Radiotracers for Bone Marrow Infection Imaging
Abstract
1. Introduction
2. Radionuclides in Nuclear Medicine Imaging
3. Established Radiotracers for Bone Infection
3.1. Gallium-Citrate (Based on Ga-67)
- Increased vascular permeability of the inflammatory tissue, allowing transferrin proteins (and thereby [67Ga]Ga-transferrin) to accumulate in the tissue.
- Uptake by leukocytes, where gallium binds to lactoferrin (another iron-binding protein). Leukocytes accumulate in the inflammatory tissue.
- Binding by siderophores (high-affinity iron-chelators), which are produced by bacteria in high amounts in iron-poor environments, such as inflammatory processes, to help the bacteria obtain sufficient iron.
- Binding by mucopolysaccharides found in the intercellular space of inflammatory sites.
- While the Ga-transferrin complex has high stability at normal pH in the blood, Ga dissociates from transferrin in environments of lower pH, as is often the case in tumours and abscesses.
3.2. Labelled Leukocytes
3.3. Bone Marrow Tracers (Labelled Nanocolloids)
3.4. Bone Scintigraphy (Labelled Phosphonates)
- First phase: Dynamic imaging right after injection (e.g., the first minute), reflecting the blood perfusion.
- Second phase: Static imaging of the relevant part of the body immediately after the first phase, reflecting the blood pool.
- Third phase: After 2–4 h, static imaging, reflecting uptake of phosphonates in the bone matrix.
3.5. FDG, a Glucose Analogue
4. Newer Tracers Considered for Bone Infection Imaging
4.1. [68Ga]Ga-Citrate
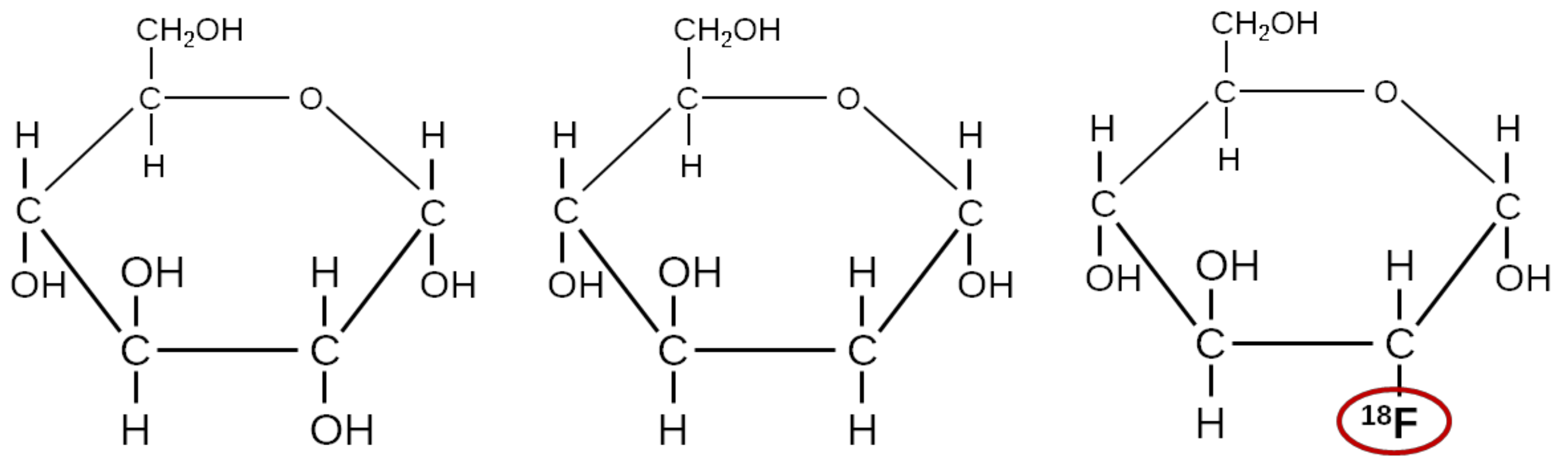
4.2. Other Sugar Analogues Than FDG
