4-Deoxy-l-erythro-5-hexoseulose Uronate (DEH) and DEH Reductase: Key Molecule and Enzyme for the Metabolism and Utilization of Alginate
Abstract
1. Introduction
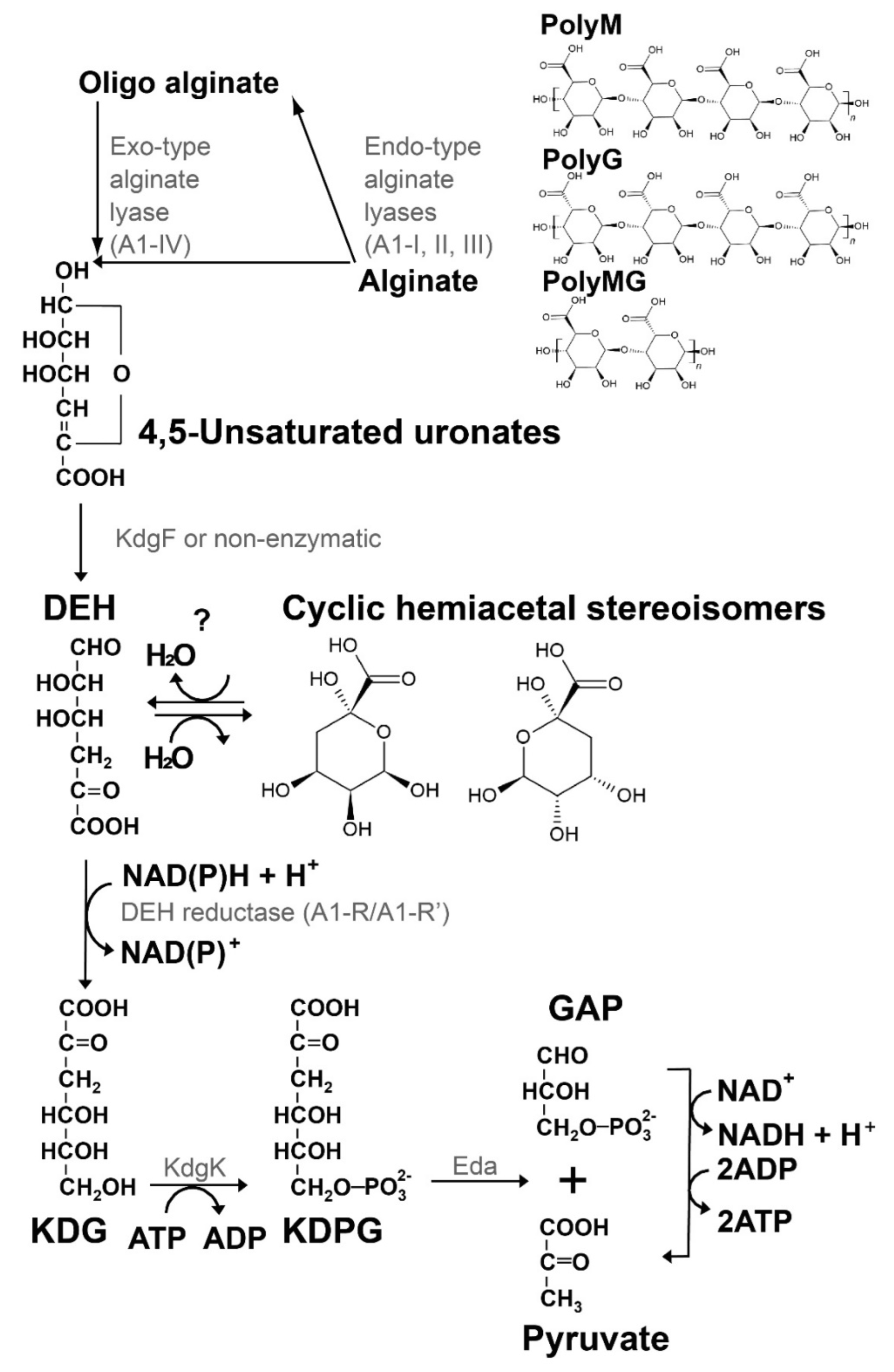
2. Formation, Structure, and Reactivity of DEH
2.1. Non-Enzymatic Formation of DEH from 4,5-Unsaturated Uronate
2.2. Enzymatic Formation of DEH from 4,5-Unsaturated Uronate
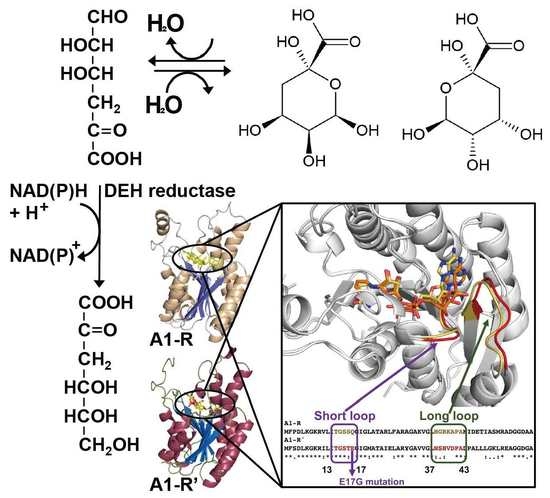
2.3. DEH as a Substrate of DEH Reductase
2.4. Structure of DEH
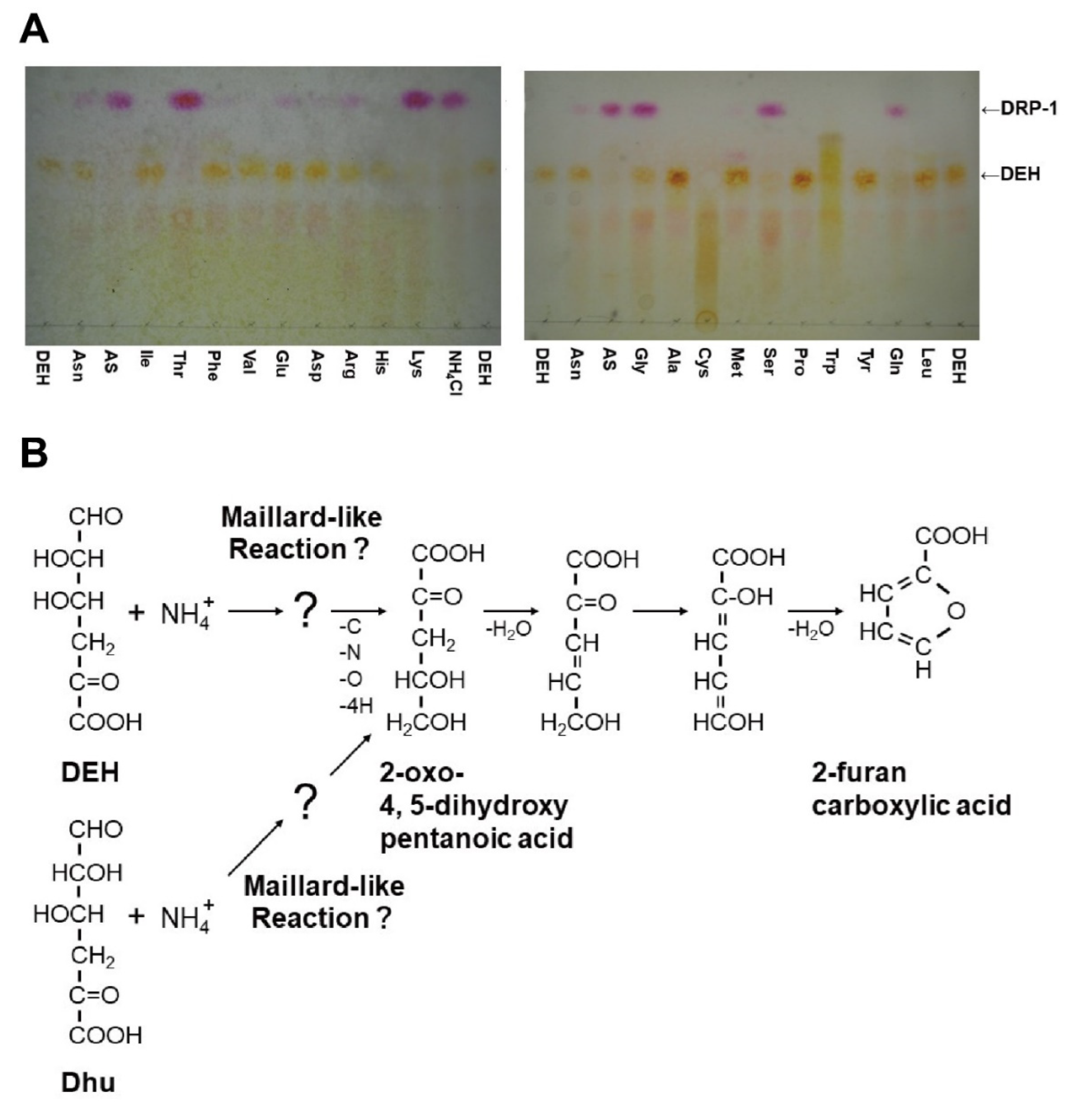
2.5. Reactivity of DEH with Specific Amino Groups
3. DEH Reductase
3.1. Discovery and Classification of DEH Reductase
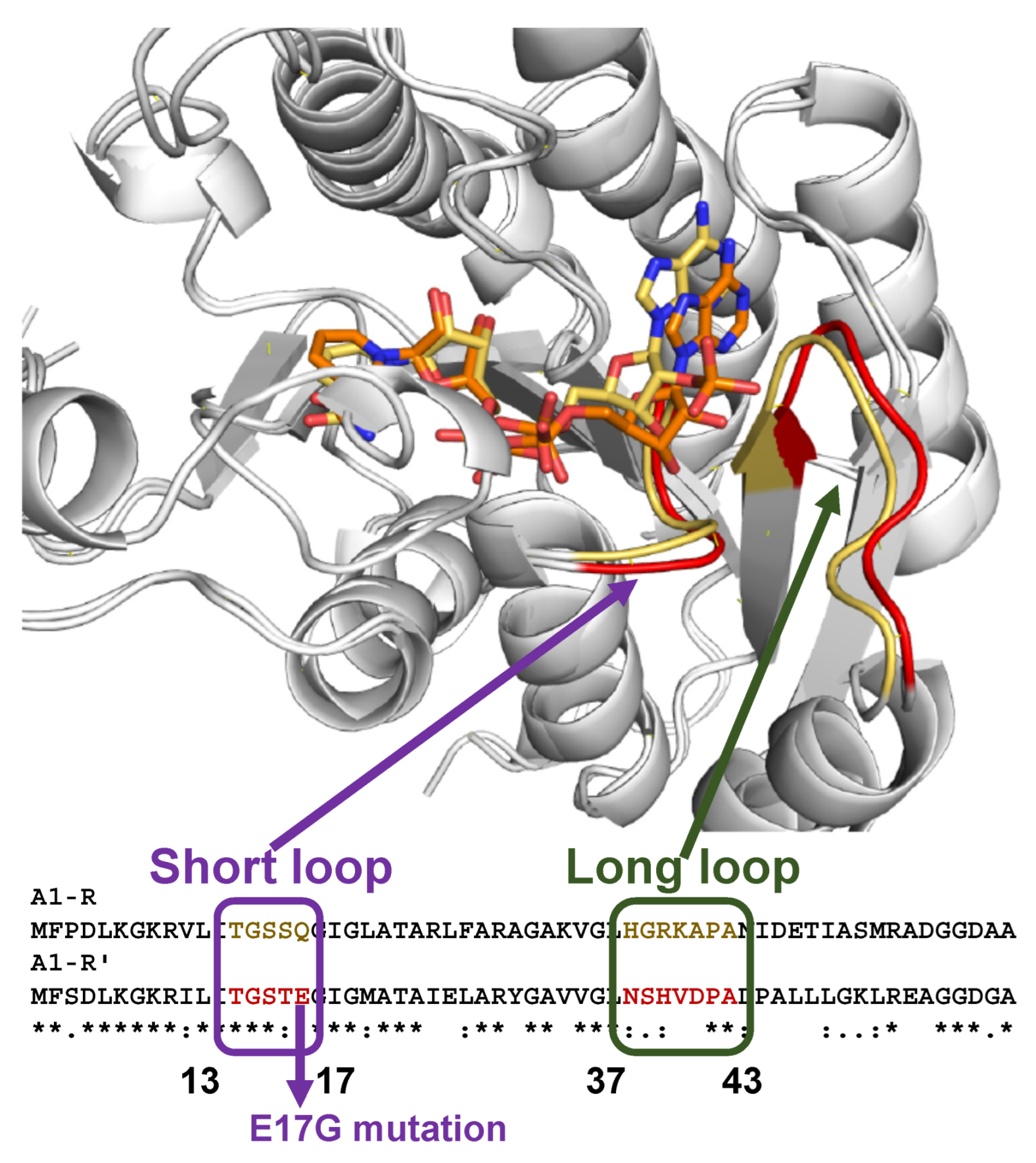
3.2. Structure of DEH Reductase
3.3. Molecular Conversion of Coenzyme Specificity in DEH Reductase
4. Significance of DEH for Biorefinery
4.1. Three Types of Hosts for the Utilization of Alginate in Biorefinery
4.2. Yeast as a Host for Biorefinery to Utilize Alginate
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawai, S.; Murata, K. Biofuel production based on carbohydrates from both brown and red macroalgae: Recent developments in key biotechnologies. Int. J. Mol. Sci. 2016, 17, 145. [Google Scholar] [CrossRef]
- Larsen, B.; Salem, D.M.S.A.; Sallam, M.A.E.; Mishrikey, M.M.; Beltagy, A.I. Characterization of the alginates from algae harvested at the Egyptian Red Sea coast. Carbohydr. Res. 2003, 338, 2325–2336. [Google Scholar] [CrossRef]
- Hashimoto, W.; Miyake, O.; Momma, K.; Kawai, S.; Murata, K. Molecular identification of oligoalginate lyase of Sphingomonas sp. strain A1 as one of the enzymes required for complete depolymerization of alginate. J. Bacteriol. 2000, 182, 4572–4577. [Google Scholar] [CrossRef]
- Kim, H.T.; Chung, J.H.; Wang, D.; Lee, J.; Woo, H.C.; Choi, I.G.; Kim, K.H. Depolymerization of alginate into a monomeric sugar acid using Alg17C, an exo-oligoalginate lyase cloned from Saccharophagus degradans 2-40. Appl. Microbiol. Biotechnol. 2012, 93, 2233–2239. [Google Scholar] [CrossRef]
