Anti-Influenza with Green Tea Catechins: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
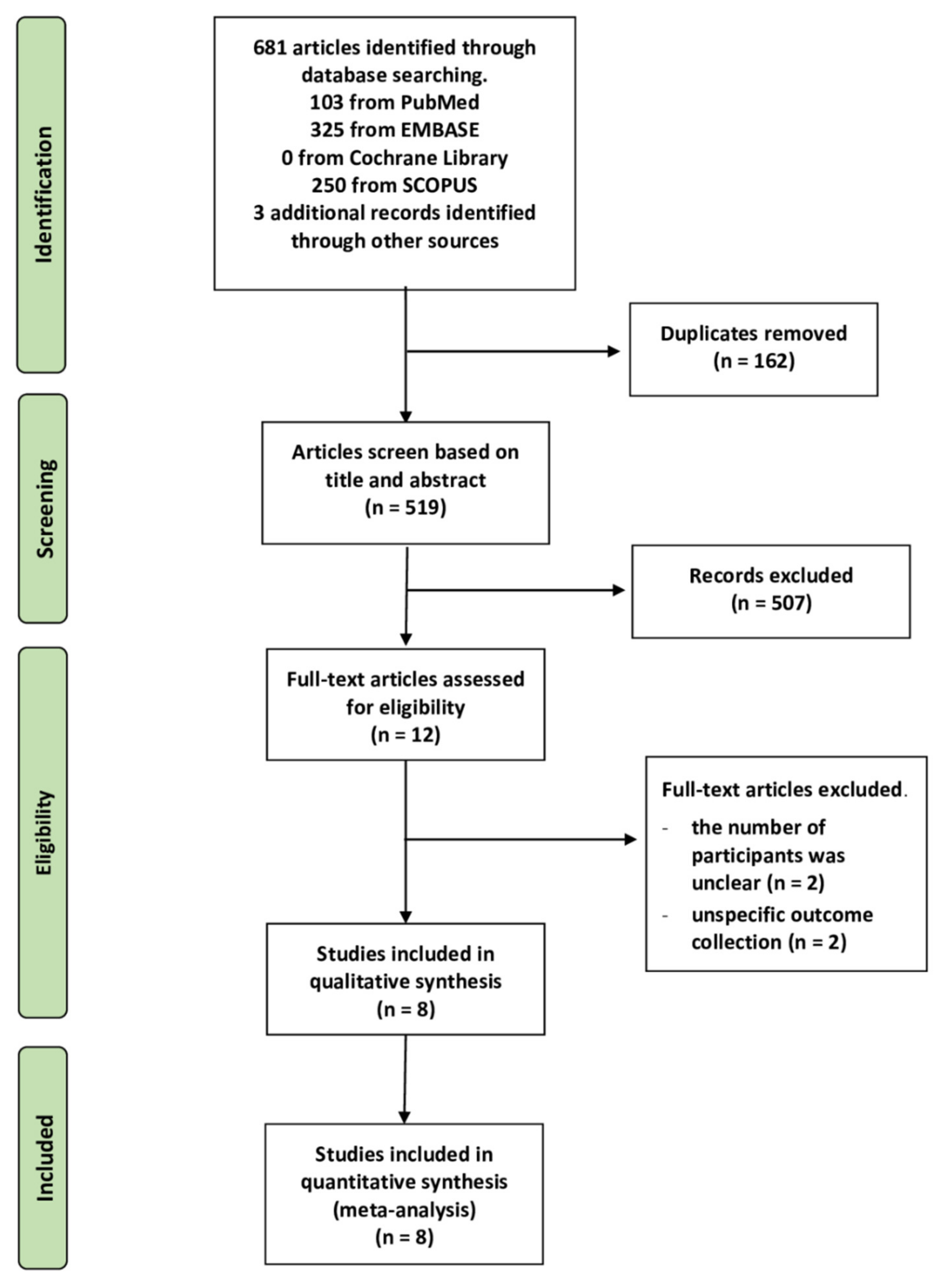
2.1. Literature Search
2.2. Characteristics of the Included Studies
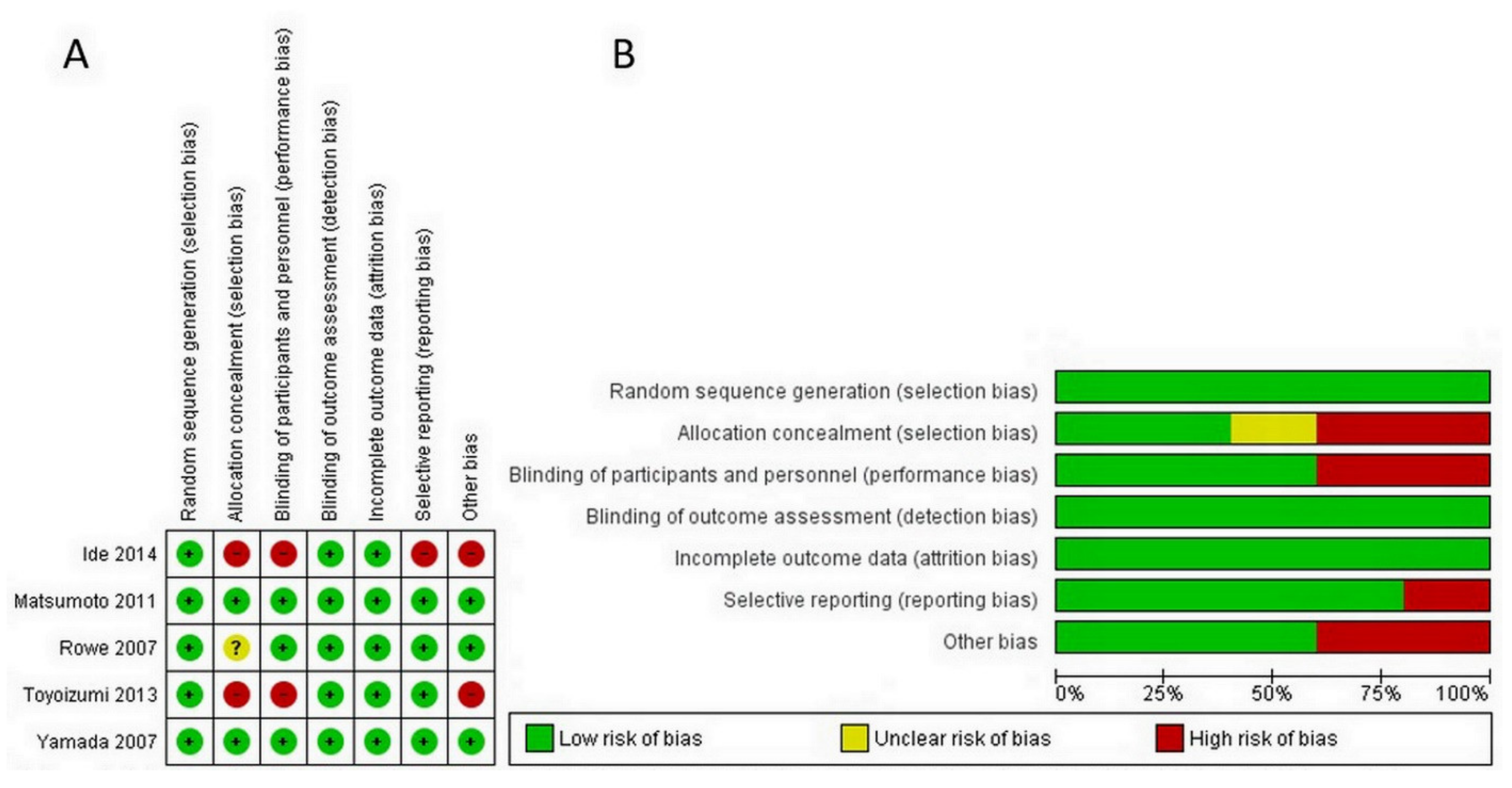
2.3. Quality Assessment
