Glyconectin Cell Adhesion Epitope, β-d-GlcpNAc3S-(1→3)-α-l-Fucp, Is Involved in Blastulation of Lytechinus pictus Sea Urchin Embryos
Abstract
1. Introduction
2. Results
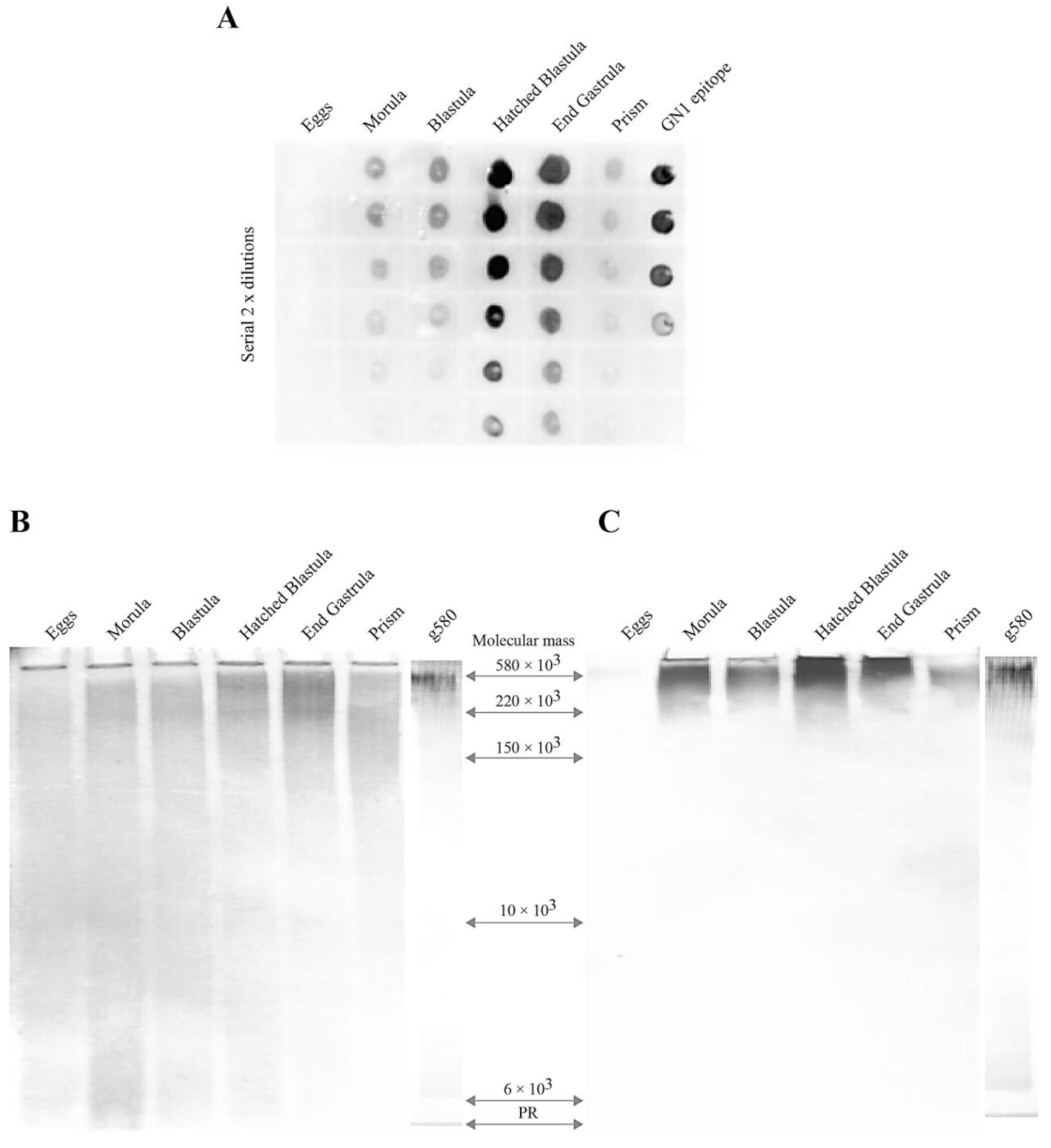
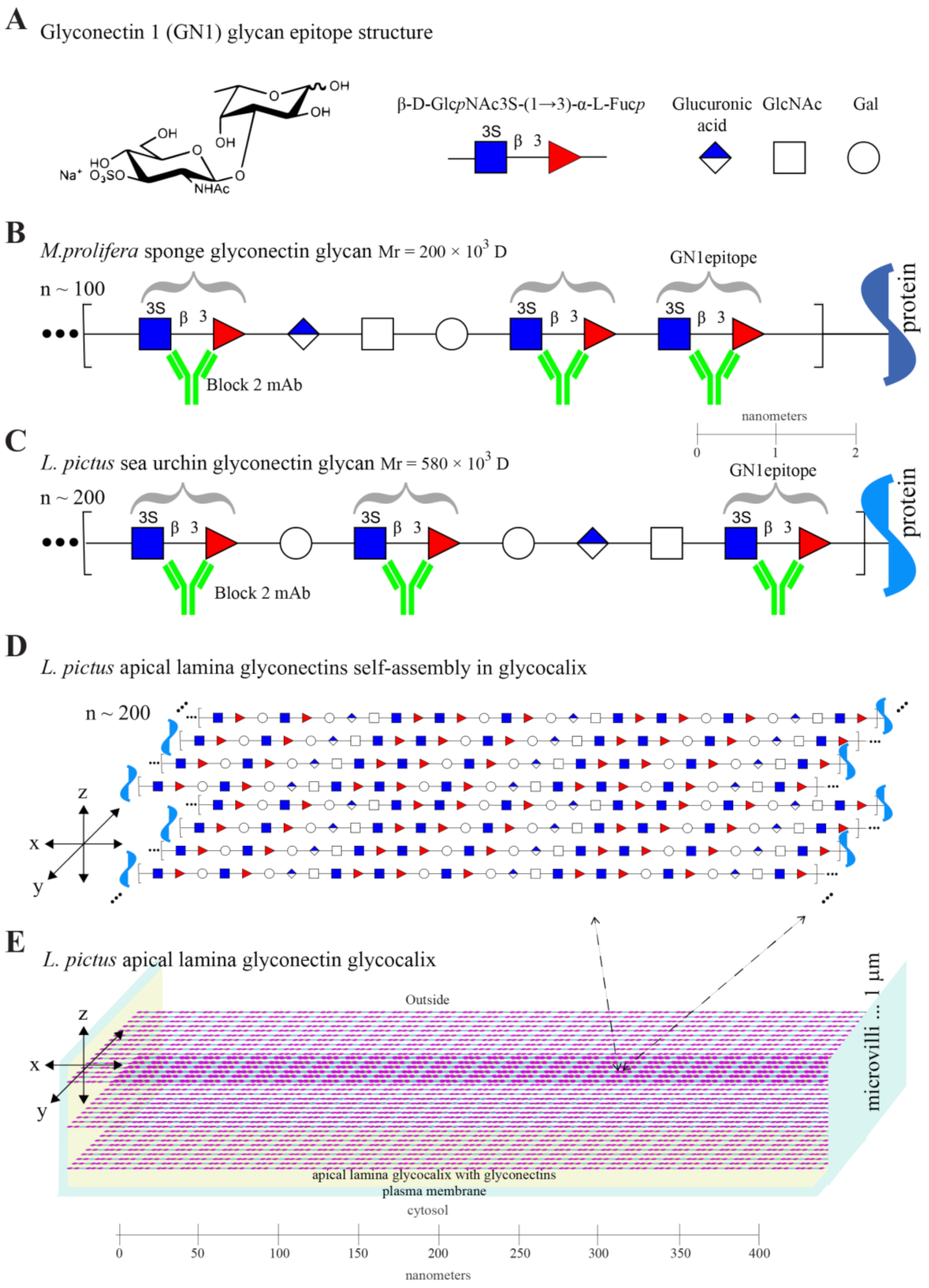
2.1. Expression of the GN1 Glycan Cell Adhesion Epitope β-d-GlcpNAc3S-(1→3)-α-l-Fucp during Embryonal Development of Sea Urchin L. pictus
2.2. Immuno-Purification of the Sea Urchin Embryonal g580 Glycan Bearing the GN1 Cell Adhesion Epitope with the Anti-GN1 Block 2 Monoclonal Antibodies
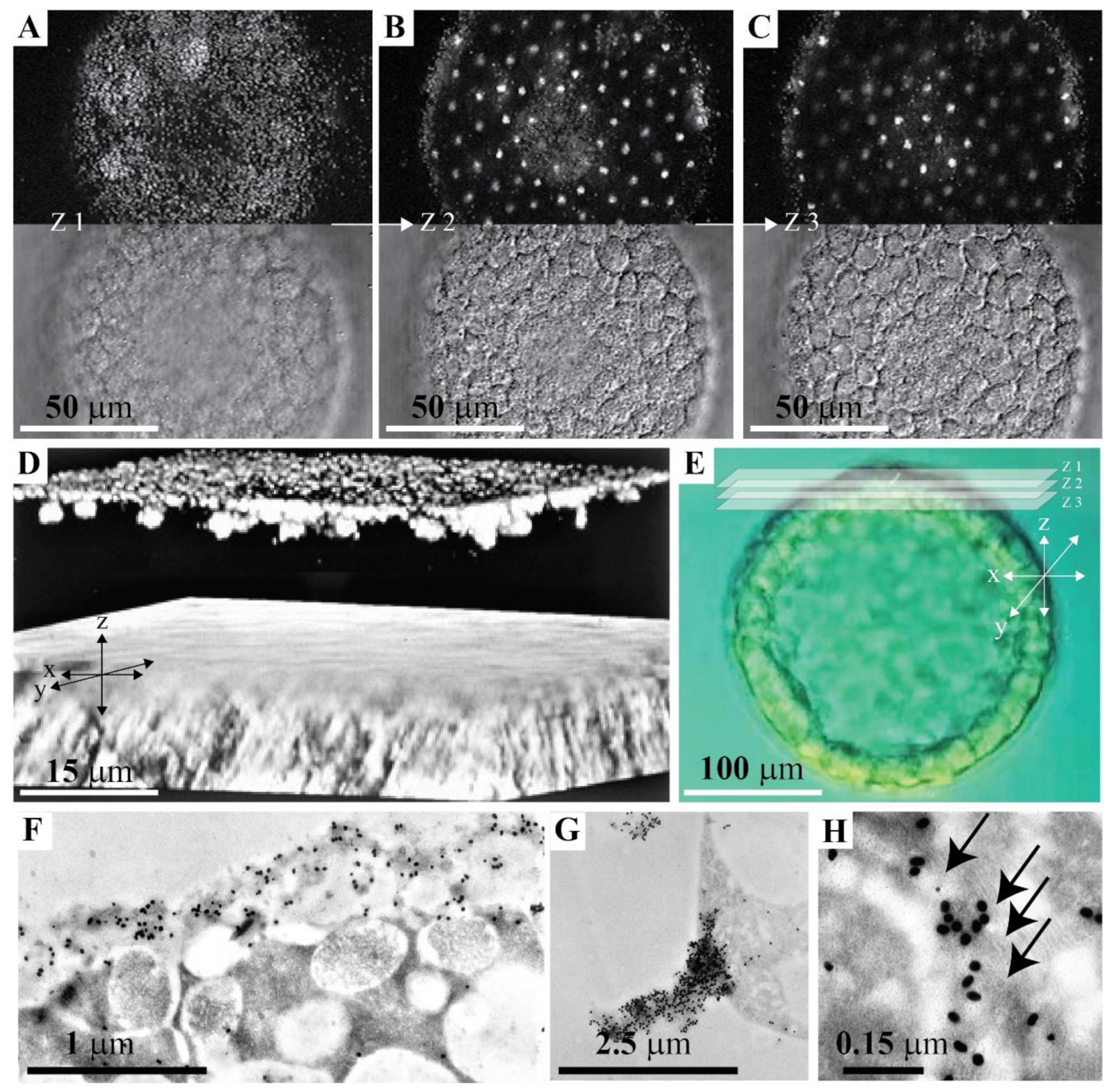
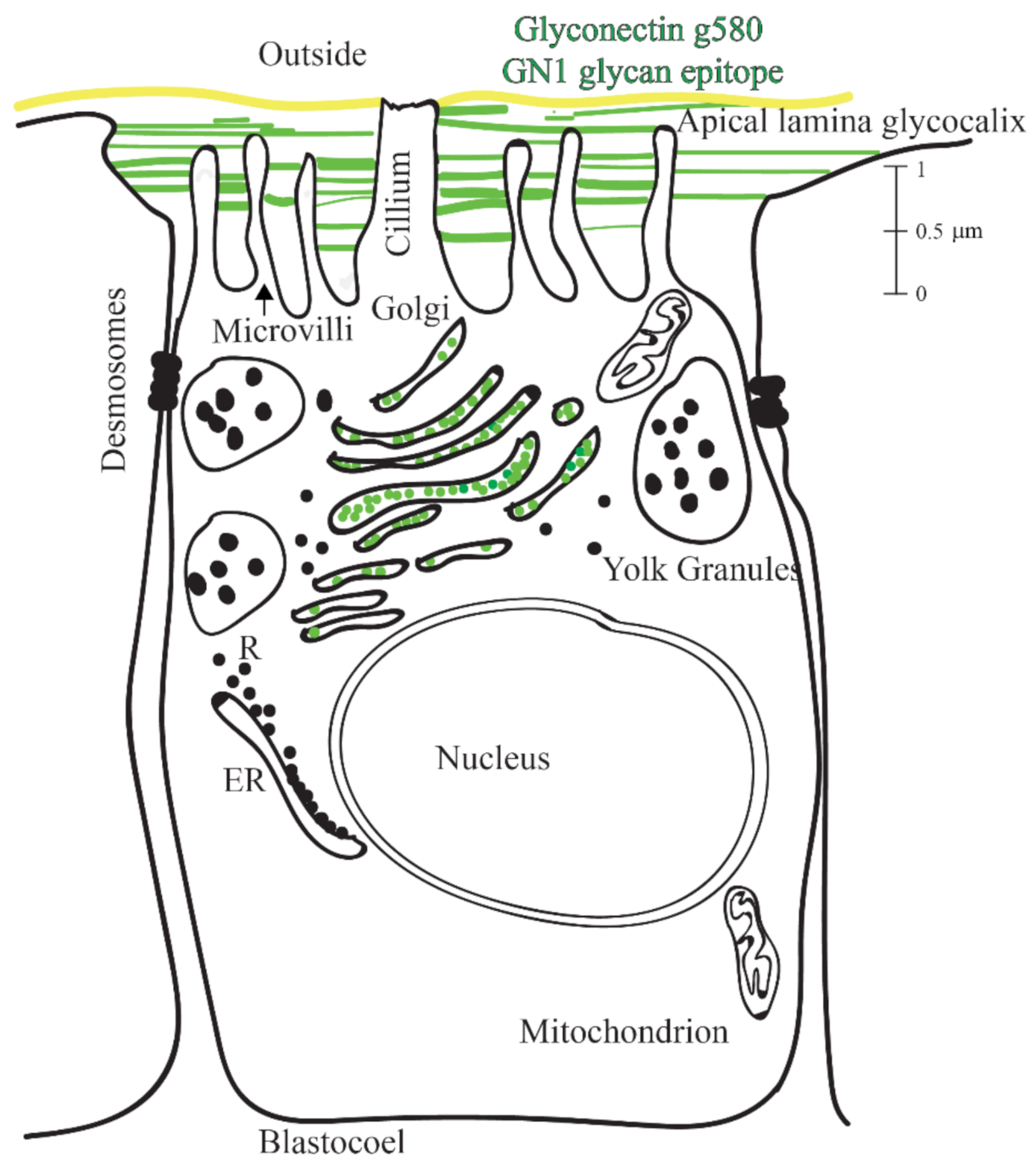
2.3. Immuno-Histological Localization of the GN1 Glycan Epitope in the Apical Lamina and Golgi of Sea Urchin Hatched Blastula Embryos with the Anti-GN1 Block 2 Monoclonal Antibodies
2.3.1. Confocal Microscopy
2.3.2. Immunogold Electron Microscopy
2.4. Hatched Blastula Cell Adhesion
2.4.1. Enhancement of Blastula Cells Reaggregation by the Glyconectin g580 Acidic Glycan
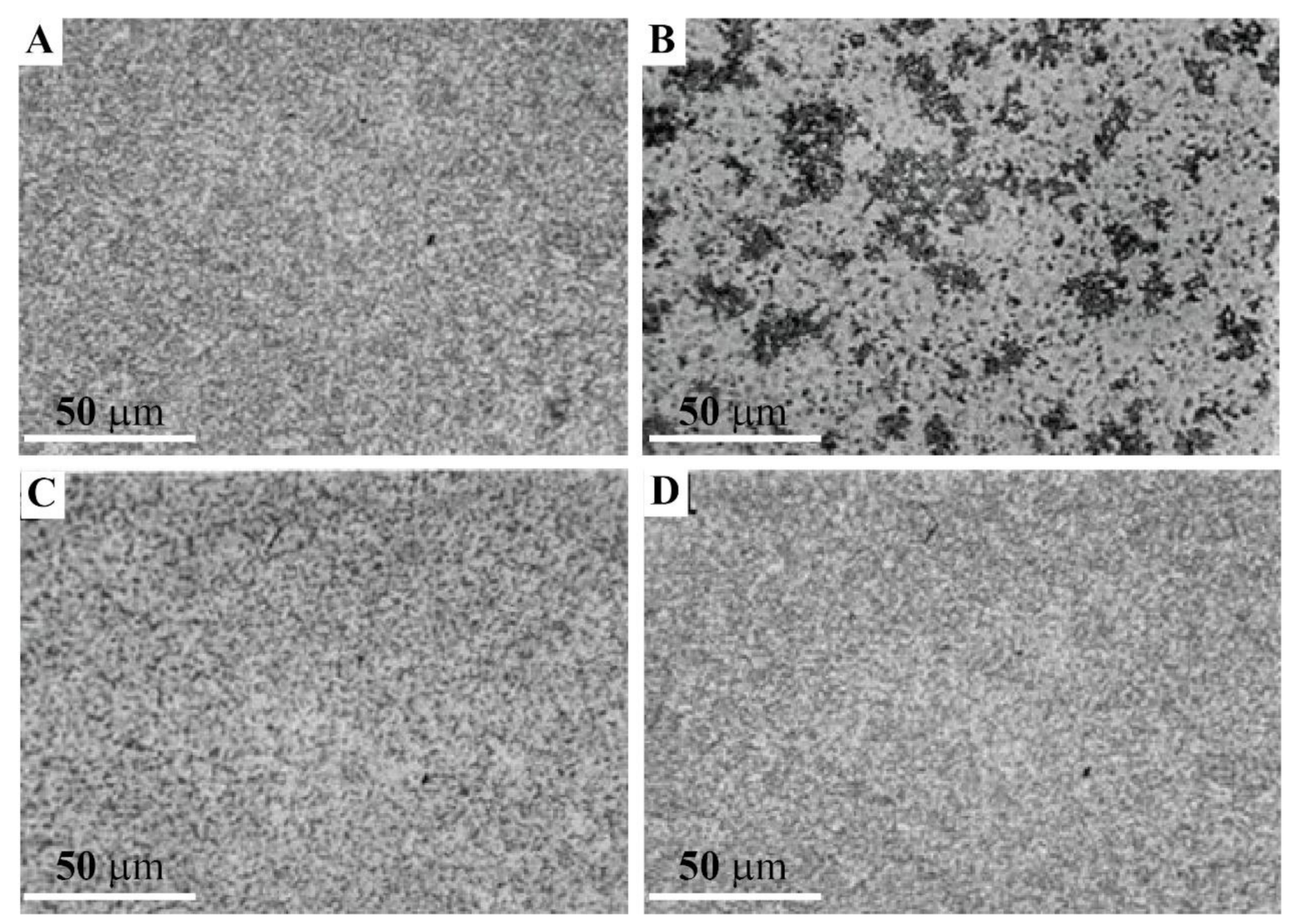
2.4.2. Inhibition of Blastula Cell Reaggregation with the Anti-GN1 Block 2 Monoclonal Antibody
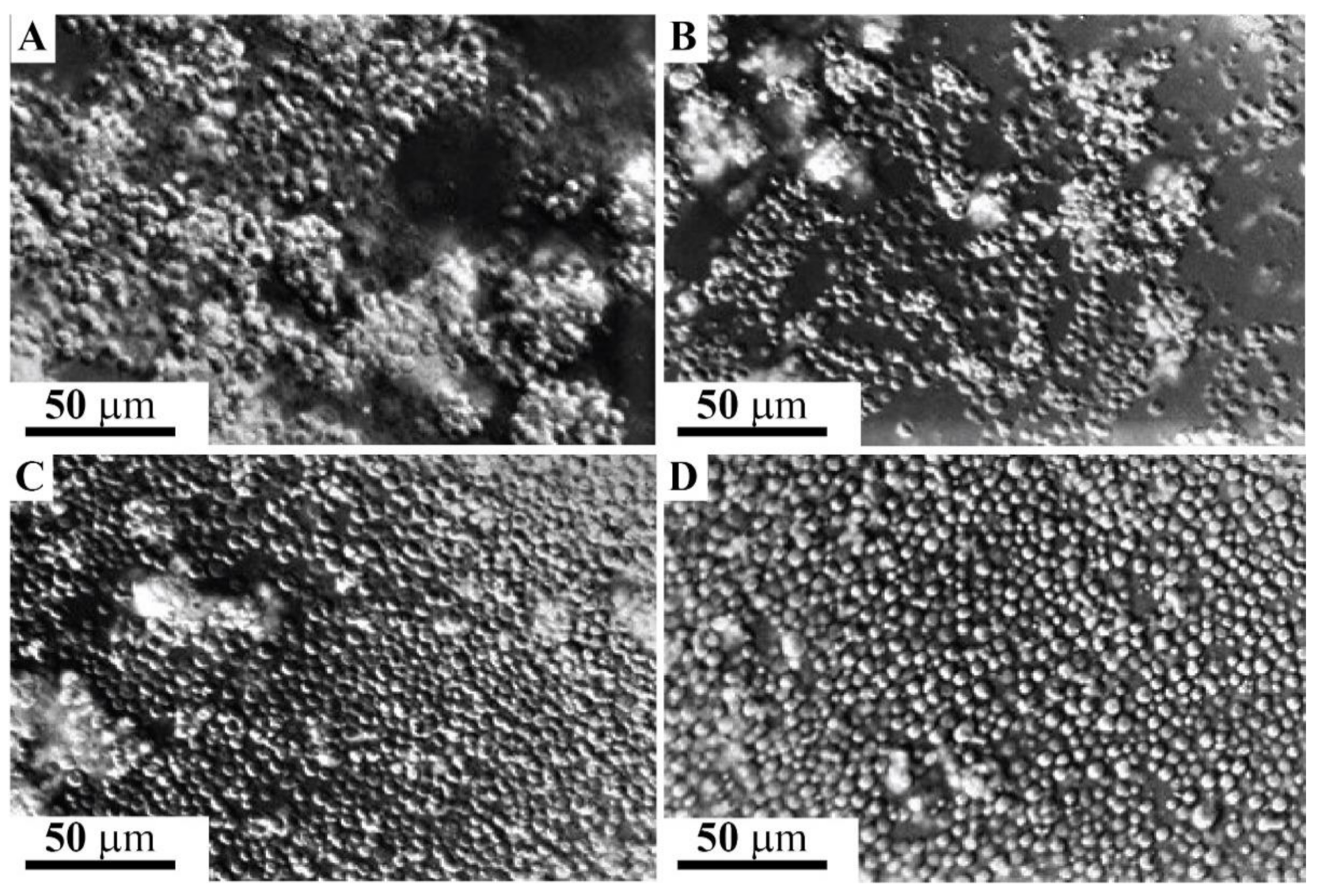
2.5. Adhesion of g580 Glyconectin Glycan Coated Beads
2.6. Alteration of Hatched Blastula Embryos Shape and Inhibition of Gastrulation by the Anti-GN1 Block 2 Monoclonal Antibody
2.7. Model and Mechanism of Blastulation and Integrity of Hatched Sea Urchin Blastula on the Basis of Glycan–Glycan Interactions via the GN1 Epitope of g580 Glyconectin Glycan
3. Discussion
4. Materials and Methods
4.1. Fertilization and Embryo Cultures
4.2. Dissociation of Embryos
4.3. Reaggregation of Dissociated Hatched Blastula Cells
4.4. In Vivo Treatment of Hatched Blastula Embryos with the Anti-GN1 Fab Fragments of the Block 2 Monoclonal Antibody
4.5. Preparation and Aggregation of g580 Coated Beads
4.6. Fixation and Embedding of Embryos
4.7. Immunogold Staining of Embryos
4.8. Confocal Immunofluorescence Microscopy of the Whole Embryo Mounts
4.9. Isolation of Glycans from Sea Urchin Embryos
4.10. Immuno-Purification, Immunoblotting, and Polyacrylamide Gel Electrophoresis of Glycans
4.11. Analytical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Misevic, G.; Garbarino, E. Glycan-to-Glycan Binding: Molecular Recognition through Polyvalent Interactions Mediates Specific Cell Adhesion. Molecules 2021, 26, 397. [Google Scholar] [CrossRef] [PubMed]
- Wickström, S.A.; Niessen, C.M. Cell adhesion and mechanics as drivers of tissue organization and differentiation: Local cues for large scale organization. Curr. Opin. Cell Biol. 2018, 54, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Cary, G.A.; Hinman, V.F. Echinoderm development and evolution in the post-genomic era. Dev. Biol. 2017, 427, 203–211. [Google Scholar] [CrossRef]
- Katow, H. Mechanisms of the epithelial-to-mesenchymal transition in sea urchin embryos. Tissue Barriers 2015, 3, e1059004. [Google Scholar] [CrossRef][Green Version]