- Ochiai, A.; Yamasaki, M.; Mikami, B.; Hashimoto, W.; Murata, K. Crystal structure of exotype alginate lyase Atu3025 from Agrobacterium tumefaciens. J. Biol. Chem. 2010, 285, 24519–24528. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, J.K.; Lee, S.M.; Robb, M.; Hof, F.; Barr, C.; Abe, K.T.; Hehemann, J.H.; McLean, R.; Abbott, D.W.; Boraston, A.B. KdgF, the missing link in the microbial metabolism of uronate sugars from pectin and alginate. Proc. Natl. Acad. Sci. USA 2016, 113, 6188–6193. [Google Scholar] [CrossRef] [PubMed]
- Preiss, J.; Ashwell, G. Alginic acid metabolism in bacteria. I. Enzymatic formation of unsaturated oligosac-charides and 4-deoxy-l-erythro-5-hexoseulose uronic acid. J. Biol. Chem. 1962, 237, 309–316. [Google Scholar] [CrossRef]
- Enquist-Newman, M.; Faust, A.M.; Bravo, D.D.; Santos, C.N.; Raisner, R.M.; Hanel, A.; Sarvabhowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 2014, 505, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, F.; Hirayama, M.; Kashihara, T.; Tanaka, H.; Hashimoto, W.; Murata, K.; Kawai, S. Crucial role of 4-deoxy-l-erythro-5-hexoseulose uronate reductase for alginate utilization revealed by adaptive evolution in engineered Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 4206. [Google Scholar] [CrossRef]
- Takagi, T.; Sasaki, Y.; Motone, K.; Shibata, T.; Tanaka, R.; Miyake, H.; Mori, T.; Kuroda, K.; Ueda, M. Construction of bioengineered yeast platform for direct bioethanol production from alginate and mannitol. Appl. Microbiol. Biotechnol. 2017, 101, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Pouyssegur, J.; Stoeber, F. Study of the common degradative pathway of hexuronates in Escherichia coli K 12. Purification, properties and individuality of 2-keto-3-deoxy-D-gluconnokinase. Biochimie 1971, 53, 771–781. [Google Scholar] [CrossRef]
- Egan, S.E.; Fliege, R.; Tong, S.; Shibata, A.; Wolf, R.E., Jr.; Conway, T. Molecular characterization of the Entner-Doudoroff pathway in Escherichia coli: Sequence analysis and localization of promoters for the edd-eda operon. J. Bacteriol. 1992, 174, 4638–4646. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jiang, C.; Xu, J.; Liu, Z.; Mao, X. Characteristics and applications of alginate lyases: A review. Int. J. Biol. Macromol. 2020, 164, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, P.; Zhang, Y.Z.; Chen, X.L. Diversity of three-dimensional structures and catalytic mechanisms of alginate lyases. Appl. Environ. Microbiol. 2018, 84, e02040-17. [Google Scholar] [CrossRef]
- Dharani, S.R.; Srinivasan, R.; Sarath, R.; Ramya, M. Recent progress on engineering microbial alginate lyases towards their versatile role in biotechnological applications. Folia Microbiol. 2020, 65, 937–954. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef]
- Takase, R.; Mikami, B.; Kawai, S.; Murata, K.; Hashimoto, W. Structure-based conversion of the coenzyme requirement of a short-chain dehydrogenase/reductase involved in bacterial alginate metabolism. J. Biol. Chem. 2014, 289, 33198–33214. [Google Scholar] [CrossRef]
- Mori, T.; Takahashi, M.; Tanaka, R.; Miyake, H.; Shibata, T.; Chow, S.; Kuroda, K.; Ueda, M.; Takeyama, H. Falsirhodobacter sp. alg1 harbors single homologs of endo and exo-type alginate lyases efficient for alginate depolymerization. PLoS ONE 2016, 11, e0155537. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, R.; Mochizuki, S.; Ojima, T. Identification of a 4-deoxy-l-erythro-5-hexoseulose uronic acid reductase, FlRed, in an alginolytic bacterium Flavobacterium sp. strain UMI-01. Mar. Drugs 2015, 13, 493–508. [Google Scholar] [CrossRef]
- Nakata, S.; Murata, K.; Hashimoto, W.; Kawai, S. Uncovering the reactive nature of 4-deoxy-l-erythro-5-hexoseulose uronate for the utilization of alginate, a promising marine biopolymer. Sci. Rep. 2019, 9, 17147. [Google Scholar] [CrossRef]
- Mochizuki, S.; Nishiyama, R.; Inoue, A.; Ojima, T. A novel aldo-keto reductase, HdRed, from the pacific abalone Haliotis discus hannai, which reduces alginate-derived 4-deoxy-l-erythro-5-hexoseulose uronic acid to 2-keto-3-deoxy-D-gluconate. J. Biol. Chem. 2015, 290, 30962–30974. [Google Scholar] [CrossRef]
