Flavanols from Nature: A Phytochemistry and Biological Activity Review
Abstract
:1. Introduction
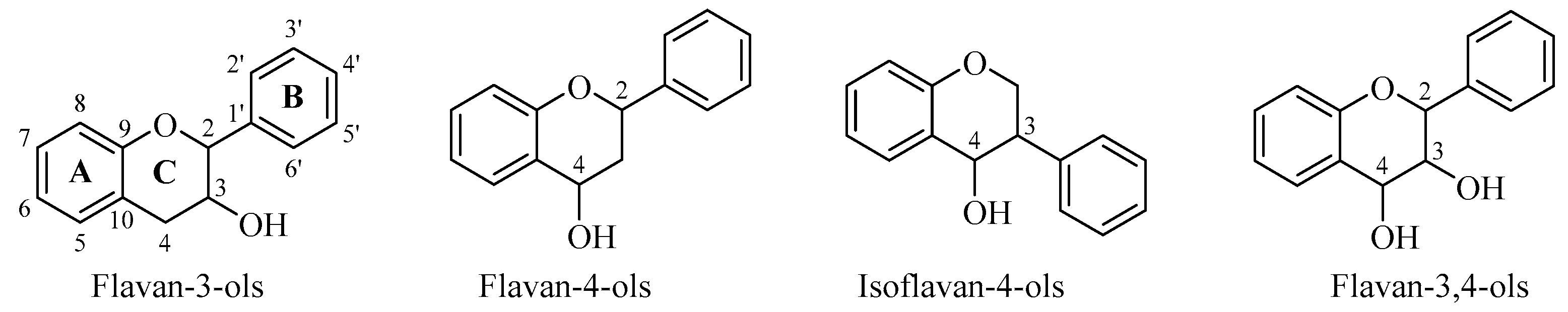
2. Flavan-3-ols
2.1. Simple Flavan-3-ols
2.2. Alkaloid Flavan-3-ols
2.3. Oligomeric Flavan-3-ols
3. Flavan-4-ols
4. Isoflavan-4-ols
5. Flavan-3,4-ols
5.1. Simple Flavan-3,4-ols
5.2. Oligomeric Flavan-3,4-ols
6. Pharmacological Activities
6.1. Anti-Oxidation
6.2. Anti-Inflammation
6.3. Anti-Cancer
6.4. Anti-Viral
6.5. Cardiovascular Protection
6.6. Vascular Protection
6.7. Miscellaneous Activities
6.8. Metabolism and Pharmacokinetics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Knaze, V.; Zamora, R.R.; Lujan, B.L. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2012, 108, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Jian, Y.; Yi, P.; Sun, J.; Wang, W. A comprehensive review on Pronephrium penangianum. Isr. J. Chem. 2019, 59, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Diana, J.; O’Leary, M.; Philip, W.; Lindberg, M.B.; Semple, S.J. Phytochemistry and bioactivity of Acacia sensu stricto (Fabaceae: Mimosoideae). Phytochem. Rev. 2018, 18, 129–172. [Google Scholar]
- Cho, J.Y.; Moon, J.H.; Eun, J.B.; Chung, S.J.; Park, K.H. Isolation and characterization of 3(Z)-dodecenedioic acid as an antibacterial substance from Hovenia dulcis thunb. Food Sci. Biotechnol. 2004, 13, 46–50. [Google Scholar]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2010, 52, 79–104. [Google Scholar] [CrossRef]
- Berends, L.M.; Velpen, V.D.; Vera, C.A. Flavan-3-ols, theobromine, and the effects of cocoa and chocolate on cardiometabolic risk factors. Curr. Opin. Lipidol. 2015, 26, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tai, B.H.; Trung, T.N.; Nhiem, N.X.; Ha, D.T.; Van Men, C.; Duong, V.B.; Van Luong, H.; Song, S.; Bae, K.; Kim, Y.H. A new flavan-3-ol and the anti-inflammatory effect of flavonoids from the fruit peels of Wisteria floribunda. J. Asian Nat. Prod. Res. 2011, 13, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Ryoyasu, S.; Katoh, M. A history of catechin chemistry with special reference to tea leaves. Chagyo Kenkyu Hokoku 2009, 2009, 1–18. [Google Scholar] [CrossRef]
- Li, G.; Min, B.S.; Zheng, C.; Lee, J.; Oh, S.R.; Ahn, K.S.; Lee, H.K. Neuroprotective and free radical scavenging activities of phenolic compounds from Hovenia dulcis. Arch. Pharmacal Res. 2005, 28, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.J.; Zhang, Y.W.; Jiang, H.Y.; Bao, Y.L.; Li, Y.X. Chemical constituents from the roots of Zizyphus jujuba Mill. var. spinosa. Biochem. Syst. Ecol. 2013, 50, 182–186. [Google Scholar] [CrossRef]
- Zeng, X.; Qiu, Q.; Jiang, C.; Jing, Y.; Qiu, G.; He, X. Antioxidant flavanes from Livistona chinensis. Fitoterapia 2011, 82, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Padumadasa, C.; Abeysekara, A.; Thabrew, I.; Ediriweera, A. Pharmacological overview of proanthocyanidins from the bark of Thespesia populnea (L.) as an antioxidant and cytotoxic agent. Int. J. Pharm. Sci. Res. 2016, 7, 85–92. [Google Scholar]
- Iida, N.; Inatomi, Y.; Murata, H.; Inada, A.; Nakanishi, T. A new flavone xyloside and two new flavan-3-ol glucosides from Juniperus communis var. depressa. Chem. Biodivers. 2007, 4, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Sautour, M.; Miyamoto, T.; Lacaille-Dubois, M.A. Steroidal saponins and flavan-3-ol glycosides from Dioscorea villosa. Biochem. Syst. Ecol. 2006, 34, 60–63. [Google Scholar] [CrossRef]
- Hori, K.; Wada, M.; Yahara, S.; Watanabe, T.; Devkota, H.P. Antioxidant phenolic compounds from the rhizomes of Astilbe rivularis. Nat. Prod. Res. 2018, 32, 453–456. [Google Scholar] [CrossRef]
- Brookes, K.B.; Katsoulis, L.C. Bioactive components of Rhoicissus tridentate: A pregnancy-related traditional medicine. S. Afr. J. Sci. 2006, 102, 267–272. [Google Scholar]
- Nguyen, V.B.; Wang, S.L.; Nguyen, A.D.; Vo, T.; Zhang, L.J.; Nguyen, Q.V.; Kuo, Y.H. Isolation and identification of novel α-amylase inhibitors from Euonymus laxiflorus Champ. Res. Chem. Intermed. 2018, 44, 1411–1424. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, S.; Hu, Z.; Chen, L.; Wan, C. Anti-Inflammatory constituents from Chaenomeles speciosa. Nat. Prod. Commun. 2020, 15, 1–5. [Google Scholar] [CrossRef]
- Hefeng, P.; Lundgren, N. Phenolic extractives from root bark of Picea abies. Phytochemistry 1995, 39, 1423–1428. [Google Scholar]
- Yang, C.S.; Huang, H.C.; Wang, S.Y.; Sung, P.J.; Huang, G.J.; Chen, J.J.; Kuo, Y.H. New diphenol and isocoumarins from the aerial part of Lawsonia inermis and their inhibitory activities against NO production. Molecules 2016, 21, 1299. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Misra, L.; Tewari, R.; Gupta, P.; Mishra, P.; Shukla, R. Anti-inflammatory flavanol glycosides from Saraca asoca bark. Nat. Prod. Res. 2016, 30, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Pinto, F.; Sousa, A.D.O.; Santiago, G. Chemical constituents and acetylcholinesterase inhibitory activity from the stems of Bauhinia pentandra. Nat. Prod. Res. 2020, 35, 5277–5281. [Google Scholar] [CrossRef]