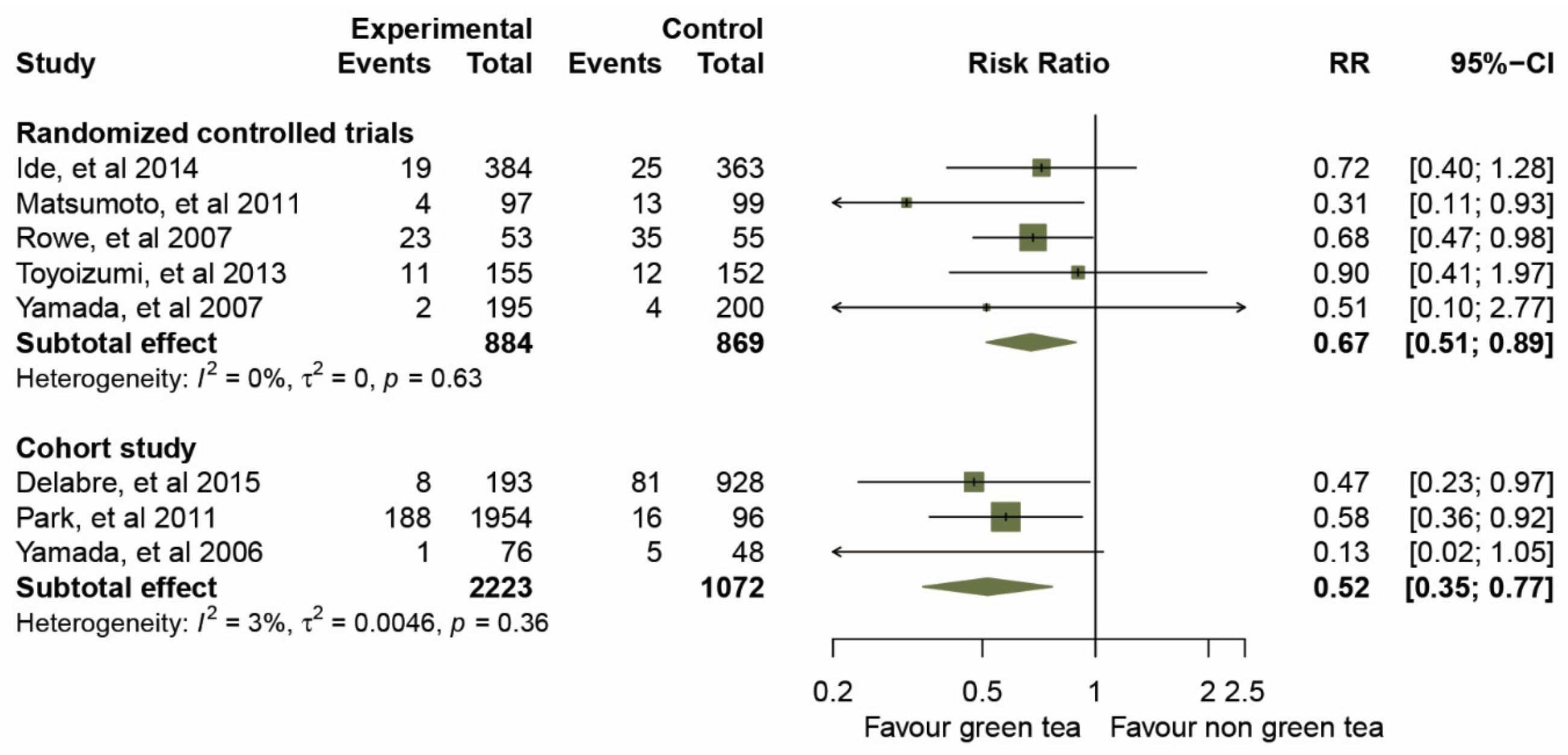
2.4. Clinical Effects of GTCs on Influenza Prevention
2.5. Sensitivity and Subgroup Analyses
2.6. Publication Bias of Included Studies
3. Discussion
3.1. Comparison with Other Studies
3.2. Clinical Implication
3.3. Strengths and Limitations
4. Materials and Methods
4.1. Search Strategies
4.2. Data Extraction and Outcome Measures
4.3. Quality Assessment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Fact Sheets Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza (accessed on 11 June 2021).