4.3. [15O]water
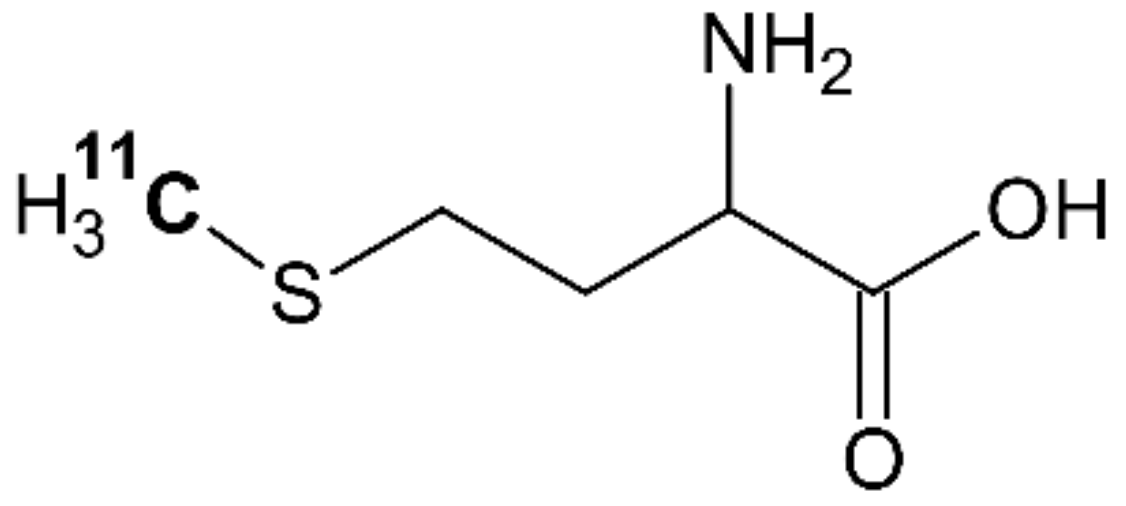
4.4. [11C]methionine
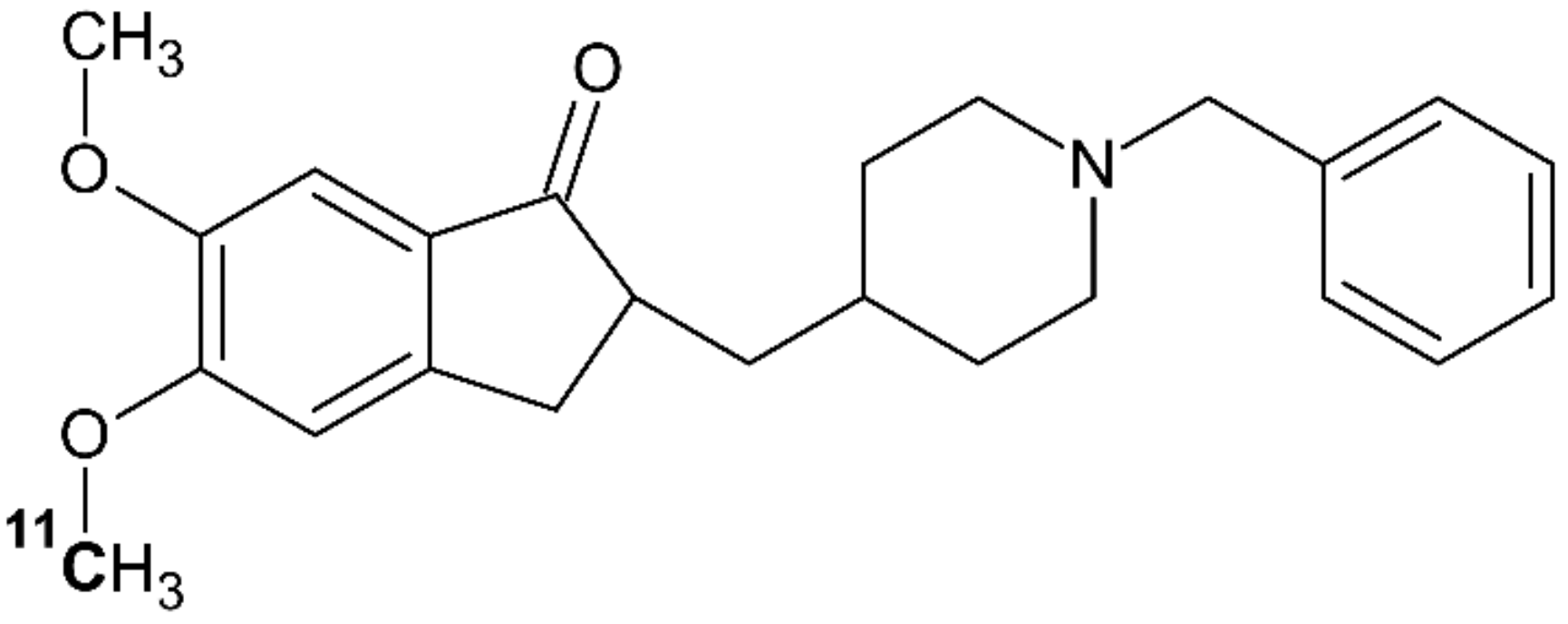
4.5. [11C]donepezil
4.6. Technetium-Labelled Interleukin-8 (IL-8)
4.7. [68Ga]Ga-DOTA-Siglec-9
4.8. 68Ga-Labelled Phage-Display Selected Peptides
4.9. Labelled Antimicrobial Peptides, Ubiquicidin
5. Discussion
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Wynn Jones, H.; Beckles, V.L.L.; Akinola, B.; Stevenson, A.J.; Harrison, W.J. Chronic haematogenous osteomyelitis in children: An Unsolved Problem. J. Bone Jt. Surg. 2011, 93, 1005–1010. [Google Scholar] [CrossRef]
- Palestro, C.J. Molecular Imaging of Infection: The first 50 years. Semin. Nucl. Med. 2020, 50, 23–34. [Google Scholar] [CrossRef]
- Johansen, L.K.; Koch, J.; Kirketerp-Møller, K.; Wamsler, O.J.; Nielsen, O.L.; Leifsson, P.S.; Frees, D.; Aalbæk, B.; Jensen, H.E. Therapy of haematogenous osteomyelitis—A comparative study in a porcine model and angolan children. In Vivo 2013, 27, 305–312. [Google Scholar]
- Eckerman, K.; Endo, A. ICRP Publication 107. Nuclear decay data for dosimetric calculations. Ann. ICRP 2008, 38, 7–96. [Google Scholar] [CrossRef] [PubMed]
- Lavender, J.P.; Lowe, J.; Barker, J.R.; Burn, J.I.; Chaudhri, M.A. Gallium 67 citrate scanning in neoplastic and inflammatory lesions. Br. J. Radiol. 1971, 44, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.J.; Vallabhajosula, S. Clinically proven radiopharmaceuticals for infection imaging: Mechanisms and applications. Semin. Nucl. Med. 2009, 39, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Palestro, C.J. Radionuclide imaging of osteomyelitis. Semin. Nucl. Med. 2015, 45, 32–46. [Google Scholar] [CrossRef]