- Shibata, T.; Fujii, R.; Miyake, H.; Tanaka, R.; Mori, T.; Takahashi, M.; Takagi, T.; Yoshikawa, H.; Kuroda, K.; Ueda, M. Development of an analysis method for 4-deoxy-l-erythro-5-hexoseulose uronic acid by LC/ESI/MS with selected ion monitoring. Nat. Prod. Commun. 2017, 12, 941–944. [Google Scholar] [CrossRef]
- Hirayama, M.; Hashimoto, W.; Murata, K.; Kawai, S. Comparative characterization of three bacterial exo-type alginate lyases. Int. J. Biol. Macromol. 2016, 86, 519–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cha, Q.Q.; Wang, X.J.; Ren, X.B.; Li, D.; Wang, P.; Li, P.Y.; Fu, H.H.; Zhang, X.Y.; Chen, X.L.; Zhang, Y.Z.; et al. Comparison of alginate utilization pathways in culturable bacteria isolated from Arctic and Antarctic marine environments. Front. Microbiol. 2021, 12, 609393. [Google Scholar] [CrossRef] [PubMed]
- Takase, R.; Ochiai, A.; Mikami, B.; Hashimoto, W.; Murata, K. Molecular identification of unsaturated uronate reductase prerequisite for alginate metabolism in Sphingomonas sp. A1. Biochim. Biophys. Acta 2010, 1804, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Han, F.; Gong, Q.; Yu, W. Cloning and characterization of the first polysaccharide lyase family 6 oligoalginate lyase from marine Shewanella sp. Kz7. J. Biochem. 2016, 159, 77–86. [Google Scholar] [CrossRef]
- Maruyama, Y.; Oiki, S.; Takase, R.; Mikami, B.; Murata, K.; Hashimoto, W. Metabolic fate of unsaturated glucuronic/iduronic acids from glycosaminoglycans: Molecular identification and structure determination of streptococcal isomerase and dehydrogenase. J. Biol. Chem. 2015, 290, 6281–6292. [Google Scholar] [CrossRef]
- Preiss, J.; Ashwell, G. Alginic acid metabolism in bacteria. II. Enzymatic reduction of 4-deoxy-l-erythro-5-hexoseulose uronic acid to 2-keto-3-deoxy-D-gluconic acid. J. Biol. Chem. 1962, 237, 317–321. [Google Scholar] [CrossRef]
- Murata, K.; Kawai, S.; Mikami, B.; Hashimoto, W. Superchannel of bacteria: Biological significance and new horizons. Biosci. Biotechnol. Biochem. 2008, 72, 265–277. [Google Scholar] [CrossRef]
