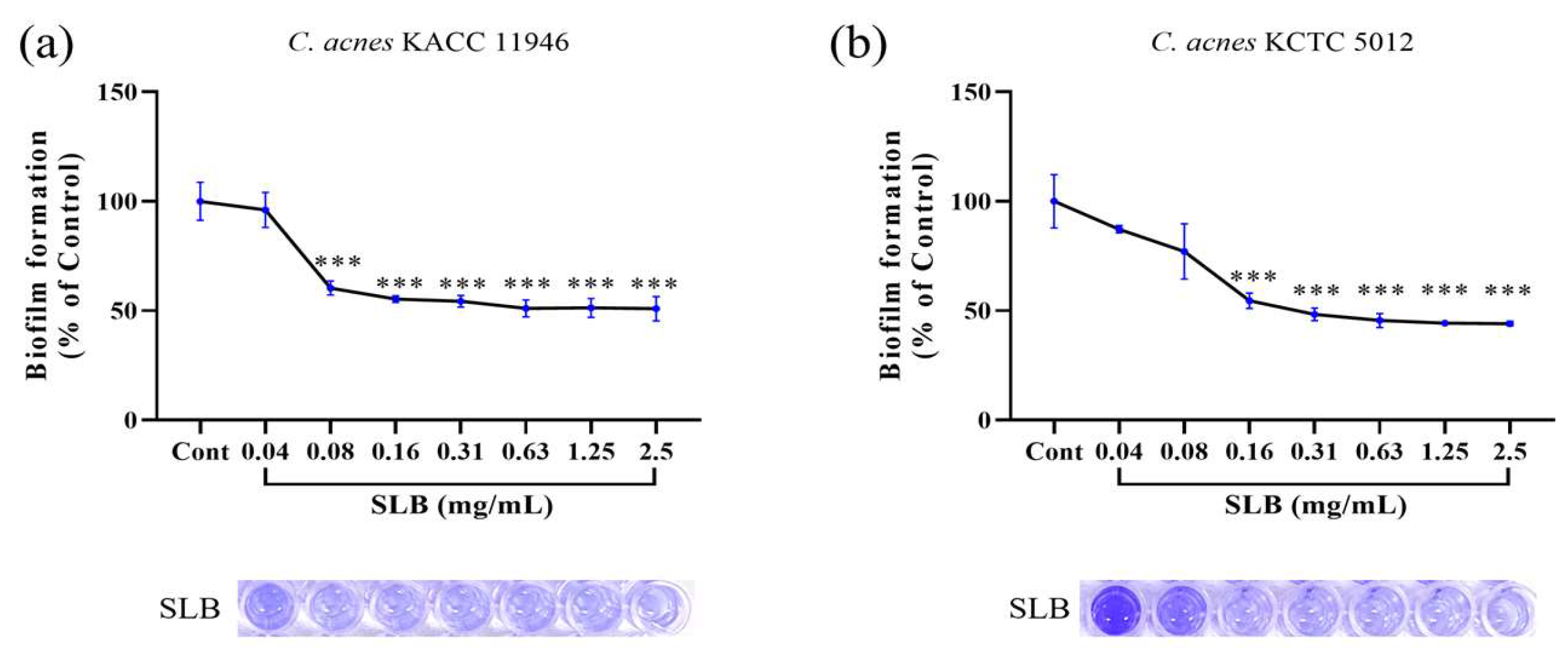
2.5. Inhibition of Biofilm Formation
2.6. Inhibitory Activity of Nitric Oxide (NO) Production
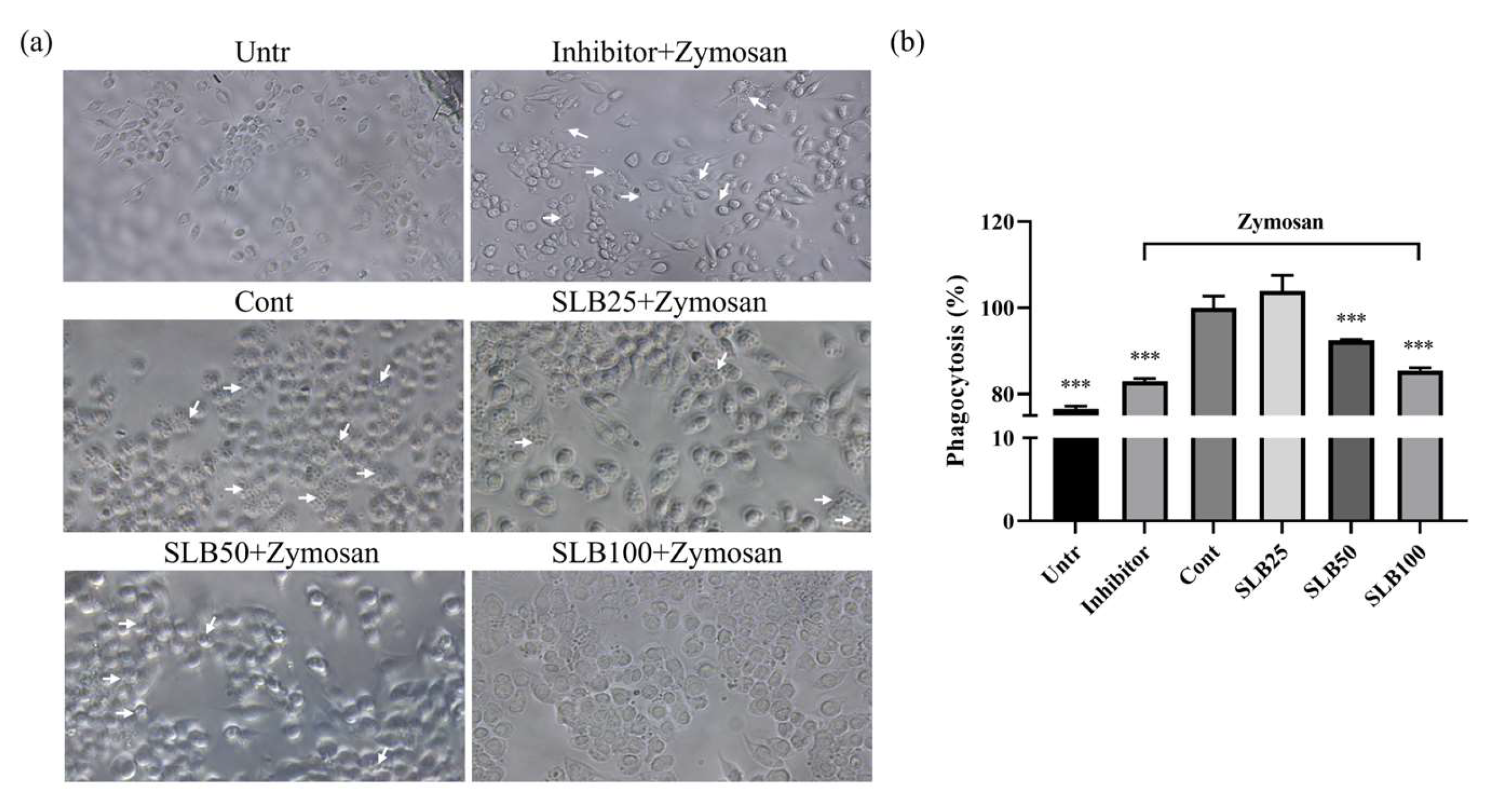
2.7. Effects of SLB on Phagocytosis
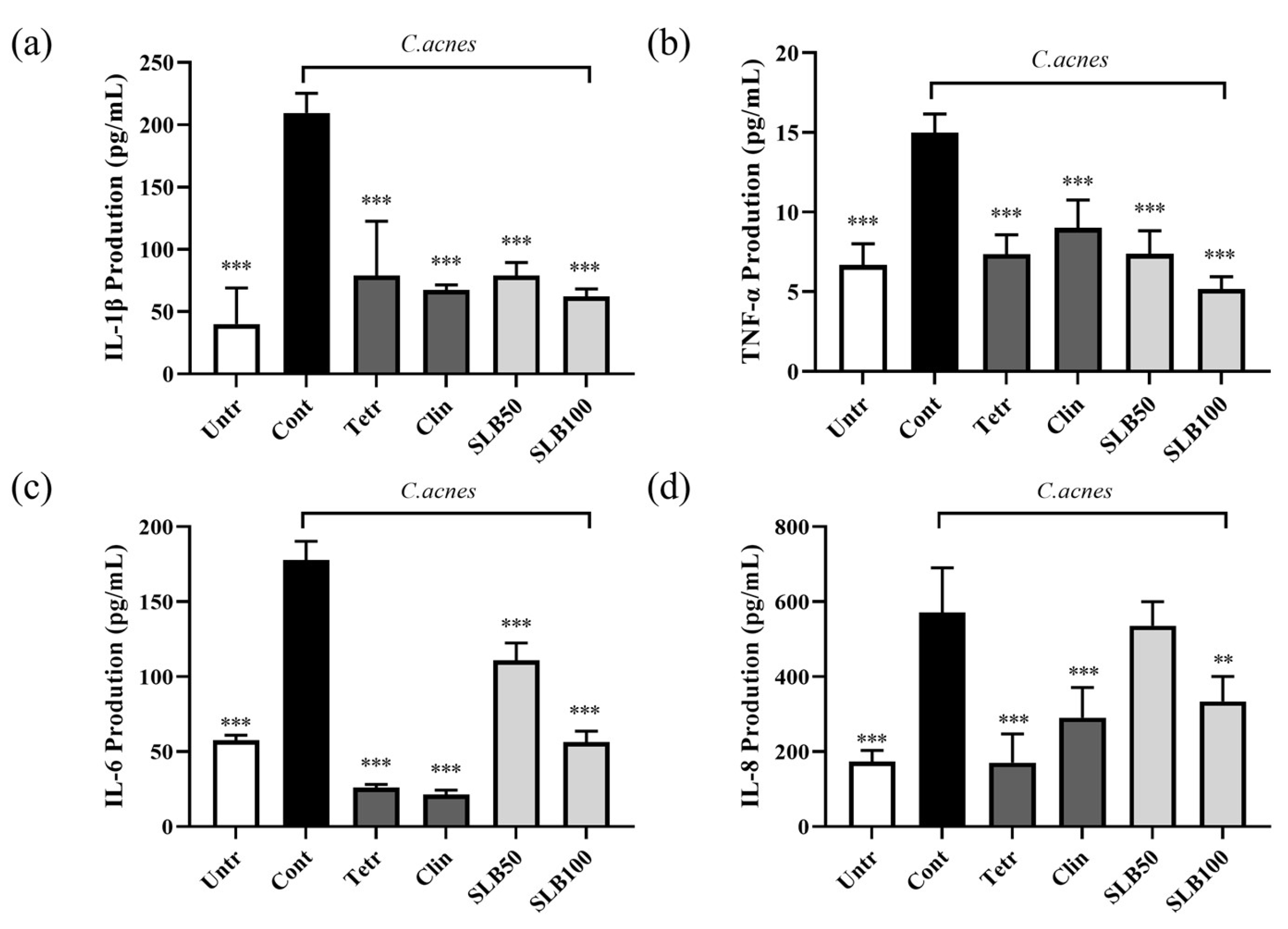
2.8. Effect of SLB on Cytokine Production in HaCaT Cells Treated with Heat-Inactivated C. acnes
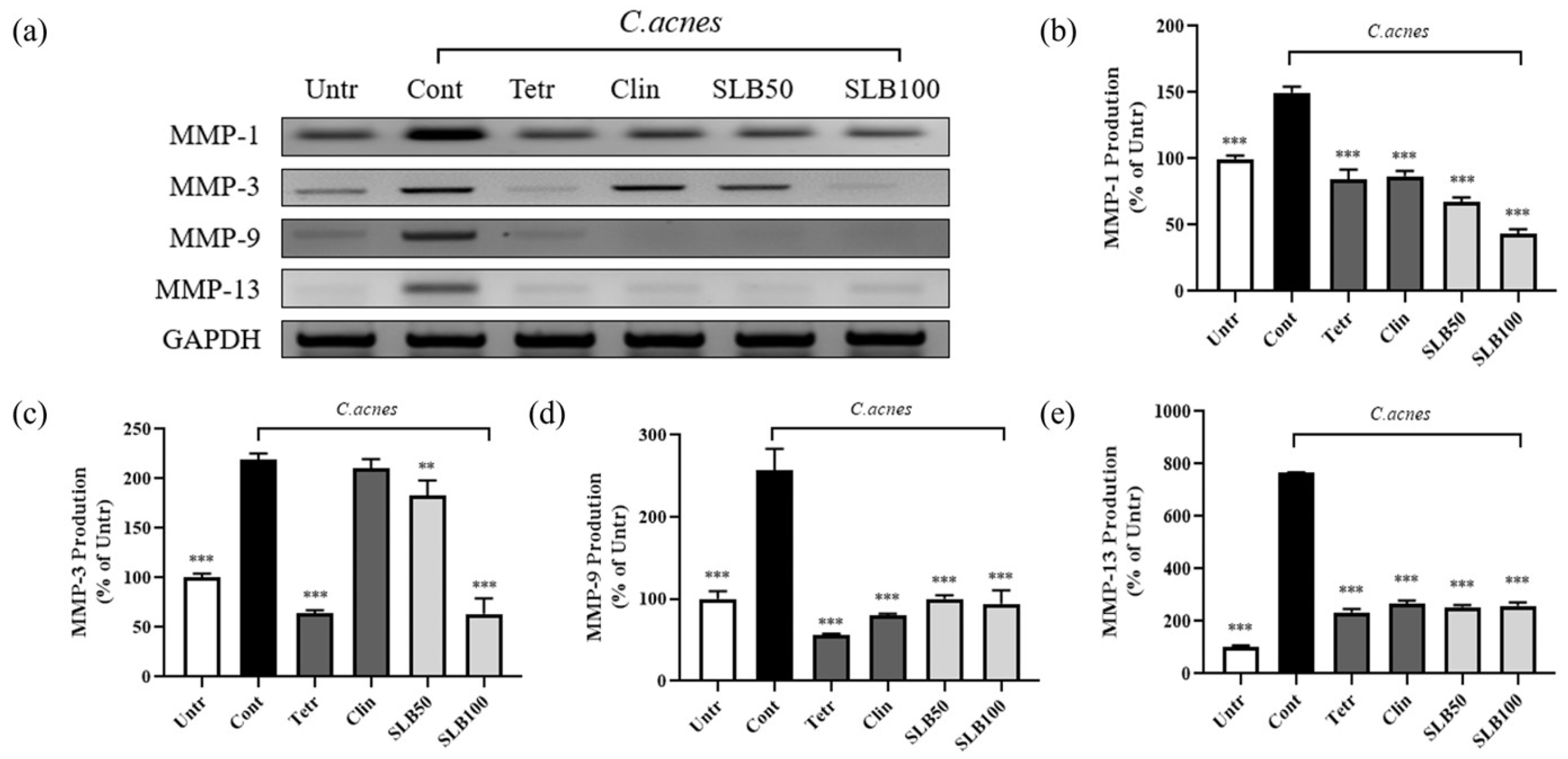
2.9. Effect of SLB on MMPs Gene Expression in C. acnes-Treated HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. SLB Extractions
4.2. HPLC
4.3. Well Diffusion Assay
4.4. MIC and MBC Assay
4.5. Scanning Electron Microscopy
4.6. Biofilm Formation
4.7. Cell Culture
4.8. NO Assay
4.9. Cytotoxicity
4.10. Phagocytosis Assay
4.11. Enzyme-Linked Immunosorbent Assay
4.12. RT-PCR
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, S.C.; Cook-Bolden, F.; Rahman, Z.; Strachan, D. Acne Vulgaris in Skin of Color. J. Am. Acad. Dermatol. 2002, 46, S98–S106. [Google Scholar] [CrossRef] [PubMed]
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R.P. Acne Vulgaris: Pathogenesis, Treatment, and Needs Assessment. Dermatol. Clin. 2012, 30, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xue, Y.; Chen, Y.; Chen, T.; Zhong, J.; Shao, X.; Chen, J. Acne and Risk of Mental Disorders: A Two-Sample Mendelian Randomization Study Based on Large Genome-Wide Association Data. Front. Public Health 2023, 11, 1156522. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, X.; Luo, Z.; Liu, M.; Zhang, H.; Zheng, H.; Li, J. Global, Regional and National Burdens of Acne Vulgaris in Adolescents and Young Adults Aged 10–24 Years from 1990 to 2021: A Trend Analysis. Br. J. Dermatol. 2024, ljae352. [Google Scholar] [CrossRef]