- Kijjoa, A.; Giesbrecht, A.M.; Gottlieb, O.R.; Gottlieb, H.E. 1,3-diaryl-propanes and propan-2-ols from Virola species. Phytochemistry 1981, 20, 1385–1388. [Google Scholar] [CrossRef]
- Roux, D.G.; Paulus, E. Isolation of (−)-7:3′:4′-trihydroxyflavan-3-ol [(−)-fisetinidol], a naturally occurring catechin from black-wattle heartwood. Biochem. J. 1961, 78, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guang, M.F.U.; Fan, L.H.; Shi-Shan, Y.U.; De-Quan, Y.U. Flavonoids from Lysidice rhodostegia Hance. J. Integr. Plant Biol. 2005, 47, 759–763. [Google Scholar] [CrossRef]
- Areej, M.A.; Shagufta, P.; Ghada, A.F.; Saleh, I.A.; Kamal, E. New flavane gallates isolated from the leaves of Plicosepalus curviflorus and their hypoglycemic activity. Fitoterapia 2012, 83, 1610–1615. [Google Scholar]
- Jin, Y.J.; Lin, C.C.; Lu, T.M.; Li, J.H.; Chen, I.S.; Kuo, Y.H.; Ko, H.H. Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis. Phytochemistry 2015, 117, 424–435. [Google Scholar] [CrossRef]
- Luan, N.Q.; Phung, N.; Khanh, V.D.; Tri, M.D.; Hanh, N.N. Novel naphthalene glycoside derivative from leaves of Cassia grandis L. Can Tho Univ. J. Sci. 2015, 31, 1733–1738. [Google Scholar]
- Kpegba, K.; Agbonon, A.; Petrovic, A.G.; Amouzou, E.; Gbeassor, M.; Proni, G.; Nesnas, N. Epiafzelechin from the root bark of Cassia sieberiana: Detection by DART mass spectrometry, spectroscopic characterization, and antioxidant properties. J. Nat. Prod. 2011, 74, 455–459. [Google Scholar] [CrossRef]
- Imtiyaz, Z.; Wang, Y.F.; Lin, Y.T.; Liu, H.K.; Lee, M.H. Isolated compounds from Turpinia formosana Nakai induce ossification. Int. J. Mol. Sci. 2019, 20, 3119. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.L.; Hu, X.; He, H.L.; Qiu, L.; Li, Y.Z.; Ding, W.B. A new epicatechin glucopyranoside derivative from Styrax suberifolius. Nat. Prod. Res. 2020, 34, 1977–1983. [Google Scholar] [CrossRef]
- Demeule, M.J.M.L.; Annabi, B.; Gingras, D.; Boivin, D.; Jodoin, J.; Lamy, S.; Bertrand, Y.; Béliveau, R. Green tea catechins as novel antitumor and antiangiogenic compounds. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Deachathai, S.; Mahabusarakam, W.; Phongpaichit, S.; Taylor, W.C. Phenolic compounds from the fruit of Garcinia dulcis. Phytochemistry 2005, 66, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Lee, G.Y.; Lee, Y.M.; Kim, Y.S.; Sun, H.; Kim, D.H.; Kim, J.S. Flavan-3-ols having a gamma-lactam from the roots of Actinidia arguta inhibit the formation of advanced glycation end products in vitro. Cheminform 2010, 40, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Zhu, H.T.; Wang, D.; Zhang, M.; Zhang, Y.J. New flavoalkaloids with potent α-Glucosidase and acetylcholinesterase inhibitory activities from Yunnan black tea ‘Jin-Ya’. J. Agric. Food Chem. 2020, 68, 7955–7963. [Google Scholar] [CrossRef]
- Homberger, K.; Hesse, M. Kopsirachin, ein ungewhnliches alkaloid aus der Apocynaceae Kopsia dasyrachis Ridl. 189. Mitteilung über organische Naturstoffe. Helv. Chim. Acta 1984, 67, 237–248. [Google Scholar] [CrossRef]
- Blair, L.M.; Calvert, M.B.; Sperry, J. Flavoalkaloids—Isolation, biological activity, and total synthesis. Alkaloids Chem. Biol. 2017, 77, 85–115. [Google Scholar] [CrossRef]
- Tripetch, K.; Ryoji, K.; Phannipha, C.; Krisana, K. Lotthanongine, an unprecedented flavonoidal indole alkaloid from the roots of Thai medicinal plant, Trigonostemon reidioides. Tetrahedron Lett. 2002, 43, 2941–2943. [Google Scholar]
- Tanaka, T.; Watarumi, S.; Fujieda, M.; Kouno, I. New black tea polyphenol having N-ethyl-2-pyrrolidinone moiety derived from tea amino acid theanine: Isolation, characterization and partial synthesis. Food Chem. 2005, 93, 81–87. [Google Scholar] [CrossRef]
- Kuhnle, G.C. Nutrition epidemiology of flavan-3-ols: The known unknowns. Mol. Asp. Med. 2017, 61, 2–11. [Google Scholar] [CrossRef]
- Hatano, T.; Miyatake, H.; Natsume, M.; Osakabe, N.; Takizawa, T.; Ito, H.; Yoshida, T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry 2002, 59, 749–758. [Google Scholar] [CrossRef]
- Zeng, Y.; Sun, Y.X.; Meng, X.H.; Yu, T.; Zhang, Y.J. A new methylene bisflavan-3-ol from the branches and leaves of Potentilla fruticosa. Nat. Prod. Res. 2019, 34, 1238–1245. [Google Scholar] [CrossRef]