- Tsan, M.-F. Mechanism of gallium-67 Accumulation in inflammatory lesions. J. Nucl. Med. 1985, 26, 88–92. [Google Scholar] [PubMed]
- Hoffer, P. Gallium: Mechanisms. J. Nucl. Med. 1980, 21, 282–285. [Google Scholar]
- Chitambar, C.R. Gallium and its competing roles with iron in biological systems. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J.; McCready, V.R.; Stoot, J.H.M.B.; Van Deurzen, D.F.P. The Mechanism of accumulation of tumour-localising radiopharmaceuticals. Eur. J. Nucl. Med. 1998, 25, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Palestro, C.J.; Brown, M.L.; Forstrom, L.A.; Greenspan, B.S.; McAfee, J.G.; Royal, H.D.; Schauwecker, D.S.; Seabold, J.E.; Signore, A. Society of Nuclear Medicine Procedure Guideline for Gallium Scintigraphy in Inflammation, Version 3.0; Society of Nuclear Medicine: Reston, VA, USA, 2004; Available online: http://snmmi.files.cms-plus.com/docs/Gallium_Scintigraphy_in_Inflammation_v3.pdf (accessed on 17 May 2021).
- Palestro, C.J.; Love, C.; Bhargava, K.K. Labeled leukocyte imaging: Current status and future directions. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 105–123. [Google Scholar]
- Palestro, C.J.; Brown, M.L.; Forstrom, L.A.; McAfee, J.G.; Royal, H.D.; Schauwecker, D.S.; Seabold, J.E.; Signore, A. Society of Nuclear Medicine Procedure Guideline for 111In-Leukocyte Scintigraphy for Suspected Infection/Inflammation; Version 3.0; Society of Nuclear Medicine: Reston, VA, USA, 2004; Available online: http://snmmi.files.cms-plus.com/docs/Leukocyte_v3.pdf (accessed on 17 May 2021).