- Cui, M.; Lin, C.Y.; Su, Y.H. Recent advances in functional perturbation and genome editing techniques in studying sea urchin development. Brief. Funct. Genom. 2017, 16, 309–318. [Google Scholar] [CrossRef]
- Balinsky, B.I. An electron microscopic investigation of the mechanisms of adhesion of the cells in a sea urchin blastula and gastrula. Exp. Cell Res. 1959, 16, 429–433. [Google Scholar] [CrossRef]
- Giudice, G. Restitution of whole larvae from disaggregated cells of sea urchin embryos. Dev. Biol. 1962, 5, 402–411. [Google Scholar] [CrossRef]
- Gustafson, T.; Wolpert, L. The Cellular Basis of Morphogenesis and Sea Urchin Development. Int. Rev. Cytol. 1963, 15, 139–214. [Google Scholar] [CrossRef]
- Wilt, F.H. Looking into the sea urchin embryo you can see local cell interactions regulate morphogenesis. BioEssays 1997, 19, 665–668. [Google Scholar] [CrossRef]
- Costa, C.; Cavalcante, C.; Zito, F.; Yokota, Y.; Matranga, V. Phylogenetic analysis and homology modelling of Paracentrotus lividus nectin. Mol. Divers. 2010, 14, 653–665. [Google Scholar] [CrossRef]
- Alliegro, M.C.; Alliegro, M.A. Echinonectin is a Del-1-like molecule with regulated expression in sea urchin embryos. Gene Expr. Patterns 2007, 7, 651–656. [Google Scholar] [CrossRef]
- Asao, M.I.; Oppenheimer, S.B. Inhibition of cell aggregation by specific carbohydrates. Exp. Cell Res. 1979, 120, 101–110. [Google Scholar] [CrossRef]
- Idoni, B.; Ghazarian, H.; Metzenberg, S.; Hutchins-Carroll, V.; Oppenheimer, S.B.; Carroll, E.J. Use of specific glycosidases to probe cellular interactions in the sea urchin embryo. Exp. Cell Res. 2010, 316, 2204–2211. [Google Scholar] [CrossRef][Green Version]
- Papakonstantinou, E.; Misevic, G.N. Isolation and characterization of a new class of acidic glycans implicated in sea urchin embryonal cell adhesion. J. Cell. Biochem. 1993, 53, 98–113. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Karakiulakis, G.; Aletras, A.J.; Misevic, G.N. A novel class of adhesion acidic glycans in sea urchin embryos. Isolation, characterization and immunological studies during early embryonal development. Eur. J. Biochem. 1994, 224, 1067–1077. [Google Scholar] [CrossRef]
- Vitorelli, M.L.; Matranga, V.; Feo, S.; Giudice, G.; Noll, H. Inverse affects on thymidine incorporation in dissociated blastula cells of the sea urchin Paracentrotus lividus induced by butanol treatment and Fab addition. Cell Differ. 1980, 9, 63–70. [Google Scholar] [CrossRef]
- Oppenheimer, S.B.; Meyer, J.T. Carbohydrate specificity of sea urchin blastula adhesion component. Exp. Cell Res. 1982, 139, 451–455. [Google Scholar] [CrossRef]
- Oppenheimer, S.B.; Meyer, J.T. Isolation of species-specific and stage-specific adhesion promoting component by disaggregation of intact sea urchin embryo cells. Exp. Cell Res. 1982, 137, 472–476. [Google Scholar] [CrossRef]