- Hoong, Y.B.; Pizzi, A.; Tahir, P.M.; Pasch, H. Characterization of Acacia mangium polyflavonoid tannins by MALDI-TOF mass spectrometry and CP-MAS 13C NMR. Eur. Polym. J. 2010, 46, 1268–1277. [Google Scholar] [CrossRef]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J. Cocoa polyphenols and their potential benefits for human health. Oxidative Med. Cell. Longev. 2012, 2012, 1–23. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Chromone and flavonoid alkaloids: Occurrence and bioactivity. Molecules 2011, 17, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, T.; Lei, X.; Chen, R.Y. Two novel flavanes from the leaves of Morus alba L. J. Asian Nat. Prod. Res. 2010, 12, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Jin, J.; Ruan, J.L.; Zhu, C.C.; Lin, C.Z. Antioxidant flavonoid glycosides from aerial parts of the fern Abacopteris penangiana. J. Nat. Prod. 2007, 70, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Jin, J.; Ruan, J.L.; Cai, Y.L.; Zhu, C.C. Two new flavan-4-ol glycosides from Abacopteris penangiana. Acta Pharm. Sin. 2010, 43, 392–395. [Google Scholar]
- Zhao, Z.; Ruan, J.; Jin, J.; Zhu, C.; Liu, Y. A novel anthocyanidin glycoside from the rhizomes of Abacopteris penangiana. Fitoterapia 2010, 81, 1171–1175. [Google Scholar] [CrossRef]
- Zhao, Z.; Ruan, J.; Jin, J.; Zou, J.; Zhou, D.; Fang, W.; Zeng, F. Flavan-4-ol glycosides from the rhizomes of Abacopteris penangiana. J. Nat. Prod. 2006, 69, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Ruan, J.L.; Jin, J.; Zhu, C.C.; Yu, Y. Two new flavonoids from the rhizomes of Abacopteris penangiana. Helv. Chim. Acta 2011, 94, 446–452. [Google Scholar] [CrossRef]
- Jiang, J.H.; Yang, J.H.; Zhang, X.J.; Wang, W.J.; Zhang, Y.H.; Liu, Y.; Chen, Y.G. Flavonoids from the fern Pronephrium penangianum. Chem. Nat. Compd. 2014, 50, 912–914. [Google Scholar] [CrossRef]
- Fang, J.B.; Chen, J.C.; Duan, H.Q. Two new flavan-4-ol glycosides from Abacopteris penangiana. J. Asian Nat. Prod. Res. 2010, 12, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Chen, X.L.; Lei, Y.F.; Wei, H.; Xiong, C.M.; Liu, Y.J.; Fu, W.; Ruan, J.L. Vascular protective potential of the total flavanol glycosides from Abacopteris penangiana via modulating nuclear transcription factor-κB signaling pathway and oxidative stress. J. Ethnopharmacol. 2011, 136, 217–223. [Google Scholar] [CrossRef]
- Lenssen, A.W.; Martin, S.S.; Townsend, C.E.; Hawkins, B. Acicerone: An isoflavone from Astragalus cicer. Phytochemistry 1994, 36, 1185–1187. [Google Scholar] [CrossRef]
- Martin, S.S.; Townsend, C.E.; Lenssen, A.W. Induced isoflavonoids in diverse populations of Astragalus cicer. Biochem. Syst. Ecol. 1994, 22, 657–661. [Google Scholar] [CrossRef]
- Subarnas, A.; Oshima, Y.; Hikino, H. Isoflavans and a pterocarpan from Astragalus mongholicus. Phytochemistry 1991, 30, 2777–2780. [Google Scholar] [CrossRef]
- Gorai, D.; Jash, S.K.; Roy, R. Flavonoids from Astragalus genus. Int. J. Pharm. Sci. Res. 2016, 7, 27–32. [Google Scholar]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Kuo, L.M.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New isoflavonoid glycosides and related constituents from astragali radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Seigler, D.S. Phytochemistry of Acacia-sensu lato. Biochem. Syst. Ecol. 2003, 31, 845–873. [Google Scholar] [CrossRef]
- Harborne, J.B. The Flavonoids: Advances in Research since 1980; Springer: Berlin/Heidelberg, Germany, 2013; Volume 111, pp. 559–565. [Google Scholar]
- Tindale, M.D.; Roux, D.G. An extended phytochemical survey of Australian species of Acacia: Chemotaxonomic and phylogenetic aspects. Phytochemistry 1974, 13, 829–839. [Google Scholar] [CrossRef]
- Foo, L.Y. Isolation of [4-O-4]-linked biflavanoids from Acacia melanoxylon: First examples of a new class of single ether-linked proanthocyanidin dimers. J. Chem. Soc. Chem. Commun. 1989, 20, 1505–1506. [Google Scholar] [CrossRef]
- Young, D.A.; Ferreira, D.; Roux, D.G. Synthesis of condensed tannins. Part 10.‘Dioxane-linked’ profisetinidins. J. Chem. Soc. Perkin Trans. 1 1983, 174, 2031–2035. [Google Scholar] [CrossRef]
- Cosme, P.; Bruyne, T.D.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Fitzpatrick, D.F.; Fleming, R.C.; Bing, B.; Maggi, D.A.; O’Malley, R.M. Isolation and characterization of endothelium-dependent vasorelaxing compounds from grape seeds. J. Agric. Food Chem. 2000, 48, 6384–6390. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Becker, L.B.; Hoek, T.L.; Schumacker, P.T.; Li, C.Q.; Zhao, D.; Wojcik, K.; Anderson, T.; Qin, Y.; Dey, L.; et al. Grape seed proanthocyanidin extract attenuates oxidant injury in cardiomyocytes. Pharmacol. Res. 2003, 47, 463–469. [Google Scholar] [CrossRef]
- Nuttall, S.L.; Kendall, M.J.; Bombardelli, E.; Morazzoni, P. An evaluation of the antioxidant activity of a standardized grape seed extract, Leucoselect. J. Clin. Pharm. Ther. 1998, 23, 385–389. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. Plant bioactives and redox signaling: (–)-Epicatechin as a paradigm. Mol. Asp. Med. 2018, 61, 31–40. [Google Scholar] [CrossRef]