- GIPHARMA. Nanocoll 500 Micrograms Kit for Radiopharmaceutical Preparation—Summary of Product Characteristics; GIPHARMA: Saluggia, Italy, 2015; Available online: https://imedi.co.uk/nanocoll-500-micrograms-kit-for-radiopharmaceutical-preparation/summary (accessed on 17 May 2021).
- Frühling, J.; Verbist, A.; Balikdjian, D. Which diphosphonate for routine bone scintigraphy (MDP, HDP or DPD)? Nucl. Med. Commun. 1986, 7, 415–425. [Google Scholar] [CrossRef]
- Ogawa, K.; Saji, H. Advances in drug design of radiometal-based imaging agents for bone disorders. Int. J. Mol. Imaging 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Jones, A.G.; Francis, M.D.; Davis, M.A. Bone scanning: Radionuclidic reaction mechanisms. Semin. Nucl. Med. 1976, 6, 3–18. [Google Scholar] [CrossRef]
- Afzelius, P.; Alstrup, A.; Nielsen, O.; Nielsen, K.; Jensen, S. Attempts to target Staphylococcus Aureus induced osteomyelitis bone lesions in a juvenile pig model by using radiotracers. Molecules 2020, 25, 4329. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, L.; Reivich, M.; Kennedy, C.; Des Rosiers, M.H.; Patlak, C.S.; Pettigrew, K.D.; Sakurada, O.; Shinohara, M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977, 28, 897–916. [Google Scholar] [CrossRef]
- Som, P.; Atkins, H.L.; Bandoypadhyay, D.; Fowler, J.S.; MacGregor, R.R.; Matsui, K.; Oster, Z.H.; Sacker, D.F.; Shiue, C.Y.; Turner, H.; et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-d-glucose (F-18): Nontoxic tracer for rapid tumor detection. J. Nucl. Med. 1980, 21, 670–675. [Google Scholar] [CrossRef]
- Potter, M.; Newport, E.; Morten, K.J. The warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef]
- Devlin, M.J. The “skinny” on brown fat, obesity, and bone. Am. J. Phys. Anthr. 2015, 156, 98–115. [Google Scholar] [CrossRef]
- Zhao, S.; Kuge, Y.; Tsukamoto, E.; Mochizuki, T.; Kato, T.; Hikosaka, K.; Nakada, K.; Hosokawa, M.; Kohanawa, M.; Tamaki, N. Fluorodeoxyglucose uptake and glucose transporter expression in experimental inflammatory lesions and malignant tumours: Effects of insulin and glucose loading. Nucl. Med. Commun. 2002, 23, 545–550. [Google Scholar] [CrossRef]
- Takeuchi, M.; Dahabreh, I.J.; Nihashi, T.; Iwata, M.; Varghese, G.M.; Terasawa, T. Nuclear imaging for classic fever of unknown origin: Meta-analysis. J. Nucl. Med. 2016, 57, 1913–1919. [Google Scholar] [CrossRef]
- Afzelius, P.; Alstrup, A.K.O.; Schønheyder, H.C.; Borghammer, P.; Bender, D.; Jensen, S.B.; Nielsen, O.L. Utility of 11C-Methionine and 11C-Donepezil for imaging of Staphylococcus Aureus induced osteomyelitis in a juvenile porcine model: Comparison to Autologous 111in-labelled leukocytes, 99mTc-DPD, and 18F-FDG. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 286–300. [Google Scholar]
- Afzelius, P.; Nielsen, O.L.; Alstrup, A.K.O.; Bender, D.; Leifsson, P.S.; Jensen, S.B.; Schønheyder, H.C. Biodistribution of the Radionuclides 18F-FDG, 11C-Methionine, 11C-PK11195, and 68Ga-Citrate in domestic juvenile female pigs and morphological and molecular imaging of the tracers in hematogenously disseminated Staphylococcus Aureus lesions. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 42–58. [Google Scholar]
- Nielsen, O.L.; Afzelius, P.; Bender, D.; Schønheyder, H.C.; Leifsson, P.S.; Nielsen, K.M.; Larsen, J.O.; Jensen, S.B.; Alstrup, A.K.O. Comparison of Autologous 111In-Leukocytes, 18F-FDG, 11C-Methionine, 11C-PK11195 and 68Ga-Citrate for diagnostic nuclear imaging in a juvenile porcine haematogenous staphylococcus aureus osteomyelitis model. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 169–182. [Google Scholar]
- Welling, M.M.; Hensbergen, A.W.; Bunschoten, A.; Velders, A.H.; Roestenberg, M.; van Leeuwen, F.W.B. An update on radiotracer development for molecular imaging of bacterial infections. Clin. Transl. Imaging 2019, 7, 105–124. [Google Scholar] [CrossRef]