- Matranga, V.; Di Ferrol, D.; Zito, F.; Cervello, M.; Nakano, E. A new extracellular matrix protein of the sea urchin embryo with properties of a substrate adhesion molecule. Roux’s Arch. Dev. Biol. 1992, 201, 173–178. [Google Scholar] [CrossRef]
- Kondo, K. Demonstration of a reaggregation inhibitor in sea urchin embryos. Exp. Cell Res. 1974, 86, 178–181. [Google Scholar] [CrossRef]
- Noll, H.; Matranga, V.; Cascino, D.; Vittorelli, L. Reconstitution of membranes and embryonic development in dissociated blastula cells of the sea urchin by reinsertion of aggregation-promoting membrane proteins extracted with butanol. Proc. Natl. Acad. Sci. USA 1979, 76, 288–292. [Google Scholar] [CrossRef]
- Romancino, D.P.; Ghersi, G.; Montana, G.; Bonura, A.; Perriera, S.; Di Carlo, M. Characterization of bep1 and bep4 antigens involved in cell interactions during Paracentrotus lividus development. Differentiation 1992, 50, 67–74. [Google Scholar] [CrossRef]
- Ageenko, N.; Kiselev, K.; Dmitrenok, P.; Odintsova, N. Pigment Cell Differentiation in Sea Urchin Blastula-Derived Primary Cell Cultures. Mar. Drugs 2014, 12, 3874–3891. [Google Scholar] [CrossRef]
- Matranga, V.; Kuwasaki, B.; Noll, H. Functional characterization of toposomes from sea urchin blastula embryos by a morphogenetic cell aggregation assay. EMBO J. 1986, 5, 3125–3132. [Google Scholar] [CrossRef]
- McCarthy, R.A.; Spiegel, M. The enhancement of reaggregation of sea urchin blastula cells by exogenous proteins. Cell Differ. 1983, 13, 103–114. [Google Scholar] [CrossRef]
- Timourian, H.; Clothier, G.; Watchmaker, G. Reaggregation of sea urchin blastula cells. I. Intrinsic differences in the blastula cells. Dev. Biol. 1973, 31, 252–263. [Google Scholar] [CrossRef]
- Spiegel, E.; Howard, L. Development of cell junctions in sea-urchin embryos. J. Cell Sci. 1983, 62, 27–48. [Google Scholar] [CrossRef]
- Ghersi, G.; Salamone, M.; Dolo, V.; Levi, G.; Vittorelli, M.L. Differential expression and function of cadherin-like proteins in the sea urchin embryo. Mech. Dev. 1993, 41, 47–55. [Google Scholar] [CrossRef]
- Stemmler, M.P. Cadherins in development and cancer. Mol. Biosyst. 2008, 4, 835–850. [Google Scholar] [CrossRef]
- DeSimone, D.W.; Spiegel, E.; Spiegel, M. The biochemical identification of fibronectin in the sea urchin embryo. Biochem. Biophys. Res. Commun. 1985, 133, 183–188. [Google Scholar] [CrossRef]
- Spiegel, E.; Burger, M.M.; Spiegel, M. Fibronectin and laminin in the extracellular matrix and basement membrane of sea urchin embryos. Exp. Cell Res. 1983, 144, 47–55. [Google Scholar] [CrossRef]
- McCarthy, R.A.; Beck, K.; Burger, M.M. Laminin is structurally conserved in the sea urchin basal lamina. EMBO J. 1987, 6, 1587–1593. [Google Scholar] [CrossRef]
- Matranga, V.; Adragna, N.; Cervello, M.; Vittorell, M.L. Different aggregation properties of sea urchin embryonic cells at different developmental stages. II. Stage specific response to fibronectin and collagen. Cell Biol. Int. Rep. 1984, 8, 797–807. [Google Scholar] [CrossRef]