- Cremonini, E.; Oteiza, P.I. (–)-Epicatechin and its metabolites prevent palmitate-induced NADPH oxidase upregulation, oxidative stress and insulin resistance in HepG2 cells, Arch. Biocheistry Biophys. 2018, 646, 55–63. [Google Scholar] [CrossRef]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochemistry Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guzman, M.; Jimenez, R.; Sanchez, M.; Romero, M.; O’Valle, F.; Lopez-Sepulveda, R.; Quintela, A.M.; Galindo, P.; Zarzuelo, M.J.; Bailon, E.; et al. Chronic (–)-epicatechin improves vascular oxidative and inflammatory status but not hypertension in chronic nitric oxide-deficient rats. Br. J. Nutr. 2011, 106, 1337–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstraeten, S.V.; Mackenzie, G.G.; Oteiza, P.I.; Fraga, C.G. (–)-Epicatechin and related procyanidins modulate intracellular calcium and prevent oxidation in Jurkat T cells. Free Radic. Res. 2008, 42, 864–872. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (–)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Romero-Perez, D.; Villarreal, F.; Ceballos, G.; Ramirez-Sanchez, I. Cell membrane mediated (–)-epicatechin effects on upstream endothelial cell signaling: Evidence for a surface receptor. Bioorg. Med. Chem. Lett. 2014, 24, 2749–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettaieb, A.; Cremonini, E.; Kang, H.; Kang, J.; Haj, F.G.; Oteiza, P.I. Anti-inflammatory actions of (–)-epicatechin in the adipose tissue of obese mice. Int. J. Biochem. Cell Biol. 2016, 81 Pt B, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Leonardo, C.C.; Agrawal, M.; Singh, N.; Moore, J.R.; Biswal, S.; Dore, S. Oral administration of the flavanol (–)-epicatechin bolsters endogenous protection against focal ischemia through the Nrf2 cytoprotective pathway. Eur. J. Neurosci. 2013, 38, 3659–3668. [Google Scholar] [CrossRef]
- Bahia, P.K.; Rattray, M.; Williams, R.J. Dietary flavonoid (-)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and upregulates glutathione in cortical astrocytes. J. Neurochem. 2008, 106, 2194–2204. [Google Scholar]
- Shah, Z.A.; Li, R.C.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Dore, S. The flavanol (–)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owczarek, A.; Kolodziejczyk, C.J.; Woźniak, S.J.; Magiera, A.; Kobiela, N.; Wąsowicz, K.; Olszewska, M.A. Aesculus hippocastanum potential activity mechanisms of bark: Antioxidant effects in chemical and biological in vitro models. Antioxidants 2021, 10, 995. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Valls, F.J.; Krisa, S.; Garcia, F.; Hornedo, O.R. Polyphenolic characterization of merlot, tannat and syrah skin extracts at different degrees of maturity and anti-inflammatory potential in RAW 264.7 Cells. Foods 2021, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Rimbach, G.; Saliou, C.; Valacchi, G.; Packer, L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000, 465, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.L.; Tsai, S.H.; Lin-Shiau, S.Y.; Ho, C.T. Theaflavin-3,3′-digallate from black tea blocks the nitric oxide synthase by down-regulating the activation of NF-kappaB in macrophages. Eur. J. Pharmacol. 1999, 367, 379–388. [Google Scholar] [CrossRef]
- Gerardo, G.M.; Fernando, C.; José, M.D.; Carl, L.K.; César, G.F.; Patricia, I.O. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. Faseb J. 2004, 18, 167–169. [Google Scholar]
- Giovanni, S.; Sergio, D.; Laura, D.R.; Antonino, D.L.; Hector, H.O.; Giuseppe, M.; Arrigo, F.C.; Salvador, G. Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients 2014, 6, 3202–3213. [Google Scholar]
- Lin, Y.L.; Lin, J.K. (-)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol. Pharmacol. 1997, 52, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.F.; Chen, J.L.; Wei, H.; Xiong, C.M.; Ruan, J.L. Hypolipidemic and anti-inflammatory properties of abacopterin A from Abacopteris penangiana in high-fat diet-induced hyperlipidemia mice. Food Chem. Toxicol. 2011, 49, 3206–3210. [Google Scholar] [CrossRef] [PubMed]
- Nihal, A.; Feyes, D.K.; Rajesh, A.; Hasan, M. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar]
- Bo, Z.; Jing, P.; Fang, D.; Zhao, C.; Zhang, L.; Wei, Q.; Yang, L.; Zheng, R.; Liu, Z. Redifferentiation of human hepatoma cells induced by green tea polyphenols. Res. Chem. Intermed. 2004, 30, 626–635. [Google Scholar]
- Gali, H.U.; Perchellet, E.M.; Gao, X.M.; Karchesy, J.J.; Perchellet, J.P. Comparison of the inhibitory effects of monomeric, dimeric, and trimeric procyanidins on the biochemical markers of skin tumor promotion in mouse epidermis in vivo. Planta Med. 1994, 60, 235–239. [Google Scholar] [CrossRef]
- Qin, Y.; Muhammad, D.; Wang, W.M.; Jian, Y.Q.; Wang, W. An enhanced silver nanocluster system for cytochrome c detection and natural drug screening targeted for cytochrome c. Sens. Actuators B Chem. 2019, 291, 485–492. [Google Scholar] [CrossRef]