- Boktor, S.W.; Hafner, J.W. Influenza. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Killingley, B.; Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza Other Respir. Viruses 2013, 7 (Suppl. 2), 42–51. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, C.; Wang, Q. Physical distancing, face masks, and eye protection for prevention of COVID-19. Lancet 2020, 395. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Firenzuoli, F. Ginseng integrative supplementation for seasonal acute upper respiratory infections: A systematic review and meta-analysis. Complementary Ther. Med. 2020, 52, 102457. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.A. Prevention and Treatment of Influenza, Influenza-Like Illness, and Common Cold by Herbal, Complementary, and Natural Therapies. J. Evid. Based. Complementary Altern. Med. 2017, 22, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wieland, L.S.; Piechotta, V.; Feinberg, T.; Ludeman, E.; Hutton, B.; Kanji, S.; Seely, D.; Garritty, C. Elderberry for prevention and treatment of viral respiratory illnesses: A systematic review. BMC Complement. Med. 2021, 21, 112. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Sodagari, H.R.; Farzaei, M.H.; Abdolghaffari, A.H.; Gooshe, M.; Rezaei, N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev. Anti Infect. 2016, 14, 57–80. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M.; Imai, K.; Nakachi, K. Green tea: Cancer preventive beverage and/or drug. Cancer Lett. 2002, 188, 9–13. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef] [Green Version]
- Furushima, D.; Ide, K.; Yamada, H. Effect of Tea Catechins on Influenza Infection and the Common Cold with a Focus on Epidemiological/Clinical Studies. Molecules 2018, 23, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules 2021, 26, 3572. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef] [Green Version]
- Chow, H.H.S.; Hakim, I.A. Pharmacokinetic and chemoprevention studies on tea in humans. Pharm. Res 2011, 64, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.B.; Tao, S.; Lee, M.-J.; Hu, Q.; Meng, X.; Lin, Y.; Yang, C.S. Effects of gut microbiota and time of treatment on tissue levels of green tea polyphenols in mice. Biofactors 2018. [Google Scholar] [CrossRef]
- Suganuma, M.; Okabe, S.; Oniyama, M.; Tada, Y.; Ito, H.; Fujiki, H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 1998, 19, 1771–1776. [Google Scholar] [CrossRef]
- Andreu-Fernández, V.; Almeida Toledano, L.; Pizarro, N.; Navarro-Tapia, E.; Gómez-Roig, M.D.; de la Torre, R.; García-Algar, Ó. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Taher, F.; Llantada, A.; Do, Q.; Sapp, T.; Sommerhalter, M. Effect of Water Hardness on Catechin and Caffeine Content in Green Tea Infusions. Molecules 2021, 26, 3485. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kozukue, N. Stability of green tea catechins in commercial tea leaves during storage for 6 months. J. Food Sci. 2009, 74, H47–H51. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.Y. Metabolism of green tea catechins: An overview. Curr. Drug. Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef] [PubMed]