- Mohiuddin, A. A Comprehensive Review of Acne Vulgaris. CRDOA 2019, 6, 1–34. [Google Scholar] [CrossRef]
- Jin, Z.; Song, Y.; He, L. A Review of Skin Immune Processes in Acne. Front. Immunol. 2023, 14, 1324930. [Google Scholar] [CrossRef]
- Jeremy, A.H.T.; Holland, D.B.; Roberts, S.G.; Thomson, K.F.; Cunliffe, W.J. Inflammatory Events Are Involved in Acne Lesion Initiation. J. Investig. Dermatol. 2003, 121, 20–27. [Google Scholar] [CrossRef]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and Extracellular Matrix Degradation Mediated by Activated Transcription Factors Nuclear Factor-κB and Activator Protein-1 in Inflammatory Acne Lesions in Vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm Formation by Propionibacterium acnes Is Associated with Increased Resistance to Antimicrobial Agents and Increased Production of Putative Virulence Factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef]
- Coenye, T.; Spittaels, K.-J.; Achermann, Y. The Role of Biofilm Formation in the Pathogenesis and Antimicrobial Susceptibility of Cutibacterium acnes. Biofilm 2022, 4, 100063. [Google Scholar] [CrossRef]
- Leyden, J.J.; McGinley, K.J.; Cavalieri, S.; Webster, G.F.; Mills, O.H.; Kligman, A.M. Propionibacterium acnes Resistance to Antibiotics in Acne Patients. J. Am. Acad. Dermatol. 1983, 8, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Soriano, E.A.; Azevedo, P.S.; Miot, H.A.; Minicucci, M.F.; Pansani, M.C.; Matsubara, L.S.; Okoshi, K.; Zornoff, L.A.M.; Matsubara, B.B.; Paiva, S.A.R. Cardiac Remodeling Induced by 13-Cis Retinoic Acid Treatment in Acne Patients. Int. J. Cardiol. 