- Kumar, V.; Boddeti, D.K. 68Ga-radiopharmaceuticals for PET imaging of infection and inflammation. In Theranostics, Gallium-68, and Other Radionuclides; Baum, R.P., Rösch, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 194, pp. 189–219. ISBN 978-3-642-27993-5. [Google Scholar]
- Jensen, S.B.; Nielsen, K.M.; Mewis, D.; Kaufmann, J. Fast and Simple one-step preparation of 68Ga Citrate for routine clinical PET. Nucl. Med. Commun. 2013, 34, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.J.; Lankinen, P.; Pöyhönen, T.; Jalava, J.; Aro, H.T.; Roivainen, A. Comparison of 18F-FDG and 68Ga PET imaging in the assessment of experimental osteomyelitis due to Staphylococcus Aureus. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Errani, C.; Boriani, L.; Fantini, L.; Ambrosini, V.; Boschi, S.; Rubello, D.; Pettinato, C.; Mercuri, M.; Gasbarrini, A.; et al. 68Ga-Citrate PET/CT for evaluating patients with infections of the bone: Preliminary results. J. Nucl. Med. 2010, 51, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Jødal, L.; Jensen, S.B.; Nielsen, O.L.; Afzelius, P.; Borghammer, P.; Alstrup, A.K.O.; Hansen, S.B. Kinetic modelling of infection tracers [18F]FDG, [68Ga]Ga-Citrate, [11C]Methionine, and [11C]Donepezil in a porcine osteomyelitis model. Contrast Media Mol. Imaging 2017, 2017, 9256858. [Google Scholar] [CrossRef]
- Ning, X.; Lee, S.; Wang, Z.; Kim, D.; Stubblefield, B.; Gilbert, E.; Murthy, N. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nat. Mater. 2011, 10, 602–607. [Google Scholar] [CrossRef]
- Weinstein, E.A.; Ordonez, A.A.; DeMarco, V.P.; Murawski, A.M.; Pokkali, S.; MacDonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging enterobacteriaceae infection in vivo with 18F-Fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6, 259ra146. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-B.; Wu, Z.; Cao, Q.; Dick, D.W.; Tseng, J.R.; Gambhir, S.S.; Chen, X. The synthesis of 18F-FDS and its potential application in molecular imaging. Mol. Imaging. Biol. 2008, 10, 92–98. [Google Scholar] [CrossRef]
- Yao, S.; Xing, H.; Zhu, W.; Wu, Z.; Zhang, Y.; Ma, Y.; Liu, Y.; Huo, L.; Zhu, Z.; Li, Z.; et al. Infection imaging with 18F-FDS and first-in-human evaluation. Nucl. Med. Biol. 2016, 43, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Boos, W.; Shuman, H. Maltose/Maltodextrin System of Escherichia coli: Transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 1998, 62, 204–229. [Google Scholar] [CrossRef]
- Ning, X.; Seo, W.; Lee, S.; Takemiya, K.; Rafi, M.; Feng, X.; Weiss, D.; Wang, X.; Williams, L.; Camp, V.M.; et al. PET imaging of bacterial infections with fluorine-18-labeled maltohexaose. Angew. Chem. Int. Ed. 2014, 53, 14096–14101. [Google Scholar] [CrossRef] [PubMed]
- Jødal, L.; Nielsen, O.L.; Afzelius, P.; Alstrup, A.K.O.; Hansen, S.B. Blood perfusion in osteomyelitis studied with [15O]water PET in a juvenile porcine model. EJNMMI Res. 2017, 7, 4. [Google Scholar] [CrossRef]
- Bergmann, S.R.; Fox, K.A.; Rand, A.L.; McElvany, K.D.; Welch, M.J.; Markham, J.; Sobel, B.E. Quantification of regional myocardial blood flow in vivo with H215O. Circulation 1984, 70, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Herscovitch, P.; Markham, J.; Raichle, M.E. Brain blood flow measured with intravenous H215O. I. Theory and error analysis. J. Nucl. Med. 1983, 24, 782–789. [Google Scholar]
- Raichle, M.E.; Martin, W.R.; Herscovitch, P.; Mintun, M.A.; Markham, J. Brain blood flow measured with intravenous H215O. II. Implementation and validation. J. Nucl. Med. 1983, 24, 790–798. [Google Scholar] [PubMed]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Leung, K. L-[Methyl-11C]Methionine–Molecular Imaging and Contrast Agent Database (MICAD); NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK23696/ (accessed on 14 October 2016).