- Hisano, T.; Kimura, N.; Hashimoto, W.; Murata, K. Pit structure on bacterial cell surface. Biochem. Biophys. Res. Commun. 1996, 220, 979–982. [Google Scholar] [CrossRef]
- Momma, K.; Okamoto, M.; Mishima, Y.; Mori, S.; Hashimoto, W.; Murata, K. A novel bacterial ATP-binding cassette transporter system that allows uptake of macromolecules. J. Bacteriol. 2000, 182, 3998–4004. [Google Scholar] [CrossRef]
- Yoon, H.J.; Hashimoto, W.; Miyake, O.; Okamoto, M.; Mikami, B.; Murata, K. Overexpression in Escherichia coli, purification, and characterization of Sphingomonas sp A1 alginate lyases. Protein Expr. Purif. 2000, 19, 84–90. [Google Scholar] [CrossRef]
- Hayashi, C.; Takase, R.; Momma, K.; Maruyama, Y.; Murata, K.; Hashimoto, W. Alginate-dependent gene expression mechanism in Sphingomonas sp. strain A1. J. Bacteriol. 2014, 196, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Yoneyama, F.; Kawai, S.; Hashimoto, W.; Murata, K. Bioethanol production from marine biomass alginate by genetically engineered bacteria. Energy Environ. Sci. 2011, 4, 2575–2581. [Google Scholar] [CrossRef]
- Hashimoto, W.; Maruyama, Y.; Itoh, T.; Takase, R.; Murata, K. Structural basis of bacterial supramolecules for import of heteropolysaccharide. Kagaku Seibutsu 2016, 5, 885–891. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Kavanagh, K.L.; Jornvall, H.; Persson, B.; Oppermann, U. Medium- and short-chain dehydrogenase/reductase gene and protein families: The SDR superfamily: Functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 2008, 65, 3895–3906. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Morisaka, H.; Aburaya, S.; Tatsukami, Y.; Kuroda, K.; Ueda, M. Putative alginate assimilation process of the marine bacterium Saccharophagus degradans 2-40 based on quantitative proteomic analysis. Mar. Biotechnol. (N.Y.) 2016, 18, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, O.K.; Lee, E.Y. Identification of 4-deoxy-l-etychro-hexoseulose uronic acid reductases in an alginolytic bacterium Vibrio splendidus and their uses for L-lactate production in an Escherichia coli cell-free system. Mar. Biotechnol. 2018, 20, 410–423. [Google Scholar] [CrossRef]