- Kaihatsu, K.; Yamabe, M.; Ebara, Y. Antiviral Mechanism of Action of Epigallocatechin-3-O-gallate and Its Fatty Acid Esters. Molecules 2018, 23, 2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.-M.; Lee, K.-H.; Seong, B.-L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Imanishi, N.; Tuji, Y.; Katada, Y.; Maruhashi, M.; Konosu, S.; Mantani, N.; Terasawa, K.; Ochiai, H. Additional inhibitory effect of tea extract on the growth of influenza A and B viruses in MDCK cells. Microbiol. Immunol. 2002, 46, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, T.; Iwai, Y.; Takahashi, H.; Hatakeyama, D.; Echigo, N. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. 2009, 1, RRN1052. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Downard, K.M. Catechin inhibition of influenza neuraminidase and its molecular basis with mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 222–230. [Google Scholar] [CrossRef]
- Nanri, A.; Nakamoto, K.; Sakamoto, N.; Imai, T.; Mizoue, T. Green tea consumption and influenza infection among Japanese employees. Eur. J. Clin. Nutr. 2021, 75, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Kawasaki, Y.; Akutagawa, M.; Yamada, H. Effects of Green Tea Gargling on the Prevention of Influenza Infection: An Analysis Using Bayesian Approaches. J. Altern. Complement. Med. 2017, 23, 116–120. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Kawasaki, Y. Effect of gargling with tea and ingredients of tea on the prevention of influenza infection: A meta-analysis. BMC Public Health 2016, 16, 396. [Google Scholar] [CrossRef] [Green Version]
- Rowe, C.A.; Nantz, M.P.; Bukowski, J.F.; Percival, S.S. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma, delta T cell function: A randomized, double-blind, placebo-controlled study. J. Am. Coll. Nutr. 2007, 26, 445–452. [Google Scholar] [CrossRef]
- Yamada, H.; Daimon, T.; Matsuda, K.; Yoshida, M.; Takuma, N.; Hara, Y. A Randomized Controlled Study on the Effects of Gargling with Tea Catechin Extracts on the Prevention of Influenza Infection in Healthy Adults. Rinsho Yakuri/Jpn. J. Clin. Pharmacol. Ther. 2007, 38, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complement. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef]
- Toyoizumi, K.; Yamada, H.; Matsumoto, K.; Sameshima, Y. Gargling with Green Tea for Influenza Prophylaxis: A Pilot Clinical Study. Rinsho Yakuri/Jpn. J. Clin. Pharmacol. Ther. 2013, 44, 459–461. [Google Scholar] [CrossRef] [Green Version]
- Ide, K.; Yamada, H.; Matsushita, K.; Ito, M.; Nojiri, K.; Toyoizumi, K.; Matsumoto, K.; Sameshima, Y. Effects of green tea gargling on the prevention of influenza infection in high school students: A randomized controlled study. PLoS ONE 2014, 9, e96373. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Takuma, N.; Daimon, T.; Hara, Y. Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: A prospective clinical study. J. Altern. Complement. Med. 2006, 12, 669–672. [Google Scholar] [CrossRef]
- Park, M.; Yamada, H.; Matsushita, K.; Kaji, S.; Goto, T.; Okada, Y.; Kosuge, K.; Kitagawa, T. Green tea consumption is inversely associated with the incidence of influenza infection among schoolchildren in a tea plantation area of Japan. J. Nutr. 2011, 141, 1862–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delabre, R.M.; Lapidus, N.; Salez, N.; Mansiaux, Y.; de Lamballerie, X.; Carrat, F. Risk factors of pandemic influenza A/H1N1 in a prospective household cohort in the general population: Results from the CoPanFlu-France cohort. Influenza Other Respir. Viruses 2015, 9, 43–50. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharm. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- NIH. Green Tea Dietary Supplement Pilot Study. Available online: https://www.dietarysupplementdatabase.usda.nih.gov/dsid_database/Res%20Summ%20Green%20Tea%201-8-2-17final.pdf (accessed on 11 June 2021).