2013, 163, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Yoon, J.Y.; Park, S.Y.; Min, S.; Suh, D.H. Comparison of Clinical and Histological Effects between Lactobacillus-Fermented Chamaecyparis Obtusa and Tea Tree Oil for the Treatment of Acne: An Eight-Week Double-Blind Randomized Controlled Split-Face Study. Dermatology 2014, 229, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhou, J.; Jie, C.; Xing, D.; Zhang, Y. Anticancer Activity and Mechanism of Scutellaria barbata Extract on Human Lung Cancer Cell Line A549. Life Sci. 2004, 75, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Suzaki, S.; Nishikawa, T.; Kihara, M.; Shibata, H.; Higuti, T. Phytochemical Flavones Isolated from Scutellaria barbata and Antibacterial Activity against Methicillin-Resistant Staphylococcus Aureus. J. Ethnopharmacol. 2000, 72, 483–488. [Google Scholar] [CrossRef]
- Liu, H.-L.; Kao, T.-H.; Shiau, C.-Y.; Chen, B.-H. Functional Components in Scutellaria barbata D. Don with Anti-Inflammatory Activity on RAW 264.7 Cells. J. Food Drug Anal. 2018, 26, 31–40. [Google Scholar] [CrossRef]
- Man, M.-Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Regulatory Role of Nitric Oxide in Cutaneous Inflammation. Inflammation 2022, 45, 949–964. [Google Scholar] [CrossRef]
- Möller, B.; Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-α in Immune-Mediated Inflammatory Diseases. Springer Semin. Immunopathol. 2006, 27, 391–408. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential Involvement of Interleukin-8 (IL-8) in Acute Inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, J.-M.; Jeong, S.K.; Jeon, J.E.; Yoon, H.-J.; Jeong, M.-K.; Lee, S.H. Protease-Activated Receptor-2 Mediates the Expression of Inflammatory Cytokines, Antimicrobial Peptides, and Matrix Metalloproteinases in Keratinocytes in Response to Propionibacterium acnes. Arch. Dermatol. Res. 2010, 302, 745–756. [Google Scholar] [CrossRef]