- D’Alessio, M.; Ramirez, F.; Suzuki, H.R.; Solursh, M.; Gambino, R. Structure and developmental expression of a sea urchin fibrillar collagen gene. Proc. Natl. Acad. Sci. USA 1989, 86, 9303–9307. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.R.; Reiter, R.S.; D’Alessio, M.; Di Liberto, M.; Ramirez, F.; Exposito, J.Y.; Gambino, R.; Solursh, M. Comparative analysis of fibrillar and basement membrane collagen expression in embryos of the sea urchin, Strongylocentrotus purpuratus. Zoolog. Sci. 1997, 14, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Expositosg, J.-Y.; Alessiosll, M.D.; Solurshll, M.; Ramirezs, F. Sea Urchin Collagen Evolutionarily Homologous to Vertebrate Pro-a2(1) Collagen. J. Biol. Chem. 1992, 267, 15559–15562. [Google Scholar] [CrossRef]
- Zito, F.; Burke, R.D.; Matranga, V. Pl-nectin, a discoidin family member, is a ligand for βC integrins in the sea urchin embryo. Matrix Biol. 2010, 29, 341–345. [Google Scholar] [CrossRef]
- Tonegawa, Y. Cell aggregation factor and endogenous lectin in sea urchin embryos. Cell Differ. 1982, 11, 335–337. [Google Scholar] [CrossRef]
- Salmivirta, M.; Jalkanen, M. Syndecan family of cell surface proteoglycans: Developmentally regulated receptors for extracellular effector molecules. Experientia 1995, 51, 863–872. [Google Scholar] [CrossRef]
- Alliegro, M.C.; Ettensohn, C.A.; Burdsal, C.A.; Erickson, H.P.; McClay, D.R. Echinonectin: A new embryonic substrate adhesion protein. J. Cell Biol. 1988, 107, 2319–2327. [Google Scholar] [CrossRef]
- Burdsal, C.A.; Alliegro, M.C.; McClay, D.R. Tissue-specific, temporal changes in cell adhesion to echinonectin in the sea urchin embryo. Dev. Biol. 1991, 144, 327–334. [Google Scholar] [CrossRef]
- Fuhrman, M.H.; Suhan, J.P.; Ettensohn, C.A. Developmental Expression of Echinonectin, an Endogenous Lectin of the Sea Urchin Embryo. Dev. Growth Differ. 1992, 34, 137–150. [Google Scholar] [CrossRef]
- McClay, D.R.; Fink, R.D. Sea urchin hyalin: Appearance and function in development. Dev. Biol. 1982, 92, 285–293. [Google Scholar] [CrossRef]
- Adelson, D.L.; Alliegro, M.C.; McClay, D.R. On the ultrastructure of hyalin, a cell adhesion protein of the sea urchin embryo extracellular matrix. J. Cell Biol. 1992, 116, 1283–1289. [Google Scholar] [CrossRef]
- Wessel, G.M.; Berg, L.; Adelson, D.L.; Cannon, G.; McClay, D.R. A molecular analysis of hyalin—A substrate for cell adhesion in the hyaline layer of the sea urchin embryo. Dev. Biol. 1998, 193, 115–126. [Google Scholar] [CrossRef]
- Alvarez, M.; Nnoli, J.; Carroll, E.J.; Hutchins-Carroll, V.; Razinia, Z.; Oppenheimer, S.B. Exogenous hyalin and sea urchin gastrulation, Part II: Hyalin, an interspecies cell adhesion molecule. Zygote 2008, 16, 73–78. [Google Scholar] [CrossRef]