- Kalus, U.; Kiesewetter, H.; Radtke, H. Effect of CYSTUS052 and green tea on subjective symptoms in patients with infection of the upper respiratory tract. Phytother. Res. 2010, 24, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Furushima, D.; Nishimura, T.; Takuma, N.; Iketani, R.; Mizuno, T.; Matsui, Y.; Yamaguchi, T.; Nakashima, Y.; Yamamoto, S.; Hibi, M.; et al. Prevention of Acute Upper Respiratory Infections by Consumption of Catechins in Healthcare Workers: A Randomized, Placebo-Controlled Trial. Nutrients 2019, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Kamath, A.B.; Wang, L.; Das, H.; Li, L.; Reinhold, V.N.; Bukowski, J.F. Antigens in tea-beverage prime human Vgamma 2Vdelta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc. Natl. Acad. Sci. USA 2003, 100, 6009–6014. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satomura, K.; Kitamura, T.; Kawamura, T.; Shimbo, T.; Watanabe, M.; Kamei, M.; Takano, Y.; Tamakoshi, A. Prevention of upper respiratory tract infections by gargling: A randomized trial. Am. J. Prev. Med. 2005, 29, 302–307. [Google Scholar] [CrossRef]
- Castilla, J.; Godoy, P.; Domínguez, Á.; Martín, V.; Delgado-Rodríguez, M.; Martínez-Baz, I.; Baricot, M.; Soldevila, N.; Mayoral, J.M.; Astray, J.; et al. Risk factors and effectiveness of preventive measures against influenza in the community. Influenza Other Respir. Viruses 2013, 7, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food. Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Marzec, Z.; Szwerc, W.; Głowniak, K. Green Tea Quality Evaluation Based on Its Catechins and Metals Composition in Combination with Chemometric Analysis. Molecules 2018, 23, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Zhao, J.; Kim, E.M.; Kim, K.H.; Kang, S.; Lee, H.; Lee, J. Comprehensive Investigation of the Effects of Brewing Conditions in Sample Preparation of Green Tea Infusions. Molecules 2019, 24, 1735. [Google Scholar] [CrossRef] [Green Version]
- Lapidus, N.; de Lamballerie, X.; Salez, N.; Setbon, M.; Delabre, R.M.; Ferrari, P.; Moyen, N.; Gougeon, M.L.; Vely, F.; Leruez-Ville, M.; et al. Factors associated with post-seasonal serological titer and risk factors for infection with the pandemic A/H1N1 virus in the French general population. PLoS ONE 2013, 8, e60127. [Google Scholar] [CrossRef] [Green Version]
- Cowling, B.J.; Chan, K.H.; Fang, V.J.; Cheng, C.K.; Fung, R.O.; Wai, W.; Sin, J.; Seto, W.H.; Yung, R.; Chu, D.W.; et al. Facemasks and hand hygiene to prevent influenza transmission in households: A cluster randomized trial. Ann. Intern. Med. 2009, 151, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowling, B.J.; Zhou, Y.; Ip, D.K.; Leung, G.M.; Aiello, A.E. Face masks to prevent transmission of influenza virus: A systematic review. Epidemiol. Infect. 2010, 138, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Bin-Reza, F.; Lopez Chavarrias, V.; Nicoll, A.; Chamberland, M.E. The use of masks and respirators to prevent transmission of influenza: A systematic review of the scientific evidence. Influenza Other Respir. Viruses 2012, 6, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.-X.; Wei, F.; Li, N.; Li, J.-L.; Chen, L.-J.; Liu, Y.-Y.; Luo, F.; Xiong, H.-R.; Hou, W.; Yang, Z.-Q. Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharm. Sin. 2012, 33, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawangkan, A.; Kengkla, K.; Duangjai, A.; Saokaew, S. Anti- influenza with green tea catechins: A systematic review and meta-analysis. PROSPERO. 2020. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020176371 (accessed on 11 June 2021).