- Zhao, S.; Kuge, K.; Kohanawa, M.; Takahashi, T.; Zhao, Y.; Yi, M.; Kanegae, K.; Seki, K.; Tamaki, N. Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: A comparison with 18F-FDG and 18F-FLT. J. Nucl. Med. 2008, 49, 135–141. [Google Scholar] [CrossRef]
- Maeda, Y.; Oguni, H.; Saitou, Y.; Mutoh, A.; Imai, K.; Osawa, M.; Fukuyama, Y.; Hori, T.; Yamane, F.; Kubo, O.; et al. Rasmussen syndrome: Multifocal spread of inflammation suggested from MRI and PET findings. Epilepsia 2003, 44, 1118–1121. [Google Scholar] [CrossRef]
- Hirata, K.; Shiga, T.; Fujima, N.; Manabe, O.; Usui, U.; Kuge, Y.; Tamaki, N. 11C-Methionine positron emission tomography may monitor the activity of encephalitis. Acta Radiol. 2012, 53, 1155–1157. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, nutrition, and microbiology of d-amino acids. J. Agric. Food Chem. 1999, 47, 3457–3479. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.D.; Villanueva-Meyer, J.E.; Mutch, C.A.; Flavell, R.R.; Blecha, J.E.; Kwak, T.; Sriram, R.; VanBrocklin, H.F.; Rosenberg, O.S.; Ohliger, M.A.; et al. Imaging active infection in vivo using d-amino acid derived PET radiotracers. Sci. Rep. 2017, 7, 7903. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.N.; Parker, M.F.L.; Jivan, S.; Luu, J.M.; Huynh, T.L.; Schulte, B.; Seo, Y.; Blecha, J.E.; Villanueva-Meyer, J.E.; Flavell, R.R.; et al. High enantiomeric excess in-loop synthesis of d-[Methyl-11C]Methionine for use as a diagnostic positron emission tomography radiotracer in bacterial infection. Acs Infect. Dis. 2020, 6, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Ogura, H.; Arai, Y.; Limura, Y.; Yamanishi, Y. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn. J. Pharm. 2002, 89, 7–20. [Google Scholar] [CrossRef]
- Hiraoka, K.; Okamura, N.; Funaki, Y.; Watanuki, S.; Tashiro, M.; Kato, M.; Hayashi, A.; Hosokai, Y.; Yamasaki, H.; Fujii, T.; et al. Quantitative analysis of donepezil binding to acetylcholinesterase using positron emission tomography and [5-11C-Methoxy]Donepezil. Neuroimage 2009, 46, 616–623. [Google Scholar] [CrossRef]
- Gjerløff, T.; Jakobsen, S.; Nahimi, A.; Munk, O.L.; Bender, D.; Alstrup, A.K.O.; Vase, K.H.; Hansen, S.B.; Brooks, D.J.; Borghammer, P. In vivo imaging of human acetylcholinesterase density in peripheral organs using 11C-Donepezil: Dosimetry, biodistribution, and kinetic analyses. J. Nucl. Med. 2014, 55, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.P.; Alstrup, A.K.O.; Mortensen, F.V.; Knudsen, K.; Jakobsen, S.; Madsen, L.B.; Bender, D.; Breining, P.; Petersen, M.S.; Schleimann, M.H.; et al. Cholinergic PET imaging in infections and inflammation using 11C-Donepezil and 18F-FEOBV. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 2012, 91, 1027–1032. [Google Scholar] [CrossRef]
- Fujii, T.; Watanabe, Y.; Fujimoto, K.; Kawashima, K. Expression of acetylcholine in lymphocytes and modulation of an independent lymphocytic cholinergic activity by immunological stimulation. Biog. Amines 2003, 17, 373–386. [Google Scholar] [CrossRef]
- Patel, L.; Charlton, S.J.; Chambers, J.K.; Macphee, C.H. Expression and functional analysis of chemokine receptors in human peripheral blood leukocyte populations. Cytokine 2001, 14, 27–36. [Google Scholar] [CrossRef]
- Lee, J.; Horuk, R.; Rice, G.C.; Bennett, G.L.; Camerato, T.; Wood, W.I. Characterization of two high affinity human interleukin-8 receptors. J. Biol. Chem. 1992, 267, 16283–16287. [Google Scholar] [CrossRef]