- Razinia, Z.; Carroll, E.J.; Oppenheimer, S.B. Microplate assay for quantifying developmental morphologies: Effects of exogenous hyalin on sea urchin gastrulation. Zygote 2007, 15, 159–164. [Google Scholar] [CrossRef][Green Version]
- Ghazarian, H.; Coyle-Thompson, C.; Dalrymple, W.; Hutchins-Carroll, V.; Metzenberg, S.; Razinia, Z.; Carroll, E.J.; Oppenheimer, S.B. Exogenous hyalin and sea urchin gastrulation. Part IV: A direct adhesion assay—Progress in identifying hyalin’s active sites. Zygote 2010, 18, 17–26. [Google Scholar] [CrossRef]
- Katow, H. Pamlin, a Primary Mesenchyme Cell Adhesion Protein, in the Basal Lamina of the Sea Urchin Embryo. Exp. Cell Res. 1995, 218, 469–478. [Google Scholar] [CrossRef]
- Contreras, A.; Vitale, J.; Hutchins-Carroll, V.; Carroll, E.J.; Oppenheimer, S.B. Exogenous hyalin and sea urchin gastrulation. Part III: Biological activity of hyalin isolated from Lytechinus pictus embryos. Zygote 2008, 16, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Sanderson, R.D. Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1990, 327, 171–186. [Google Scholar] [PubMed]
- Akasaka, K.; Terayama, H. A proteoglycan fraction isolated from the EDTA extract of sea urchin (Hemicentrotus pulcherrimus) gastrulae stimulates reaggeration of dissociated embryonic cells. Exp. Cell Res. 1984, 150, 226–233. [Google Scholar] [CrossRef]
- Tomita, K.; Yamasu, K.; Suyemitsu, T. Role of syndecan in the elongation of postoral arms in sea urchin larvae. Dev. Growth Differ. 2002, 44, 45–53. [Google Scholar] [CrossRef]
- Kinoshita, S.; Yoshii, K. The role of proteoglycan synthesis in the development of sea urchins. II. The effect of administration of exogenous proteoglycan. Exp. Cell Res. 1979, 124, 361–369. [Google Scholar] [CrossRef]
- Akasaka, K.; Amemiya, S.; Terayama, H. Scanning electron microscopical study of the inside of sea urchin embryos (Pseudocentrotus depressus): Effects of aryl β-xyloside, Tunicamycin and deprivation of sulfate ions. Exp. Cell Res. 1980, 129, 1–13. [Google Scholar] [CrossRef]
- Spiegel, E.; Howard, L.; Spiegel, M. Extracellular matrix of sea urchin and other marine invertebrate embryos. J. Morphol. 1989, 199, 71–92. [Google Scholar] [CrossRef]
- Spiegel, E.; Howard, L.; Spiegel, M. Elongated microvilli support the sea urchin embryo concentrically within the perivitelline space until hatching. Roux’s Arch. Dev. Biol. 1989, 198, 85–91. [Google Scholar] [CrossRef]
- Spiegel, E.; Howard, L.; Spiegel, M. The contractility of elongated microvilli in early sea urchin embryos. Roux’s Arch. Dev. Biol. 1990, 199, 228–236. [Google Scholar] [CrossRef]
- Wessel, G.M.; Marchase, R.B.; McClay, D.R. Ontogeny of the basal lamina in the sea urchin embryo. Dev. Biol. 1984, 103, 235–245. [Google Scholar] [CrossRef]
- Spiegel, M.; Burger, M.M. Cell adhesion during gastrulation. A new approach. Exp. Cell Res. 1982, 139, 377–382. [Google Scholar] [CrossRef]