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: Melbourne, Australia, 2011. [Google Scholar]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 12 March 2020. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 March 2021).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Authors (Year) | Study Design | Duration of Study (Months) | Route of Administration | Experimental Intervention GTCs mg/day | Age (Years) | Sex (M/F) | Received Influenza Vaccination | Clinical Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | N/A | Flu+ | Flu− | Flu+ | Flu− | Total | |||||||
| Yamada et al. (2006) [37] | Cohort | 3 | Gargling | 100 mg By 200 µg/mL ×3 times, 500 mL | 65–83 | 40/84 | 124 | 0 | 0 | 1 | 75 | 5 | 43 | 124 |
| Rowe et al. (2007) [32] | RCT | 3 | Taking capsules | 1500 mg By 2 capsules * | 18–70 | 43/65 | 0 | 108 | 0 | 23 | 30 | 35 | 20 | 108 |
| Yamada et al. (2007) [33] | RCT | 3 | Gargling | 120 mg By 400 µg/mL ×3 times, 300 mL | 20–65 | NA | 395 | 0 | 0 | 2 | 193 | 4 | 196 | 395 |
| Matsumoto et al. (2011) [34] | RCT | 5 | Taking capsules | 378 mg By 63 mg ×6 capsules | 21–69 | 44/152 | 182 | 14 | 0 | 4 | 93 | 13 | 86 | 196 |
| Park et al. (2011) [38] | Cohort | 4 | Drinking | 137–685 mg By 1–5 cups/day | 6–13 | 991/1059 | 1141 | 854 | 55 | 188 | 1766 | 16 | 80 | 2050 |
| Toyoizumi et al. (2013) [35] | RCT | 3 | Gargling | 280 mg By 560 µg/mL ×3 times, 500 mL | 15–20 | 184/124 | 130 | 177 | 0 | 11 | 144 | 12 | 140 | 307 |
| Ide et al. (2014) [36] | RCT | 3 | Gargling | 185 mg By 370 µg/mL ×3 times, 500 mL | 15–17 | 423/324 | 197 | 550 | 0 | 19 | 365 | 25 | 338 | 747 |
| Delabre et al. (2015) [39] | Cohort | 8 | Drinking | 300 mg By ≤2 cups/week | 15–50 | 520/601 | 0 | 1121 | 0 | 8 | 185 | 81 | 847 | 1121 |
| Study Criterion | Yamada et al. (2006) [37] | Park et al. (2011) [38] | Delabre et al. (2015) [39] |
|---|---|---|---|
| Selection (maximum ****) | **** | *** | **** |
| Comparability (maximum **) | ** | * | * |
| Outcome (maximum ***) | *** | ** | *** |
| Characteristics | All Studies | Randomized Control Trials | Cohort Studies | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk Ratio (95% CIs) | Heterogeneity | Risk Ratio (95% CIs) | Heterogeneity | Risk Ratio (95% CIs) | Heterogeneity | ||||
| I2-Index | p-Value | I2-Index | p-Value | I2-Index | p-Value | ||||
| Models | |||||||||
| ∙ Fixed effects model | 0.59 (0.47. 0.74) | 0.0% | 0.55 | 0.66 (0.50, 0.88) | 0.0% | 0.63 | 0.49 (0.33, 0.73) | 3.0% | 0.36 |
| ∙ Random effects model | 0.62 (0.49. 0.77) | 0.0% | 0.55 | 0.67 (0.51, 0.89) | 0.0% | 0.63 | 0.52 (0.35, 0.77) | 3.0% | 0.36 |
| Omission of Ide, et al. and Toyoizumi, et al. in the analysis of randomized control trials a | |||||||||
| ∙ Before omission | 0.62 (0.49. 0.77) | 0.0% | 0.55 | 0.67 (0.51, 0.89) | 0.0% | 0.63 | 0.52 (0.35, 0.77) | 3.0% | 0.36 |