- Ge, M.; Xiao, Y.; Chen, H.; Luo, F.; Du, G.; Zeng, F. Multiple antiviral approaches of (-)-epigallocatechin-3-gallate (EGCG) against porcine reproductive and respiratory syndrome virus infection in vitro. Antivir. Res. 2018, 158, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Choy, K.W.; Pang, C.P.; Rogers, M.S. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007, 22, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Lu, H.; Zhao, Q.; He, Y.; Niu, J.; Debnath, A.K.; Wu, S.; Jiang, S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Et Biophys. Acta Gen. Subj. 2005, 1723, 270–281. [Google Scholar] [CrossRef]
- Chowdhury, P.; Sahuc, M.E.; Rouillé, Y.; Rivière, C.; Bonneau, N.; Vandeputte, A.; Brodin, P.; Goswami, M.; Bandyopadhyay, T.; Dubuisson, J. Theaflavins, polyphenols of black tea, inhibit entry of hepatitis C virus in cell culture. PLoS ONE 2018, 13, e0198226. [Google Scholar] [CrossRef] [Green Version]
- Okada, F.; Takeo, T.; Okada, S.; Tamemasa, O. Antiviral effect of theaflavins on tobacco mosaic virus. Agric. Biol. Chem. 1977, 41, 791–794. [Google Scholar]
- Ohba, M.; Oka, T.; Ando, T.; Arahata, S.; Ikegaya, A.; Takagi, H.; Ogo, N.; Zhu, C.; Owada, K.; Kawamori, F.; et al. Antiviral effect of theaflavins against caliciviruses. J. Antibiot. 2017, 70, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 85, 153286. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, S.; Naik, S.; Patravale, V. A molecular docking study of EGCG and theaflavin digallate with the druggable targets of SARS-CoV-2. Comput. Biol. Med. 2021, 129, 104137. [Google Scholar] [CrossRef]
- Akiko, T.; Fumio, N. Effects of metabolites produced from (-)-epigallocatechin gallate by rat intestinal bacteria on angiotensin I-converting enzyme activity and blood pressure in spontaneously hypertensive rats. J. Agric. Food Chem. 2015, 63, 8262–8266. [Google Scholar]
- Actis, G.L.; Ottaviani, J.I.; Fraga, C.G. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem 2006, 54, 229–234. [Google Scholar] [CrossRef]
- Debasis, B.; Sen, C.K.; Ray, S.D.; Das, D.K.; Vinson, J.A. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 523–524, 87–97. [Google Scholar]
- Tomera, J.F. Current knowledge of the health benefits and disadvantages of wine consumption. Trends Food Sci. Technol. 1999, 10, 129–138. [Google Scholar] [CrossRef]
- Qiang, W.J.; Chen, Y. He, F.Y.; Xiao, M.F.; Cai, W.Y.; Dai, Y.F.; Yang, Q.; Li, Y.J.; Weng, X.G.; Li, Q.; et al. Molecular biological mechanisms of yuan zhi powder in the treatment of alzheimer’s disease: An analysis based on network pharmacology. Digit. Chin. Med. 2018, 1, 90–101. [Google Scholar] [CrossRef]
- Cecarini, V.; Bonfili, L.; Cuccioloni, M.; Mozzicafreddo, M.; Angeletti, M.; Keller, J.N.; Eleuteri, A.M. The fine-tuning of proteolytic pathways in Alzheimer’s disease. Cell. Mol. Life Sci. 2016, 73, 3433–3451. [Google Scholar] [CrossRef]
- Cecarini, V.; Cuccioloni, M.; Zheng, Y.; Bonfili, L.; Gong, C.; Angeletti, M.; Mena, P.; Del Rio, D.; Eleuteri, A.M. Flavan-3-ol microbial metabolites modulate proteolysis in neuronal cells reducing amyloid-beta (1-42) levels. Mol. Nutr. Food Res. 2021, 65, e2100380. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.; Huang, Y.W.; Lin, Y. Tannins and human health: A review—critical reviews in food science and nutrition. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Oshima, Y.; Watanabe, H. The mecanisms of catechins metabolism II. neutral substances in the urine of rabbits administered (+)-catechin. J. Biochem. 1958, 45, 973–977. [Google Scholar] [CrossRef]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood-Brain Barrier Permeability of Green Tea Catechin Metabolites and their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Otani, S.; Nanjo, F. Antioxidative activity of microbial metabolites of (-)-epigallocatechin gallate produced in rat intestines. Biosci. Biotechnol. Biochem. 2011, 75, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Hun, K.J.; Seung, K.J.; Sil, O.Y.; Min, H.S.; Yoon, P.J.H.; Won, L.K.; Yong, L.C. 5-(3′,4′-Dihydroxyphenyl-γ-valerolactone), a major microbial metabolite of proanthocyanidin, attenuates THP-1 monocyte-endothelial adhesion. Int. J. Mol. Sci. 2017, 18, 1363. [Google Scholar] [CrossRef] [Green Version]
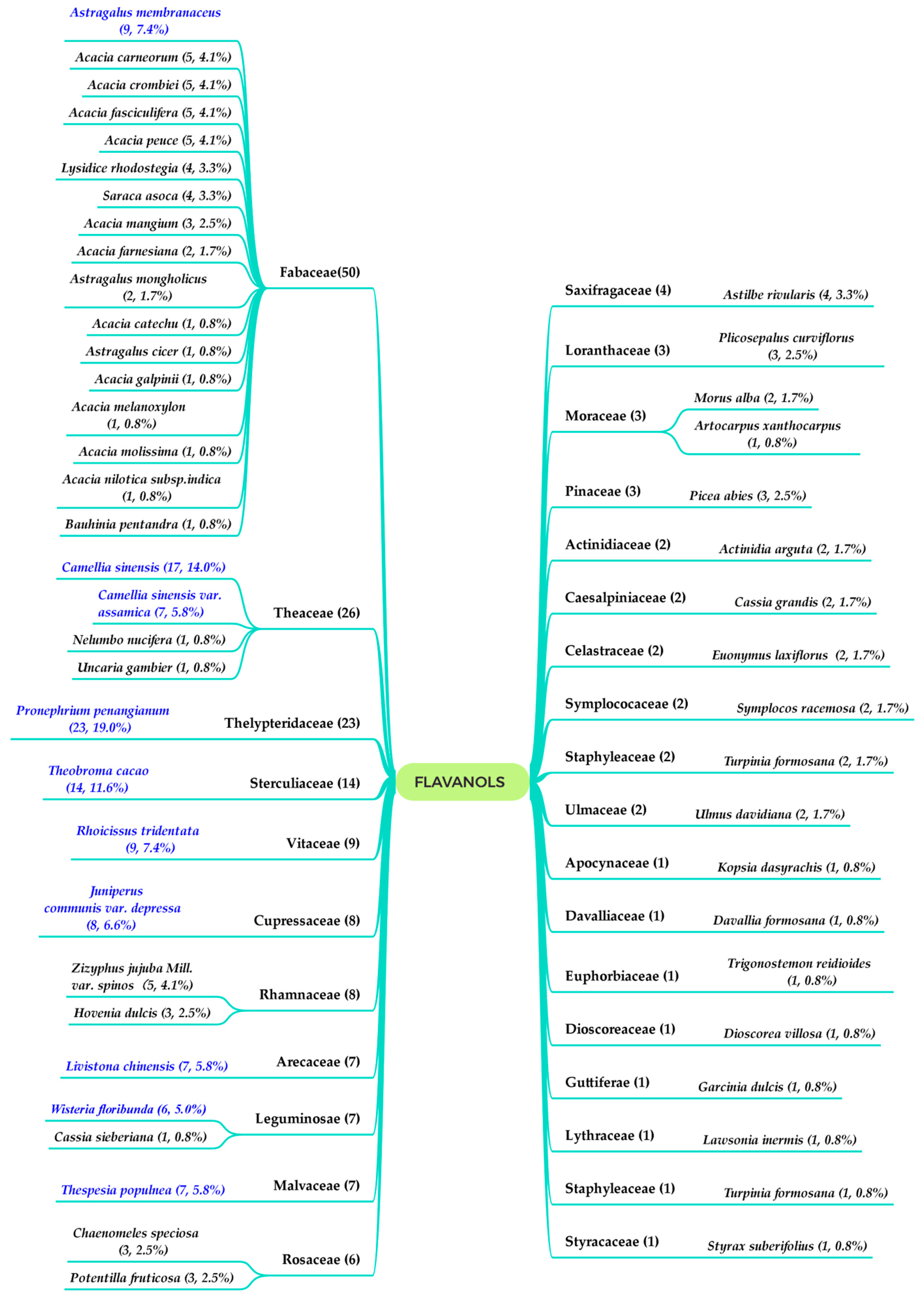
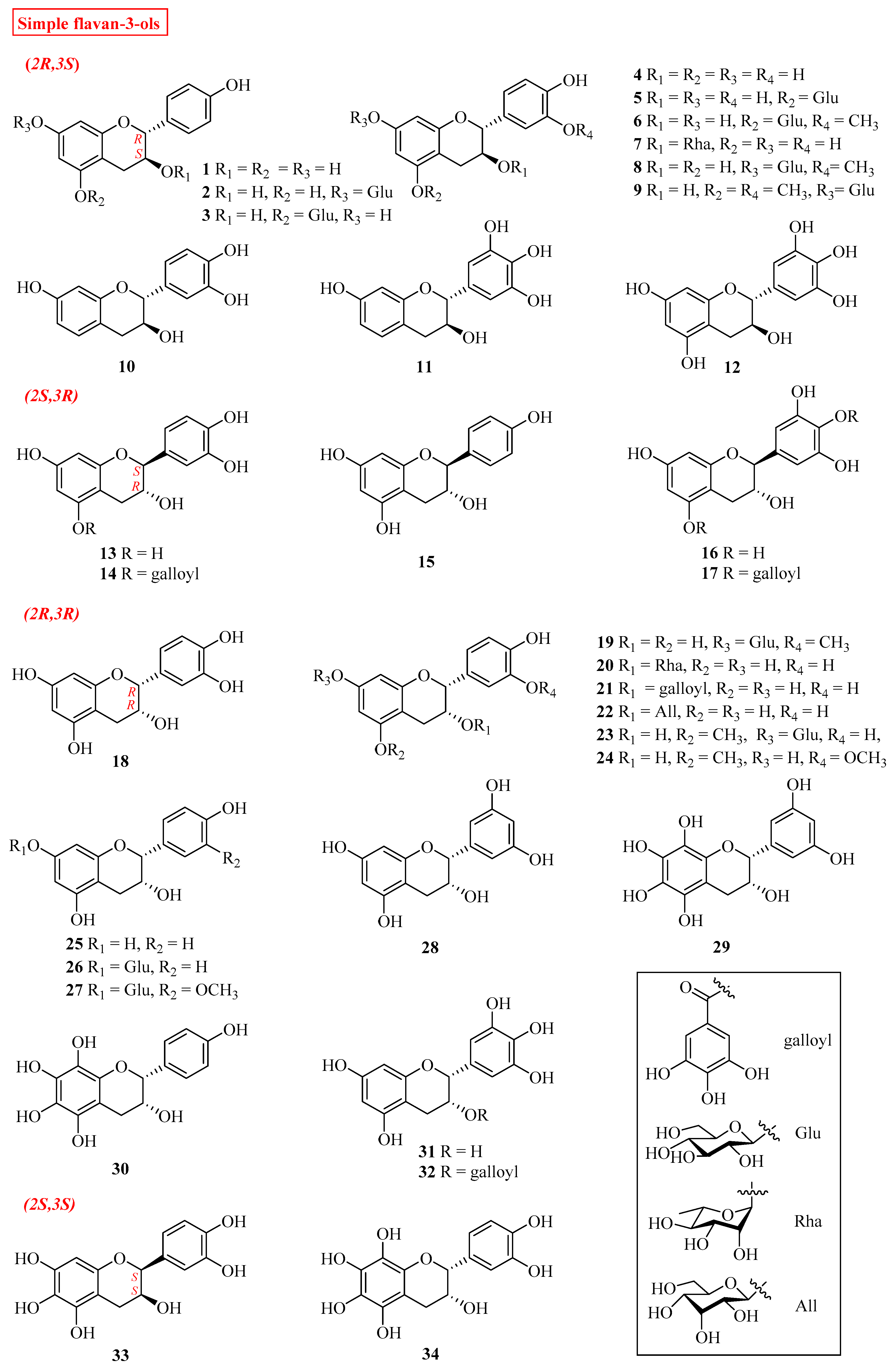
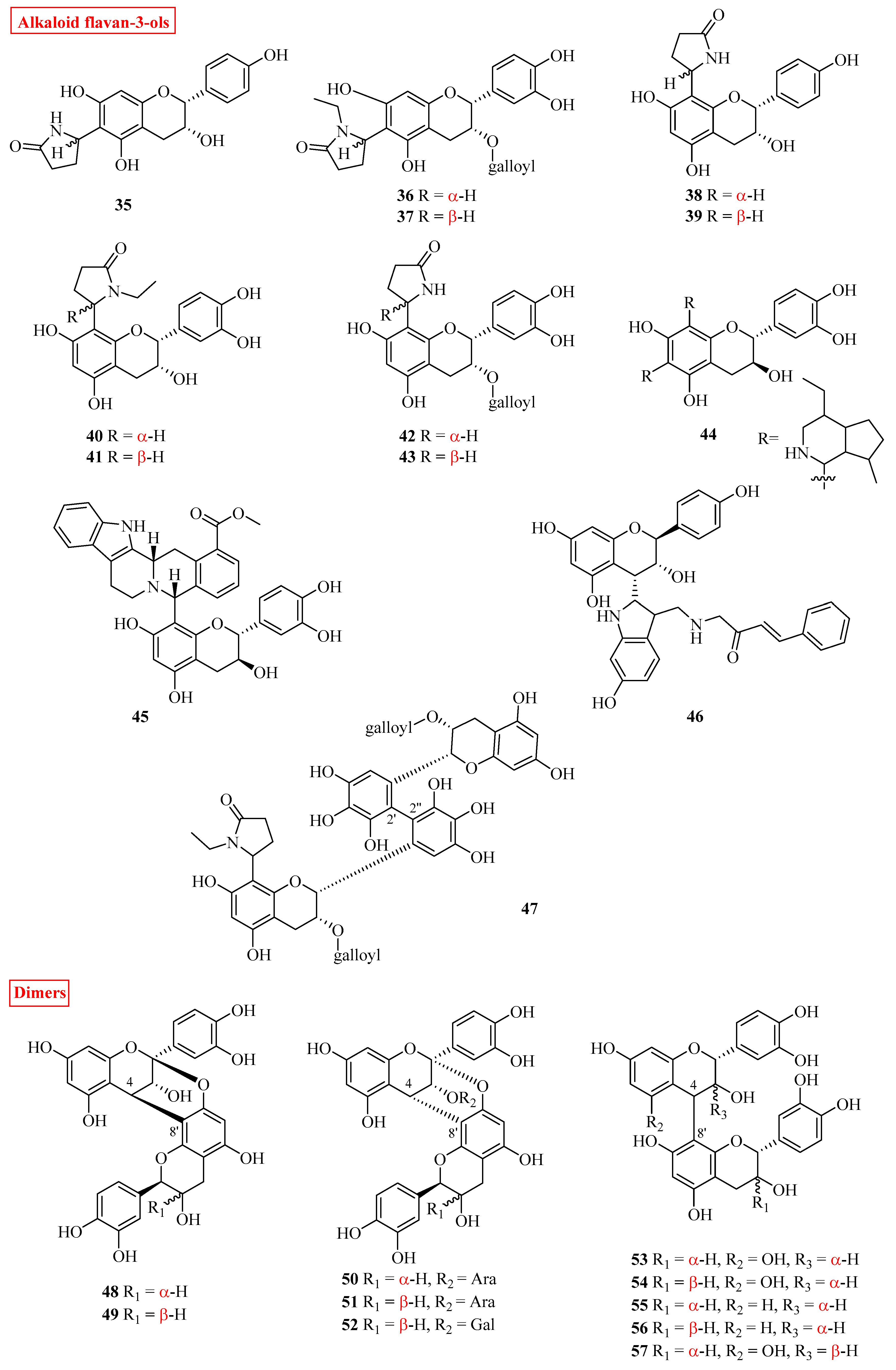

| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
| 1 | (+)-afzelechin | Wisteria floribunda | fruit peels | [8] |
| Nelumbo nucifera; | seed epicarp | [9] | ||
| Hovenia dulcis; | stem barks | [10] | ||
| Zizyphus jujuba Mill. | roots | [11] | ||
| var. spinosa | ||||