- Inoue, A.; Ojima, T. Functional identification of the 4-deoxy-l-erythro-5-hexoseulose uronate reductase from a brown alga, Saccharina japonica. Biochem. Biophys. Res. Commun. 2021, 545, 112–118. [Google Scholar] [CrossRef]
- Penning, T.M.; Wangtrakuldee, P.; Auchus, R.J. Structural and functional biology of aldo-keto reductase steroid-transforming enzymes. Endocr. Rev. 2019, 40, 447–475. [Google Scholar] [CrossRef]
- Hashimoto, W.; Kawai, S.; Murata, K. Bacterial supersystem for alginate import/metabolism and its environmental and bioenergy applications. Bioeng. Bugs 2010, 1, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Takase, R.; Maruyama, Y.; Oiki, S.; Mikami, B.; Murata, K.; Hashimoto, W. Structural determinants in bacterial 2-keto-3-deoxy-D-gluconate dehydrogenase KduD for dual-coenzyme specificity. Proteins 2016, 84, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Kuroda, K.; Ueda, M. Platform construction of molecular breeding for utilization of brown macroalgae. J. Biosci. Bioeng. 2018, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Ohashi, K.; Yoshida, S.; Fujii, M.; Mikami, S.; Sato, N.; Murata, K. Bacterial pyruvate production from alginate, a promising carbon source from marine brown macroalgae. J. Biosci. Bioeng. 2014, 117, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.G.; Kwak, D.H.; Park, S.; Woo, S.; Yang, J.S.; Kang, C.W.; Kim, B.; Noh, M.H.; Seo, S.W.; Jung, G.Y. Vibrio sp. dhg as a platform for the biorefinery of brown macroalgae. Nat. Commun. 2019, 10, 2486. [Google Scholar] [CrossRef] [PubMed]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef]
- Sharma, S.; Hansen, L.D.; Hansen, J.O.; Mydland, L.T.; Horn, S.J.; Overland, M.; Eijsink, V.G.H.; Vuoristo, K.S. Microbial protein produced from brown seaweed and spruce wood as a feed ingredient. J. Agric. Food Chem. 2018, 66, 8328–8335. [Google Scholar] [CrossRef]
- Manns, D.; Nielsen, M.M.; Bruhn, A.; Saake, B.; Meyer, A.S. Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J. Appl. Phycol. 2017, 29, 1493–1506. [Google Scholar] [CrossRef]