- Ebong, E.E.; Macaluso, F.P.; Spray, D.C.; Tarbell, J.M. Imaging the Endothelial Glycocalyx In Vitro by Rapid Freezing/Freeze Substitution Transmission Electron Microscopy. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1908–1915. [Google Scholar] [CrossRef]
- Kesimer, M.; Ehre, C.; Burns, K.A.; Davis, C.W.; Sheehan, J.K.; Pickles, R.J. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 2013, 6, 379–392. [Google Scholar] [CrossRef]
- Mitchell, M.J.; King, M.R. Physical Biology in Cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am. J. Physiol. Cell Physiol. 2014, 306, C89–C97. [Google Scholar] [CrossRef]
- de Mesy Bentley, K.L. An 11-μm-Thick Glycocalyx? It’s all in the technique! Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1712–1713. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Paul, O.; Rehm, M.; Welsch, U.; Stoeckelhuber, M.; Conzen, P.; Becker, B.F. The glycocalyx of the human umbilical vein endothelial cell: An impressive structure ex vivo but not in culture. Circ. Res. 2009, 104, 1313–1317. [Google Scholar] [CrossRef]
- Dzamba, B.J.; DeSimone, D.W. Extracellular Matrix (ECM) and the Sculpting of Embryonic Tissues. Curr. Top. Dev. Biol. 2018, 130, 245–274. [Google Scholar]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Dammer, U.; Popescu, O.; Wagner, P.; Anselmetti, D.; Guntherodt, H.-J.; Misevic, G.N. Binding Strength Between Cell Adhesion Proteoglycans Measured by Atomac Force Microscopy. Science 1995, 267, 1173–1175. [Google Scholar] [CrossRef]
- Guerardel, Y.; Czeszak, X.; Sumanovski, L.T.; Karamanos, Y.; Popescu, O.; Strecker, G.; Misevic, G.N. Molecular Fingerprinting of Carbohydrate Structure Phenotypes of Three Porifera Proteoglycan-like Glyconectins. J. Biol. Chem. 2004, 279, 15591–15603. [Google Scholar] [CrossRef]
- Misevic, G.N.; Guerardel, Y.; Sumanovski, L.T.; Slomianny, M.-C.; Demarty, M.; Ripoll, C.; Karamanos, Y.; Maes, E.; Popescu, O.; Strecker, G. Molecular recognition between glyconectins as an adhesion self-assembly pathway to multicellularity. J. Biol. Chem. 2004, 279, 15579–15590. [Google Scholar] [CrossRef]
- Misevic, G.N.; Burger, M.M. Carbohydrate-carbohydrate interactions of a novel acidic glycan can mediate sponge cell adhesion. J. Biol. Chem. 1993, 268, 4922–4929. [Google Scholar] [CrossRef]
- Spillmann, D.; Thomas-Oates, J.E.; Van Kuik, J.A.; Vliegenthart, J.F.G.; Misevic, G.; Burger, M.M.; Finne, J. Characterization of a novel sulfated carbohydrate unit implicated in the carbohydrate-carbohydrate-mediated cell aggregation of the marine sponge Microciona prolifera. J. Biol. Chem. 1995, 270, 5089–5097. [Google Scholar] [CrossRef]
- Misevic, G.N.; Popescu, O. A novel class of embryonic cell adhesion glycan epitopes is expressed in human colon carcinomas. J. Mol. Recognit. 1995, 8, 100–105. [Google Scholar] [CrossRef]