| Livistona chinensis | fruits | [12] | ||
| Thespesia populnea | barks | [13] | ||
| 2 | (+)-afzelechin-7-O-β-d-glucopyranoside | Juniperus | stems and leaves rhizomes | [14] |
| communis var. depressa | ||||
| Dioscorea villosa | [15] | |||
| 3 | (+)-afzelechin-5-O-β-d-glucopyranoside | Wisteria floribunda | fruit peels | [8] |
| Dioscorea villosa | rhizomes | [15] | ||
| 4 | (+)-catechin | Wisteria floribunda | fruit peels | [8] |
| Astilbe rivularis | rhizomes | [16] | ||
| Uncaria gambier | leaves | [9] | ||
| Rhoicissus tridentata | roots | [17] | ||
| Zizyphus jujuba Mill. | roots | [11] | ||
| var. spinosa | ||||
| Euonymus laxiflorus | trunk barks | [18] | ||
| Livistona chinensis | fruits | [12] | ||
| Thespesia populnea | barks | [13] | ||
| Chaenomeles speciosa | fruits | [19] | ||
| Picea abies | root barks | [20] | ||
| Lawsonia inermis | aerial parts | [21] | ||
| 5 | catechin-5-O-β-d-glucoside | Chaenomeles speciosa | fruits | [19] |
| 6 | (+)-3′-O-methylcatechin-5-O-β-d-glucopyranoside | Juniperus | stems and leaves | [14] |
| communis var. depressa | ||||
| 7 | 3′- deoxycatechin-3-O-α-l-rhamnopyranoside | Saraca asoca | barks | [22] |
| 8 | (+)-4′-O-methylcatechin 5-O-β-d-glucopyranoside | Juniperus | stems and leaves | [14] |
| communis var. depressa | ||||
| 9 | (+)-gallocatechin | Rhoicissus tridentata | roots | [17] |
| Thespesia populnea | barks | [13] | ||
| 10 | (−)-fisetinidol | Bauhinia pentandra | stems | [23] |
| Rhoicissus tridentata | roots | [17] | ||
| Acacia molissima | woods | [24,25] | ||
| 11 | (−)-robinetinidol | Lysidice rhodostegia | barks | [26] |
| 12 | (−)-epigallocatechin | Thespesia populnea Zizyphus jujuba Mill. var. spinosa | barks | [13] |
| roots | [11] | |||
| 13 | (−)-catechin | Astilbe rivularis; | rhizomes | [16] |
| Hovenia dulcis | stem barks | [10] | ||
| Juniperus | stems and | [14] | ||
| communis var. depressa | leaves | |||
| Plicosepalus curviflorus | leaves | [27] | ||
| Euonymus laxiflorus | trunk bark | [18] | ||
| Artocarpus xanthocarpus | leaves | [28] | ||
| Saraca asoca | ||||
| roots | [21] | |||
| 14 | (2S,3R)-3,3′,4′,5,7-pentahydroxyflavane-5-O-gallate | Plicosepalus curviflorus | leaves | [26] |
| 15 | (−)-afzelechin | Astilbe rivularis | rhizomes | [16] |
| 16 | (−)-gallocatechin | Euonymus laxiflorus | trunk barks | [18] |
| Champ | ||||
| Livistona sinensis | leaves | [12] | ||
| 17 | (2S,3R)-3,3′,4′,5,5′,7-hexahydroxyflavane-4′,5-di-O-gallate | Plicosepalus curviflorus | leaves | [26] |
| 18 | (−)-epicatechin | Wisteria floribunda | fruit peels leaves leaves roots roots stems and leaves fruits roots barks fruits | [8] |
| Cassia grandis | [29] | |||
| Acacia catechu | [9] | |||
| Rhoicissus tridentata | [17] | |||
| Lysidice rhodostegia | [25] | |||
| Juniperus | [14] | |||
| communis var. depressa | ||||
| Livistona chinensis | [12] | |||
| Cassia sieberiana | [30] | |||
| Thespesia populnea | [13] | |||
| Chaenomeles speciosa | [19] | |||
| 19 | 3′-O-methylepicatechin-7-O-β-d-glucopyranoside | Juniperus communis var.depressa | stems and leaves | [14] |
| 20 | 3′-O-deoxycatechin-3-O-α-l-rhamnopyranoside | Turpinia formosana | roots | [31] |
| 21 | (−)-epicatechin-3-O-gallate | Camellia sinensis | leaves | [9] |
| Rhoicissus tridentata | roots | [17] | ||
| Lysidice rhodostegia | roots | [25] | ||
| 22 | (−)-epicatechin-3-O-β-d-allopyranoside | Turpinia formosana | roots | [31] |
| 23 | 5-O-methylepicatechin-7-O-β-d-glucopyranoside | Juniperus communis var.depressa | stems and leaves | [14] |
| 24 | 3,7,4′-trihydroxy-5,3′-dimethoxyflavan-7-O-β-d-glucopyranoside | Styrax suberifolius | barks | [32] |
| 25 | (−)-epiafzelechin | Wisteria floribunda | fruit peels | [8] |
| Astilbe rivularis | rhizomes | [16] | ||
| Cassia grandis | leaves | [29] | ||
| Zizyphus jujuba Mill. | roots | [11] | ||
| var. spinosa | ||||
| Thespesia populnea | barks | [13] | ||
| Saraca asoca | barks | [21] | ||
| 26 | (+)-epiafzelechin 7-O-β-d-gluco-pyranoside | Wisteria floribunda | fruit peels | [8] |
| Saraca asoca | barks | [21] | ||
| 27 | 3′-O-methylepicatechin-7-O-β-d-glucopyranoside | Juniperus communis var.depressa | stems | [14] |
| 28 | (2R,3R)-3,5,7,3′,5′-penthahydroxyflavane | Camellia sinensis | leaves | [13] |
| 29 | (2R,3R)-3,5,6,7,8,3′,5′-heptahydroxyflavane | Livistona chinensis | fruits | [12] |
| 30 | (2R,3R)-3,5,6,7,8,4′-hexahydroxyflavane | Livistona chinensis | fruits | [12] |
| 31 | (−)-epigallocatechin | Rhoicissus tridentata | roots | [17] |
| Camellia sinensis | leaves | [9] | ||
| Zizyphus jujuba Mill. | roots | [11] | ||
| var. spinosa | ||||
| Thespesia populnea | barks | [13] | ||
| 32 | (−)-epigallocatechin gallate | Camellia sinensis. | leaves | [33] |
| 33 | (2S,3S)-3,5,7,3′,5′-pentahydroxyflavane | Livistona. chinensis | fruits | [12] |