| ∙ After omission | 0.58 (0.45, 0.74) | 0.0% | 0.45 | 0.62 (0.43, 0.88) | 1.0% | 0.36 | 0.52 (0.35, 0.77) | 3.0% | 0.36 |
| Omission of Park, et al. in the analysis of cohort study b | |||||||||
| ∙ Before omission | 0.62 (0.49. 0.77) | 0.0% | 0.55 | 0.67 (0.51, 0.89) | 0.0% | 0.63 | 0.52 (0.35, 0.77) | 3.0% | 0.36 |
| ∙ After omission | 0.63 (0.49, 0.81) | 0.0% | 0.44 | 0.67 (0.51, 0.89) | 0.0% | 0.63 | 0.36 (0.13, 1.03) | 26.0% | 0.24 |
| Dose of EGCG (mg/day) | |||||||||
| ∙ > 338 mg/day | 0.68 (0.47, 0.98) | N/A | N/A | 0.68 (0.47, 0.98) | N/A | N/A | N/A | N/A | N/A |
| ∙ ≤ 338 mg/day | 0.58 (0.44, 0.77) | 0.0% | 0.50 | 0.66 (0.44, 1.01) | 0.0% | 0.46 | 0.52 (0.35, 0.77) | 3.0% | 0.36 |
| Route of administration | |||||||||
| ∙ Gargling | 0.70 (0.44, 1.09) | 2.0% | 0.38 | 0.75 (0.48, 1.18) | 0.0% | 0.81 | 0.13 (0.02 1.05) | N/A | N/A |
| ∙ Taking capsules | 0.54 (0.26, 1.13) | 49.0% | 0.16 | 0.54 (0.26, 1.13) | 49.0% | 0.16 | N/A | N/A | N/A |
| ∙ Drinking | 0.54 (0.37, 0.80) | 0.0% | 0.64 | N/A | N/A | N/A | 0.54 (0.37, 0.80) | 0.0% | 0.64 |
| Type of Influenza virus | |||||||||
| ∙ Type A | 0.51 (0.10, 2.77) | N/A | N/A | 0.51 (0.10, 2.77) | N/A | N/A | N/A | N/A | N/A |
| ∙ Type B | 0.13 (0.02 1.05) | N/A | N/A | N/A | N/A | N/A | 0.13 (0.02 1.05) | N/A | N/A |
| ∙ Type A or B | 0.63 (0.50, 0.79) | 0.0% | 0.62 | 0.68 (0.51, 0.90) | 0.0% | 0.48 | 0.54 (0.37, 0.80) | 0.0% | 0.64 |
| Received influenza vaccinations (more than 80 percent of participants) | |||||||||
| ∙ Yes | 0.31 (0.13, 0.71) | 0.0% | 0.59 | 0.36 (0.15, 0.90) | 0.0% | 0.63 | 0.13 (0.02 1.05) | N/A | N/A |
| ∙ No | 0.65 (0.52, 0.82) | 0.0% | 0.76 | 0.72 (0.54, 0.96) | 0.0% | 0.82 | 0.54 (0.37, 0.80) | 0.0% | 0.64 |
| Age over 65 years | |||||||||
| ∙ Yes | 0.13 (0.02 1.05) | N/A | N/A | N/A | N/A | N/A | 0.13 (0.02 1.05) | N/A | N/A |
| ∙ No | 0.63 (0.50, 0.79) | 0.0% | 0.73 | 0.67 (0.51, 0.89) | 0.0% | 0.63 | 0.54 (0.37, 0.80) | 0.0% | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawangkan, A.; Kengkla, K.; Kanchanasurakit, S.; Duangjai, A.; Saokaew, S. Anti-Influenza with Green Tea Catechins: A Systematic Review and Meta-Analysis. Molecules 2021, 26, 4014. https://doi.org/10.3390/molecules26134014
Rawangkan A, Kengkla K, Kanchanasurakit S, Duangjai A, Saokaew S. Anti-Influenza with Green Tea Catechins: A Systematic Review and Meta-Analysis. Molecules. 2021; 26(13):4014. https://doi.org/10.3390/molecules26134014
Chicago/Turabian StyleRawangkan, Anchalee, Kirati Kengkla, Sukrit Kanchanasurakit, Acharaporn Duangjai, and Surasak Saokaew. 2021. "Anti-Influenza with Green Tea Catechins: A Systematic Review and Meta-Analysis" Molecules 26, no. 13: 4014. https://doi.org/10.3390/molecules26134014