- Misevic, G.N.; Finne, J.; Burger, M.M. Involvement of carbohydrates as multiple low affinity interaction sites in the self-association of the aggregation factor from the marine sponge Microciona prolifera. J. Biol. Chem. 1987, 262, 5870–5877. [Google Scholar] [CrossRef]
- Rini, J.M.; Esko, J.D. Glycosyltransferases and Glycan-Processing Enzymes. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Huntington, NY, USA, 2015–2017; Chapter 6. [Google Scholar] [CrossRef]
- Haseley, S.R.; Vermeer, H.J.; Kamerling, J.P.; Vliegenthart, J.F. Carbohydrate self-recognition mediates marine sponge cellular adhesion. Proc. Natl. Acad. Sci. USA 2001, 98, 9419–9424. [Google Scholar] [CrossRef]
- Carvalho de Souza, A.; Ganchev, D.N.; Snel, M.M.E.; Van Der Eerden, J.P.J.M.; Vliegenthart, J.F.G.; Kamerling, J.P. Adhesion forces in the self-recognition of oligosaccharide epitopes of the proteoglycan aggregation factor of the marine sponge Microciona prolifera. Glycoconj. J. 2009, 26, 457–465. [Google Scholar] [CrossRef]
- Lorenz, B.; Álvarez de Cienfuegos, L.; Oelkers, M.; Kriemen, E.; Brand, C.; Stephan, M.; Sunnick, E.; Yüksel, D.; Kalsani, V.; Kumar, K.; et al. Model System for Cell Adhesion Mediated by Weak Carbohydrate–Carbohydrate Interactions. J. Am. Chem. Soc. 2012, 134, 3326–3329. [Google Scholar] [CrossRef][Green Version]
- Kamerling, J.P.; Carvalho de Souza, A.C. Studying Carbohydrate Self-Recognition in Marine Sponges Using Synthetic Aggregation Factor Epitopes. In The Molecular Immunology of Complex Carbohydrates-3; Springer: New York, NY, USA, 2011; pp. 493–510. [Google Scholar]
- Misevic, G.N.; Karamanos, Y.; Misevic, N.J. Atomic force microscopy measurements of intermolecular binding forces. Methods Mol. Biol. 2009, 522, 143–150. [Google Scholar] [CrossRef]
- Popescu, O.; Misevic, G.N. Self-recognition by proteoglycans. Nature 1997, 386, 231–232. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.L.; Nguyen-Ba-Charvet, K.T.; Parray, A.; Badea, T.C.; Chédotal, A.; Kolodkin, A.L. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature 2011, 470, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Maes, E.; Florea, D.; Coppin, A.; Strecker, G. Structural analysis of 20 oligosaccharide-alditols released from the jelly coat of Rana palustris eggs by reductive beta-elimination. Characterization of the polymerized sequence [Gal(beta1,3)GalNAc(alpha1-4)]n. Eur. J. Biochem. 1999, 264, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Maes, E.; Plancke, Y.; Delplace, F.; Strecker, G. Primary Structure of Acidic Oligosaccharide-alditols Derived from the Jelly Coat of Ambystoma tigrinum: Occurrence of Oligosaccharides with Fucosyl(α1–5)[Fucosyl(α1–4)]–3–Deoxy-d-Glycero-d-Galacto-Nonulosonic Acid and Fucosyl (α1–2) Galactosyl (α1–3)-N-Acetylgalactosamine Sequences. Eur. J. Biochem. 1995, 230, 146–156. [Google Scholar] [CrossRef]