| 34 | dulcisflavan | Garcinia dulcis | fruits | [34] |
| 35 | (−)-6-(5′’S)-2-pyrrolidinone-epiafzelechin | Actinidia arguta | roots | [35] |
| 36 | (−)-6-(5′’R)-N-ethyl-2-pyrrolidinone-epicatechin-3-O-gallate | Camellia sinensis var. assamica | leaves | [36] |
| 37 | (−)-6-(5′’S)-N-ethyl-2-pyrrolidinone-epicatechin-3-O-gallate | Camellia sinensis var. assamica | leaves | [36] |
| 38 | (−)-8-(5′’R)-(2-pyrrolidinone-5-yl)-(−)-epicatechin | Camellia sinensis var. assamica | leaves | [36] |
| Actinidia arguta | roots | [36] | ||
| 39 | (−)-8-(5′’S)-(2-pyrrolidinone-5-yl)-(−)-epicatechin | Camellia sinensis var. assamica | leaves | [36] |
| Actinidia arguta | roots | [35] | ||
| 40 | (−)-8-(5′’R)-N-ethyl-2-pyrrolidinone-epicatechin | Camellia sinensis var. assamica | leaves | [36] |
| 41 | (−)-8-(5′’R)-N-ethyl-2-pyrrolidinone-epicatechin | Camellia sinensis var. assamica | leaves | [36] |
| 42 | (−)-8-(5′’R)-N-ethyl-2-pyrrolidinone-epicatechin-3-O-gallate | Camellia sinensis var. assamica | leaves | [36] |
| 43 | (−)-8-(5′’S)-N-ethyl-2-pyrrolidinone-epicatechin-3-O-gallate | Camellia sinensis var. assamica | leaves | [36] |
| 44 | kopsirachin | Kopsia dasyrachis | leaves | [37] |
| 45 | (−)-uncariagambiriine | Uncaria gambir | leaves | [38] |
| 46 | lotthanongine | Trigonostemon reidioides; | leaves | [39] |
| Baliospermum reidioides | leaves | [39] | ||
| 47 | 8′-ethylpyrrolidinonyltheasinensin A | Camellia sinensis | leaves | [40] |
| 48 | (−)-epicatechin-(2β→O-7; 4β→8)-(+)-catechin | Camellia sinensis | leaves | [41] |
| 49 | (−)-epicatechin-(2β→O-7; 4β→8)-(−)-catechin | Theobroma cacao | fruits | [42] |
| 50 | 3T-O-α-l-arabinopyranosyl-ent-epicatechin-(2α→7,4α→8)-epicatechin | Theobroma cacao | fruits | [42] |
| 51 | 3T-O-β-d-galactopyranosyl-ent-epicatechin-(2α→7,4α→8)-epicatechin | Theobroma cacao | fruits | [42] |
| 52 | 3T-O-α-l-arabinopyranosyl-ent-epicatechin-(2α→7,4α→8)-epicatechin | Theobroma cacao | fruits | [42] |
| 53 | procyanidin B4 | Rhoicissus tridentata | roots | [17] |
| Picea abies | root barks | [20] | ||
| Camellia sinensis | leaves | [41] | ||
| 54 | procyanidin B3 | Rhoicissus tridentata | roots | [17] |
| Picea abies | root barks | [21] | ||
| 55 | fisetinidol-(4β→8)-catechin | Rhoicissus tridentata | roots | [17] |
| 56 | fisetinidol-(4α→8) | Rhoicissus tridentata | roots | [17] |
| 57 | procyanidin B2 | Theobroma cacao | fruits | [42] |
| 58 | procyanidin B5 | Theobroma cacao | fruits | [42] |
| 59 | methylene 8,8-bis-(+)-catechin | Potentilla fruticosa | leaves | [43] |
| 60 | methylene-6,8-bis-(+)-catechin | Potentilla fruticosa | leaves | [43] |
| 61 | methylene 6,8-bis-(7-O-glucosyl)-(+)-catechin | Potentilla fruticosa | leaves | [43] |
| 62 | theaflavin | Camellia sinensis | leaves | [41] |
| 63 | theaflavin-3-galloyl | Camellia sinensis | leaves | [41] |
| 64 | theaflavin-3,3′-galloyl | Camellia sinensis | leaves | [41] |
| 65 | theaflavin-3′-galloyl | Camellia sinensis | leaves | [41] |
| 66 | procyanidin C1 | Theobroma cacao | fruits | [41] |
| 67 | procyanidin-C1-tri-gallate | Theobroma cacao | fruits | [42] |
| 68 | Theobroma cacao | fruits | [42] | |
| 69 | Theobroma cacao | fruits | [42] | |
| 70 | 3T-O-α-l-arabinopyranosylcinnamtannin B1 | Theobroma cacao | fruits | [42] |
| 71 | 3T-O-β-d-galactopyranosylcinnamtannin B1 | Theobroma cacao | fruits | [42] |
| 72 | cinnamtannin B1 | Theobroma cacao | fruits | [42] |
| 73 | cinnamtannin A2 | Theobroma cacao | fruits | [42] |
| 74 | angular tannin | Acacia mangium | barks | [44] |
| 75 | linear tannin | Acacia mangium | barks | [44] |
| 76 | twice-angular tannin | Acacia mangium | barks | [44] |
| No. | Name | Plant source | Plant Organ | Ref. |
|---|---|---|---|---|
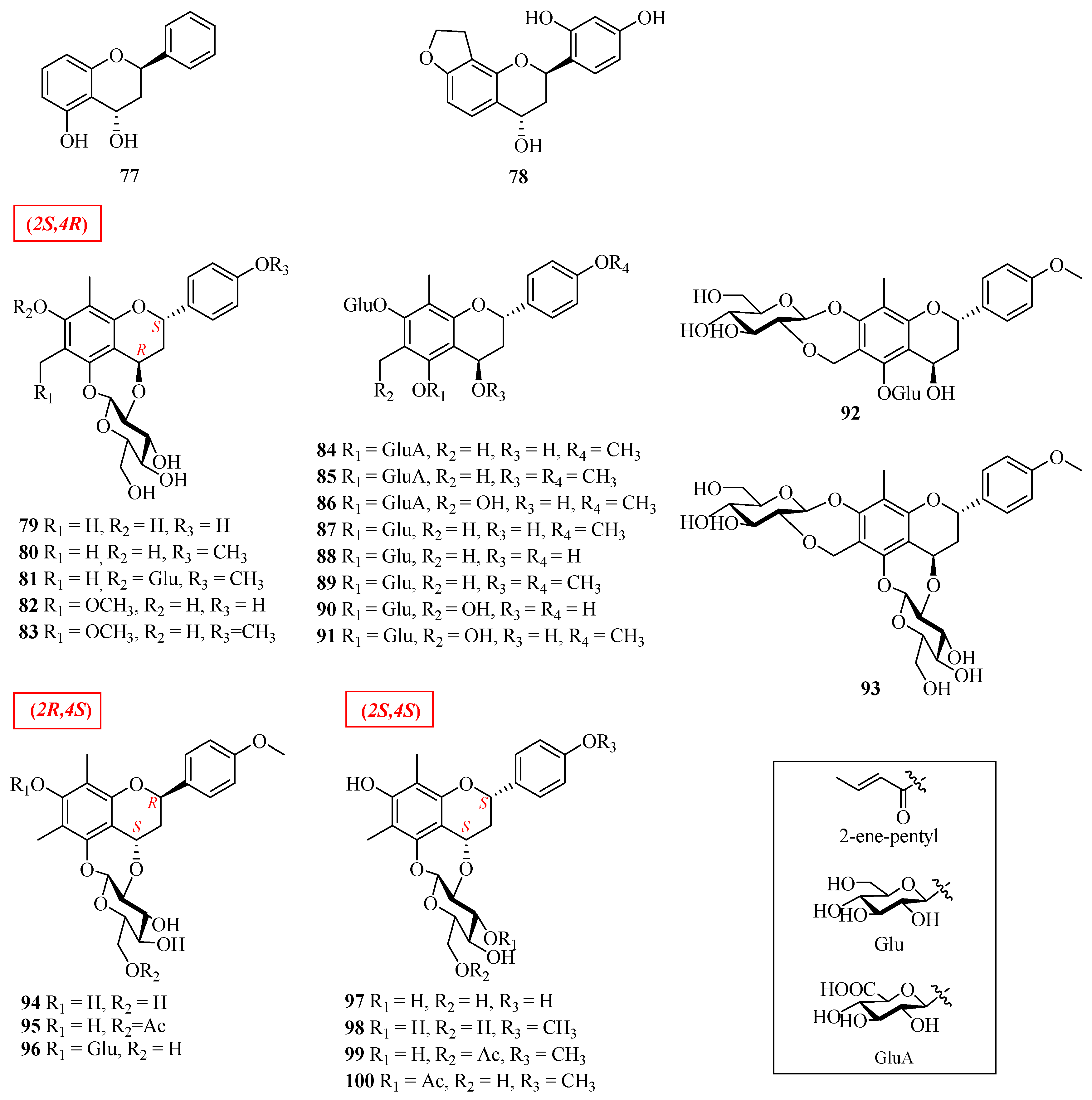
| 77 | (2R,4S)-7-hydroxy-flavan-4-ol | Morus alba | leaves | [49] |
| 78 | (2R,4S)-2′,4′-dihydroxy-2H-furan-(3′’,4′’:8,7)-flavan-4-ol | Morus alba | leaves | [49] |
| 79 | (2S,4R)-7,4′-dihydroxy-6,8-dimethyl-4,2′’-oxidoflavan-5-O-β-d-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 80 | eruberin A | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 81 | (2S,4R)-6,8-dimethyl-4′-methoxy-4,2′’-oxidoflavan-5,7-di-O-β-d-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 82 | abacopterin G | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 83 | (2S,4R)-7,4′-dihydroxy-6-methoxymethyl-8-methyl-4,2′’-oxidoflavan-5-O-β-d-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 84 | (2S,4R)-5,7-dihydroxy-4,4′-dimethoxy-6,8-dimethylflavan-5-O-β-d-6-acetylglucopyranoside-7-O-β-d-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 85 | (2S,4R)-4,5,7-trihydroxy-4′-methoxy-6,8-dimethylflavan-5-O-β-d-6-glucopyranoside-7-O-β-d-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 86 | 6′’-O-acetyltriphyllin A | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [51] |