- Cerretti, D.P.; Kozlosky, C.J.; Bos, T.V.; Nelson, N.; Gearing, D.P.; Beckmann, M.P. Molecular characterization of receptors for human interleukin-8, GRO/melanoma growth-stimulatory activity and neutrophil activating peptide-2. Mol. Immunol. 1993, 30, 359–367. [Google Scholar] [CrossRef]
- Laursen, H.; Jensen, H.E.; Leifsson, P.S.; Jensen, L.K.; Christiansen, J.G.; Trebbien, R.; Nielsen, O.L. Immunohistochemical detection of interleukin-8 in inflamed porcine tissues. Vet. Immunol. Immunopathol. 2014, 159, 97–102. [Google Scholar] [CrossRef]
- Rennen, H.J.J.M.; Boerman, O.C.; Oyen, W.J.; van der Meer, J.W.; Corstens, F.H. Specific and rapid scintigraphic detection of infection with 99mTc-labeled interleukin-8. J. Nucl. Med. 2001, 42, 117–123. [Google Scholar] [PubMed]
- Gratz, S.; Rennen, H.J.J.M.; Boerman, O.C.; Oyen, W.J.; Burma, P.; Corstens, F.H. 99mTc-Interleukin-8 for imaging acute osteomyelitis. J. Nucl. Med. 2001, 42, 1257–1264. [Google Scholar]
- Bleeker-Rovers, C.P.; Rennen, H.J.J.M.; Boerman, O.C.; Wymenga, A.B.; Visser, E.P.; Bakker, J.H.; van der Meer, J.W.M.; Corstens, F.H.M.; Oyen, W.J.G. 99mTc-labeled interleukin 8 for the scintigraphic detection of infection and inflammation: First clinical evaluation. J. Nucl. Med. 2007, 48, 337–343. [Google Scholar] [PubMed]
- Afzelius, P.; Heegaard, P.M.H.; Jensen, S.B.; Alstrup, A.K.O.; Schønheyder, H.C.; Eek, A.; Boerman, O.; Nielsen, O.L. [99mTc]-Labelled interleukin-8 as a diagnostic tool compared to [18F]FDG and CT in an experimental porcine osteomyelitis model. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 32–46. [Google Scholar] [PubMed]
- Salmi, M.; Jalkanen, S. VAP-1: An adhesin and an enzyme. Trends Immunol. 2001, 22, 211–216. [Google Scholar] [CrossRef]
- Jalkanen, S.; Salmi, M. VAP-1 and CD73, endothelial cell surface enzymes in leukocyte extravasation. ATVB 2008, 28, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Käkelä, M.; Jødal, L.; Moisio, O.; Alstrup, A.K.O.; Jalkanen, S.; Roivainen, A. Exploring the radiosynthesis and in vitro characteristics of [68Ga]Ga-DOTA-Siglec-9. J. Label. Compd. Radiopharm. 2017, 60, 439–449. [Google Scholar] [CrossRef]
- Aalto, K.; Autio, A.; Kiss, E.A.; Elima, K.; Nymalm, Y.; Veres, T.Z.; Marttila-Ichihara, F.; Elovaara, H.; Saanijoki, T.; Crocker, P.R.; et al. Siglec-9 Is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer. Blood 2011, 118, 3725–3733. [Google Scholar] [CrossRef]
- Ahtinen, H.; Kulkova, J.; Lindholm, L.; Eerola, E.; Hakanen, A.J.; Moritz, N.; Söderström, M.; Saanijoki, T.; Jalkanen, S.; Roivainen, A.; et al. 68Ga-DOTA-Siglec-9 PET/CT imaging of peri-implant tissue responses and staphylococcal infections. Ejnmmi Res. 2014, 4, 45. [Google Scholar] [CrossRef]
- Roivainen, A.; Jalkanen, S.; Nanni, C. Gallium-labelled peptides for imaging of inflammation. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Jødal, L.; Roivainen, A.; Oikonen, V.; Jalkanen, S.; Hansen, S.B.; Afzelius, P.; Alstrup, A.K.O.; Nielsen, O.L.; Jensen, S.B. Kinetic Modelling of [68Ga]Ga-DOTA-Siglec-9 in porcine osteomyelitis and soft tissue infections. Molecules 2019, 24, 4094. [Google Scholar] [CrossRef]