- Bhattacharjee, O.; Ayyangar, U.; Kurbet, A.S.; Ashok, D.; Raghavan, S. Unraveling the ECM-Immune Cell Crosstalk in Skin Diseases. Front. Cell Dev. Biol. 2019, 7, 68. [Google Scholar] [CrossRef]
- Gupta, P.D.; Birdi, T.J. Development of Botanicals to Combat Antibiotic Resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Baldwin, H.; Tan, J. Effects of Diet on Acne and Its Response to Treatment. Am. J. Clin. Dermatol. 2021, 22, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Alhatlani, B.Y.; De Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Cobian, N.; Garlet, A.; Hidalgo-Cantabrana, C.; Barrangou, R. Comparative Genomic Analyses and CRISPR-Cas Characterization of Cutibacterium acnes Provide Insights Into Genetic Diversity and Typing Applications. Front. Microbiol. 2021, 12, 758749. [Google Scholar] [CrossRef] [PubMed]
- Platsidaki, E.; Dessinioti, C. Recent Advances in Understanding Propionibacterium Acnes (Cutibacterium acnes) in Acne. F1000Res 2018, 7, 1953. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and Acne Vulgaris: A Brief Look at the Latest Updates. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Lee, J. Antibiofilm Activities of Fatty Acids Including Myristoleic Acid against Cutibacterium acnes via Reduced Cell Hydrophobicity. Phytomedicine 2021, 91, 153710. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Kircik, L. The Primary Role of Sebum in the Pathophysiology of Acne Vulgaris and Its Therapeutic Relevance in Acne Management. J. Dermatol. Treat. 2024, 35, 2296855. [Google Scholar] [CrossRef]