- Ding, J.; Fang, Y.; Xiang, Z. Antigen/IgG immune complex-primed mucosal mast cells mediate antigen-specific activation of co-cultured T cells. Immunology 2015, 144, 387–394. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Biological Functions of Glycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Huntington, NY, USA, 2015–2017; Chapter 7. [Google Scholar] [CrossRef]
- Tyler, A. A simple, non-injurious, method for inducing repeated spawning of sea urchins and send dollars. Collect. Net 1949, 19, 19–20. [Google Scholar]
- Svennerholm, L.; Fredman, P. A procedure for the quantitative isolation of brain gangliosides. Biochim. Biophys. Acta Lipids Lipid Metab. 1980, 617, 97–109. [Google Scholar] [CrossRef]
- Misevic, G.N. Immunoblotting and immunobinding of acidic polysaccharides separated by gel electrophoresis. Methods Enzymol. 1989, 95–104. [Google Scholar] [CrossRef]
- Shields, R.; Buknett, W. Determination of Protein-Bound Carbohydrate in Serum by a Modified Anthrone Method. Anal. Chem. 1960, 32, 885–886. [Google Scholar] [CrossRef]
- Dische, Z. A new specific color reaction of hexuronic acids. J. Biol. Chem. 1947, 167, 189–198. [Google Scholar] [CrossRef]
- Heinrikson, R.L.; Meredith, S.C. Amino acid analysis by reverse-phase high-performance liquid chromatography: Precolumn derivatization with phenylisothiocyanate. Anal. Biochem. 1984, 136, 65–74. [Google Scholar] [CrossRef]
- Kamerling, J.P.; Gerwig, G.J.; Vliegenthart, J.F.G.; Clamp, J.R. Characterization by gas liquid chromatography mass spectrometry and protonmagnetic resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem. J. 1975, 151, 491–495. [Google Scholar] [CrossRef]
- Zanetta, J.P.; Timmerman, P.; Leroy, Y. Gas-liquid chromatography of the heptafluorobutyrate derivatives of the O-methyl-glycosides on capillary columns: A method for the quantitative determination of the monosaccharide composition of glycoproteins and glycolipids. Glycobiology 1999, 9, 255–266. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misevic, G.; Checiu, I.; Popescu, O. Glyconectin Cell Adhesion Epitope, β-d-GlcpNAc3S-(1→3)-α-l-Fucp, Is Involved in Blastulation of Lytechinus pictus Sea Urchin Embryos. Molecules 2021, 26, 4012. https://doi.org/10.3390/molecules26134012
Misevic G, Checiu I, Popescu O. Glyconectin Cell Adhesion Epitope, β-d-GlcpNAc3S-(1→3)-α-l-Fucp, Is Involved in Blastulation of Lytechinus pictus Sea Urchin Embryos. Molecules. 2021; 26(13):4012. https://doi.org/10.3390/molecules26134012
Chicago/Turabian StyleMisevic, Gradimir, Iacob Checiu, and Octavian Popescu. 2021. "Glyconectin Cell Adhesion Epitope, β-d-GlcpNAc3S-(1→3)-α-l-Fucp, Is Involved in Blastulation of Lytechinus pictus Sea Urchin Embryos" Molecules 26, no. 13: 4012. https://doi.org/10.3390/molecules26134012
APA StyleMisevic, G., Checiu, I., & Popescu, O. (2021). Glyconectin Cell Adhesion Epitope, β-d-GlcpNAc3S-(1→3)-α-l-Fucp, Is Involved in Blastulation of Lytechinus pictus Sea Urchin Embryos. Molecules, 26(13), 4012. https://doi.org/10.3390/molecules26134012