- Viitanen, R.; Moisio, O.; Lankinen, P.; Li, X.-G.; Koivumäki, M.; Suilamo, S.; Tolvanen, T.; Taimen, K.; Mali, M.; Kohonen, I.; et al. First-in-humans study of 68Ga-DOTA-Siglec-9, a PET ligand targeting vascular adhesion protein 1. J. Nucl. Med. 2021, 62, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.M.; Kyneb, M.H.; Alstrup, A.K.O.; Jensen, J.J.; Bender, D.; Schønheyder, H.C.; Afzelius, P.; Nielsen, O.L.; Jensen, S.B. 68Ga-Labeled phage-display selected peptides as tracers for positron emission tomography imaging of Staphylococcus Aureus biofilm-associated infections: Selection, radiolabelling and preliminary biological evaluation. Nucl. Med. Biol. 2016, 43, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.M.; Jørgensen, N.P.; Kyneb, M.H.; Borghammer, P.; Meyer, R.L.; Thomsen, T.R.; Bender, D.; Jensen, S.B.; Nielsen, O.L.; Alstrup, A.K.O. Preclinical evaluation of potential infection-imaging probe [68Ga]Ga-DOTA-K-A9 in sterile and infectious inflammation. J. Label. Compd. Radiopharm. 2018, 61, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Ebenhan, T.; Chadwick, N.; Sathekge, M.M.; Govender, P.; Govender, T.; Kruger, H.G.; Marjanovic-Painter, B.; Zeevaart, J.R. Peptide synthesis, characterization and 68Ga-radiolabeling of NOTA-conjugated ubiquicidin fragments for prospective infection imaging with PET/CT. Nucl. Med. Biol. 2014, 41, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; van den Barselaar, M.T.; Roest, M.; Nibbering, P.H.; Van Furth, R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 1999, 66, 423–428. [Google Scholar] [CrossRef]
- Welling, M.M.; Paulusma-Annema, A.; Balter, H.S.; Pauwels, E.K.J.; Nibbering, P.H. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 2000, 27, 292–301. [Google Scholar] [CrossRef]
- Welling, M.M.; Lupetti, A.; Balter, H.S.; Lanzzeri, S.; Souto, B.; Rey, A.M.; Savio, E.O.; Paulusma-Annema, A.; Pauwels, E.K.; Nibbering, P.H. 99mTc-labeled antimicrobial peptides for detection of bacterial and Candida Albicans infections. J. Nucl. Med. 2001, 42, 788–794. [Google Scholar]
- Ebenhan, T.; Sathekge, M.M.; Lengana, T.; Koole, M.; Gheysens, O.; Govender, T.; Zeevaart, J.R. 68Ga-NOTA-functionalized ubiquicidin: Cytotoxicity, biodistribution, radiation dosimetry, and first-in-human PET/CT imaging of infections. J. Nucl. Med. 2018, 59, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; Avila-Rodríguez, M.A.; García-Pérez, F.O. Imaging of bacteria with radiolabeled ubiquicidin by SPECT and PET techniques. Clin. Transl. Imaging 2016, 4, 175–182. [Google Scholar] [CrossRef]
| Radionuclide | Half-Life 1 | Imaging Modality 2 |
|---|---|---|
| 11C | 20.39 min | PET |
| 15O | 122 s | PET |
| 18F | 109.77 min | PET |
| 67Ga | 3.26 d | SPECT |
| 68Ga | 67.7 min | PET |
| 99mTc | 6.02 h | SPECT |
| 111In | 2.80 d | SPECT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jødal, L.; Afzelius, P.; Alstrup, A.K.O.; Jensen, S.B. Radiotracers for Bone Marrow Infection Imaging. Molecules 2021, 26, 3159. https://doi.org/10.3390/molecules26113159
Jødal L, Afzelius P, Alstrup AKO, Jensen SB. Radiotracers for Bone Marrow Infection Imaging. Molecules. 2021; 26(11):3159. https://doi.org/10.3390/molecules26113159
Chicago/Turabian StyleJødal, Lars, Pia Afzelius, Aage Kristian Olsen Alstrup, and Svend Borup Jensen. 2021. "Radiotracers for Bone Marrow Infection Imaging" Molecules 26, no. 11: 3159. https://doi.org/10.3390/molecules26113159
APA StyleJødal, L., Afzelius, P., Alstrup, A. K. O., & Jensen, S. B. (2021). Radiotracers for Bone Marrow Infection Imaging. Molecules, 26(11), 3159. https://doi.org/10.3390/molecules26113159