- Selway, J.L.; Kurczab, T.; Kealey, T.; Langlands, K. Toll-like Receptor 2 Activation and Comedogenesis: Implications for the Pathogenesis of Acne. BMC Dermatol. 2013, 13, 10. [Google Scholar] [CrossRef]
- Shibata, M.; Katsuyama, M.; Onodera, T.; Ehama, R.; Hosoi, J.; Tagami, H. Glucocorticoids Enhance Toll-Like Receptor 2 Expression in Human Keratinocytes Stimulated with Propionibacterium acnes or Proinflammatory Cytokines. J. Investig. Dermatol. 2009, 129, 375–382. [Google Scholar] [CrossRef]
- Thiboutot, D.; Del Rosso, J.Q. Acne Vulgaris and the Epidermal Barrier: Is Acne Vulgaris Associated with Inherent Epidermal Abnormalities That Cause Impairment of Barrier Functions? Do Any Topical Acne Therapies Alter the Structural and/or Functional Integrity of the Epidermal Barrier? J. Clin. Aesthet. Dermatol. 2013, 6, 18–24. [Google Scholar]
- Xue, M.; Jackson, C.J. Novel Functions of the Anticoagulant Activated Protein C in Maintaining Skin Barrier Integrity to Impact on Skin Disease. Pathobiology 2015, 82, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Aletras, A.J.; Glass, E.; Tsogas, P.; Dionyssopoulos, A.; Adjaye, J.; Fimmel, S.; Gouvousis, P.; Herwig, R.; Lehrach, H.; et al. Matrix Metalloproteinases of Epithelial Origin in Facial Sebum of Patients with Acne and Their Regulation by Isotretinoin. J. Investig. Dermatol. 2005, 125, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Ray Jalian, H.; Liu, P.T.; Kanchanapoomi, M.; Phan, J.N.; Legaspi, A.J.; Kim, J. All-Trans Retinoic Acid Shifts Propionibacterium acnes-Induced Matrix Degradation Expression Profile toward Matrix Preservation in Human Monocytes. J. Investig. Dermatol. 