| 87 | eruberin B | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50,52] |
| 88 | 4′-hydroxypneumatopterin B | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [51] |
| 89 | (2S,4R)-4,5,7-trihydroxy-4′-methoxy-6,8-dimethylflavan-5-O-β-d-6-acetylglucopyranoside-7-O-β-d-glucopyranosid | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [50] |
| 90 | triphyllin A | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [53] |
| 91 | eruberin C | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [52] |
| 92 | abacopterin D | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [53] |
| 93 | abacopterin K | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [54] |
| 94 | (2R,4S)-6,8-dimethyl-7-hydroxy-4′-methoxy-4,2′’-oxidoflavan-5-O-β-d-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [55] |
| 95 | (2R,4S)-6,8-dimethyl-7-hydroxy-4′-methoxy-4,2′’-oxidoflavan-5-O-β-d-6′’-O-acetyl-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [56] |
| 96 | (2S,4R)-7-hydroxy-6-methoxymethyl-8-methyl-4,2′’-oxidoflavan-5-O-β-d-glucopyranoside | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [56] |
| 97 | abacopterin E | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [57] |
| 98 | abacopterin C | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [56] |
| 99 | abacopterin A | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [56] |
| 100 | abacopterin B | Abacopteris penangiana or Pronephrium penangiana | rhizomes | [56] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
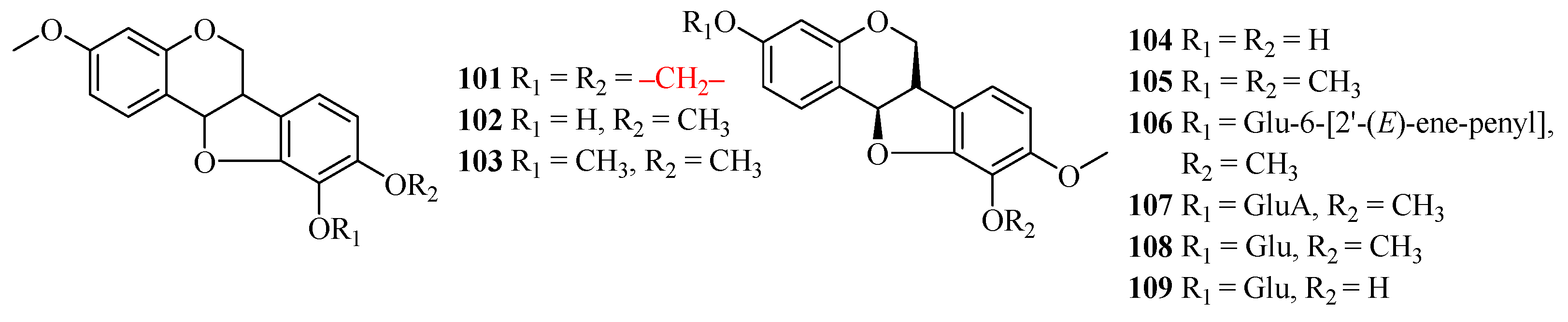
| 101 | maackiain | Astragalus cicer; | roots | [58,59] |
| Astragalus membranaceus; | roots | |||
| Astragalus mongholicus; | ||||
| Astragalus trojanus | roots | |||
| 102 | (6aR,11aR)-10-hydroxy-3,9,10-dimeth oxypterocarpan | Astragalus membranaceus; | roots | [60,61] |
| 103 | (6aR,11aR)-3,9,10-trimethoxypterocarpan | Astragalus membranaceus; | roots | [61] |
| Astragalus mongholicus | roots | |||
| 104 | vesticarpan | Astragalus membranaceus | roots | [62] |
| 105 | (−)-methylinissolin | Astragalus membranaceus | roots | [62] |
| 106 | (−)-methylinissolin-3-O-β-d-{6′-[2′’-(E)-ene-penyl]}-glucoside | Astragalus membranaceus | roots | [62] |
| 107 | (−)-methylinissolin-3-O-β-d-(6′-acetyl)-glucoside | Astragalus membranaceus | roots | [62] |
| 108 | (−)-methylinissolin-3-O-β-d-glucoside | Astragalus membranaceus | roots | [62] |
| 109 | licoagroside D | Astragalus membranaceus | roots | [62] |
| No. | Name | Plant Source | Plant Organ | Ref. |
|---|---|---|---|---|
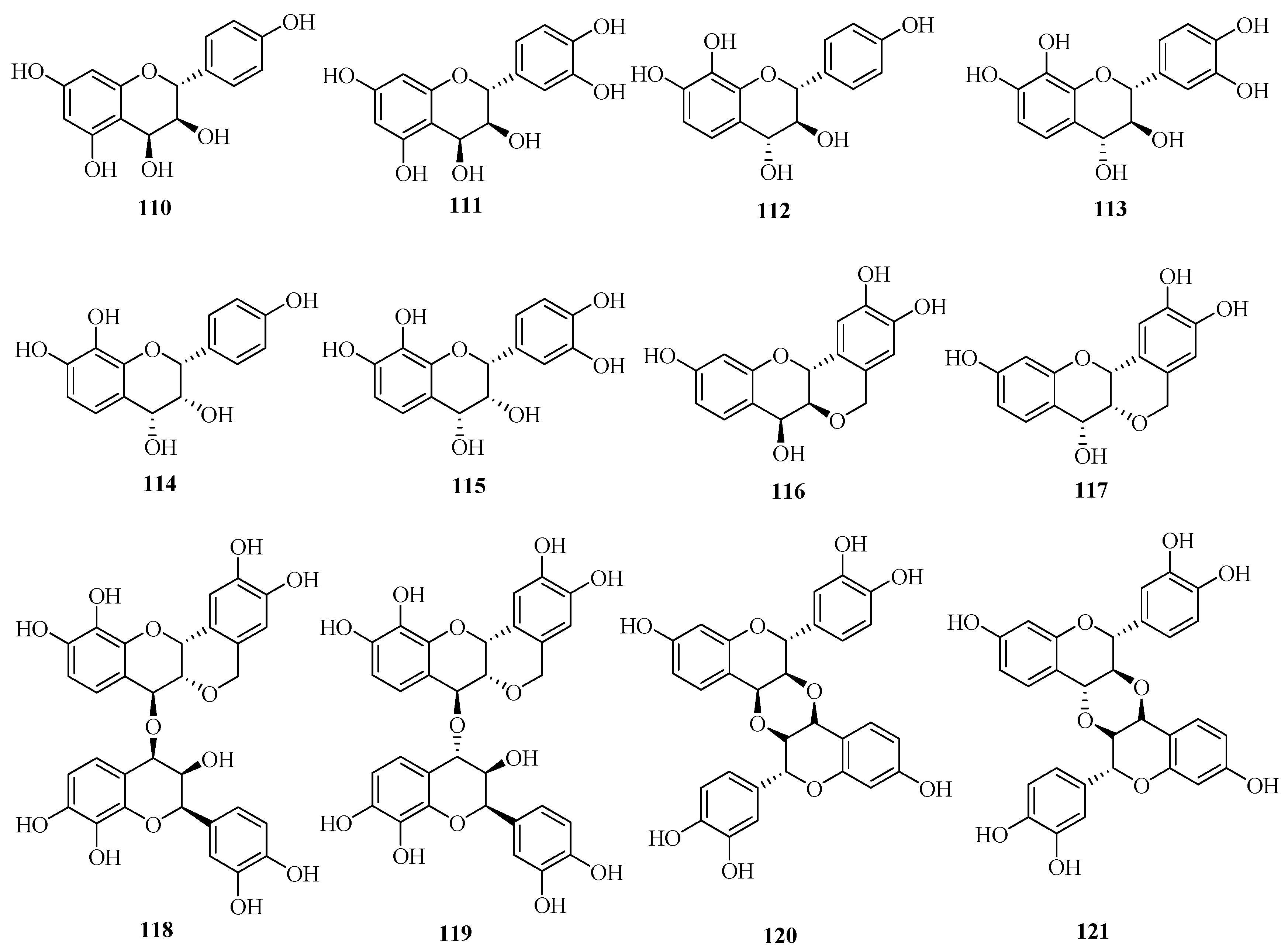
| 110 | guibourtacacidin | Acacia farnesiana | heartwoods | [63] |
| 111 | mollisacacidin | Acacia farnesiana | heartwoods | [63] |
| 112 | leucopelargonidin | Saraca asoca | barks | [25] |
| 113 | leucocyanidin | Saraca asoca | barks | [25] |
| 114 | teracacidin | Acacia galpinii | heartwoods | [63] |
| 115 | melacacidin | Acacia nilotica subsp. indica | heartwoods | [63] |
| 116 | (+)-2,3-trans-3,4-cis-peltogynol | Acacia melanoxylon | barks | [64] [65] |
| 117 | (−)-2,3-cis-3,4-cis-peltogynol | Acacia peuce; | barks | [64] [65] |
| Acacia carneorum; | barks | |||
| Acacia crombiei; | barks | |||
| Acacia fasciculifera | barks | |||
| 118 | [4-O-4]-bis-(2,3-cis-3,4-trans-3,3′,4′,7,8)-pentahydroxyflavan | Acacia peuce; | barks | [66] |
| Acacia carneorum; | barks | |||
| Acacia crombiei; | barks | |||
| Acacia fasciculifer | barks | |||
| 119 | 2,3-cis-3,4-trans-3,3′,4′,7,8-pentahydroxflavan-[4-O-4]-2,3-cis-3,4-cis-3,3′,4′,7,8-pentahydroxyflavan | Acacia peuce; | barks | [66] |
| Acacia carneorum; | barks | |||
| Acacia crombiei; | barks | |||
| Acacia fasciculifera | barks | |||
| 120 | [3,4,3′,4]-O,O-linked-bis-(2,3-trans-3,4-cis-3′,4′,7-trihydroxyflavan) | Acacia peuce; | barks | [67] |
| Acacia carneorum; | barks | |||
| Acacia crombiei; | barks | |||
| Acacia fasciculifera | barks | |||
| 121 | 2,3-trans-3,4-trans-2′,3′-trans-3′,4′-cis-diastereoisomer | Acacia peuce; | barks | [67] |
| Acacia carneorum; | barks | |||
| Acacia crombiei; | barks | |||
| Acacia fasciculifera | barks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules 2022, 27, 719. https://doi.org/10.3390/molecules27030719
Luo Y, Jian Y, Liu Y, Jiang S, Muhammad D, Wang W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules. 2022; 27(3):719. https://doi.org/10.3390/molecules27030719
Chicago/Turabian StyleLuo, Yu, Yuqing Jian, Yingkai Liu, Sai Jiang, Daniyal Muhammad, and Wei Wang. 2022. "Flavanols from Nature: A Phytochemistry and Biological Activity Review" Molecules 27, no. 3: 719. https://doi.org/10.3390/molecules27030719
APA StyleLuo, Y., Jian, Y., Liu, Y., Jiang, S., Muhammad, D., & Wang, W. (2022). Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules, 27(3), 719. https://doi.org/10.3390/molecules27030719