- Silvestrini, L.; Rossi, B.; Gallmetzer, A.; Mathieu, M.; Scazzocchio, C.; Berardi, E.; Strauss, J. Interaction of Yna1 and Yna2 is required for nuclear accumulation and transcriptional activation of the nitrate assimilation pathway in the yeast Hansenula polymorpha. PLoS ONE 2015, 10, e0135416. [Google Scholar] [CrossRef]
- De Barros Pita, W.; Leite, F.C.; de Souza Liberal, A.T.; Simoes, D.A.; de Morais, M.A., Jr. The ability to use nitrate confers advantage to Dekkera bruxellensis over S. cerevisiae and can explain its adaptation to industrial fermentation processes. Antonie Van Leeuwenhoek 2011, 100, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; Qureshi, N. Biofuel demand realization. In Biomass to Biofuels: Strategies for Global Industries; Vertès, A., Qureshi, N., Yukawa, H., Blaschek, H.P., Eds.; Wiley: Hoboken, NJ, USA, 2010; pp. 55–69. [Google Scholar]
- Nielsen, J.; Larsson, C.; van Maris, A.; Pronk, J. Metabolic engineering of yeast for production of fuels and chemicals. Curr. Opin. Biotechnol. 2013, 24, 398–404. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawai, S.; Hashimoto, W. 4-Deoxy-l-erythro-5-hexoseulose Uronate (DEH) and DEH Reductase: Key Molecule and Enzyme for the Metabolism and Utilization of Alginate. Molecules 2022, 27, 338. https://doi.org/10.3390/molecules27020338
Kawai S, Hashimoto W. 4-Deoxy-l-erythro-5-hexoseulose Uronate (DEH) and DEH Reductase: Key Molecule and Enzyme for the Metabolism and Utilization of Alginate. Molecules. 2022; 27(2):338. https://doi.org/10.3390/molecules27020338
Chicago/Turabian StyleKawai, Shigeyuki, and Wataru Hashimoto. 2022. "4-Deoxy-l-erythro-5-hexoseulose Uronate (DEH) and DEH Reductase: Key Molecule and Enzyme for the Metabolism and Utilization of Alginate" Molecules 27, no. 2: 338. https://doi.org/10.3390/molecules27020338
APA StyleKawai, S., & Hashimoto, W. (2022). 4-Deoxy-l-erythro-5-hexoseulose Uronate (DEH) and DEH Reductase: Key Molecule and Enzyme for the Metabolism and Utilization of Alginate. Molecules, 27(2), 338. https://doi.org/10.3390/molecules27020338