2008, 128, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Kähäri, V.; Saarialho-Kere, U. Matrix Metalloproteinases in Skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix Remodeling by MMPs during Wound Repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The Role of Matrix Metalloproteinases in Aging: Tissue Remodeling and Beyond. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Toriseva, M.; Kähäri, V.-M. Proteinases in Cutaneous Wound Healing. Cell. Mol. Life Sci. 2009, 66, 203–224. [Google Scholar] [CrossRef]

| Strain | MIC (mg/mL) | |
|---|---|---|
| SLB | C. acnes KACC 11946 | C. acnes KCTC 5012 |
| 0.04 | 0.08 | |
| MBC (mg/mL) | ||
| C. acnes KACC 11946 | C. acnes KCTC 5012 | |
| 0.16 | 0.16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Jin, X.; Nguyen, T.T.M.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. An In Vitro Study of the Anti-Acne Effects of Scutellaria barbata. Molecules 2025, 30, 515. https://doi.org/10.3390/molecules30030515
Zheng Q, Jin X, Nguyen TTM, Park S-J, Yi G-S, Yang S-J, Yi T-H. An In Vitro Study of the Anti-Acne Effects of Scutellaria barbata. Molecules. 2025; 30(3):515. https://doi.org/10.3390/molecules30030515
Chicago/Turabian StyleZheng, Qiwen, Xiangji Jin, Trang Thi Minh Nguyen, Se-Jig Park, Gyeong-Seon Yi, Su-Jin Yang, and Tae-Hoo Yi. 2025. "An In Vitro Study of the Anti-Acne Effects of Scutellaria barbata" Molecules 30, no. 3: 515. https://doi.org/10.3390/molecules30030515
APA StyleZheng, Q., Jin, X., Nguyen, T. T. M., Park, S.-J., Yi, G.-S., Yang, S.-J., & Yi, T.-H. (2025). An In Vitro Study of the Anti-Acne Effects of Scutellaria barbata. Molecules, 30(3), 515. https://doi.org/10.3390/molecules30030515










