1. Introduction
In recent years, the massive interest in polyphenolic compounds as substances with potential beneficial health properties resulted in an increase in the number of studies aimed at their identification and quantification, especially in the plants that were used for centuries in traditional medicine [
1,
2,
3,
4,
5,
6]. Among them is hawthorn,
Crataegus L., of the
Rosaceae family comprising of about 200 species of deciduous shrubs and trees growing wild in the forests of Europe, North Africa, West Asia, and North America. Native Americans used
Crataegus berries, flowers, and bark as diuretics for kidney and bladder diseases, to treat stomach pains, and to stimulate appetite, while in Europe, it was used to treat asthma, pharyngitis, and insomnia [
6,
7]. Today,
Crataegus berries and flowers are officially recognized as herbal raw materials and have been included in European, American, and Canadian pharmacopeias. Recent research confirmed and extended the possibilities of the traditional use of this plant, among others, to anti-inflammatory [
8,
9], anti-oxidant [
10,
11], anti-aging [
12], anti-depressant [
13], anti-viral, anti-fungal, anti-bacterial [
14], hypoglycemic, hepatoprotective [
15], gastroprotective [
16], immunostimulatory, hypolipidemic, hypocholesterolemic, and anti-atherosclerotic effects [
17]. Furthermore,
Crataegus extracts are considered as putative therapeutic agents for the treatment of hepatocellular carcinoma, breast cancer, melanoma, and colorectal cancer [
18,
19,
20]. Thus,
Crataegus has a high pro-health potential, which could be used in the prevention and/or the treatment of many diseases, including cancer. Health-promoting hawthorn properties are mainly attributed to the high content of biologically active ingredients, of which the polyphenolic compounds are the most important ones. The most essential polyphenolic constituents so far identified in
Crataegus are phenolic acids (e.g., chlorogenic, caffeic, ferulic,
p-coumaric, gallic, syringic acids), flavonoids, and flavonoids glycosides (rutin, quercetin 3-
O-glucoside, luteolin 7-
O-glucoside, naringenin, C-hexosides, vitexin, isovitexin, kaempferol 3-
O-glucuronide, myricitrin), anthocyanidins, proanthocyanidins, and their derivatives (epicatechin, procyanidin dimers, procyanidin trimer, cyanidin, cinchonains) [
10,
11,
18,
19,
20].
Numerous studies indicated that polyphenolic compounds of the extracts of
Crataegus and several other plants could be behind the observed anti-cancer effects [
19,
20,
21,
22,
23]. In order to address this notion, we performed comparative studies on polyphenol-rich
Crataegus extracts obtained from berries, leaves, and flowers of its six species and simultaneously assessed their impact on the viability, morphology, and invasive potential on the human glioblastoma U87MG cell line derived from an aggressive primary malignant brain tumor. All the examined extracts were analyzed for content and chromatographic profiles of secondary metabolites in order to correlate the levels of the particular group of compounds with the observed cellular effects. To date, it has been the first such contemporaneous examination of the effects of
Crataegus extracts on the glioblastoma cells with the use of both cell biology and phytochemistry techniques.
2. Results
2.1. Crataegus Extracts Inhibit U87MG Human Glioblastoma Cells Viability
In the light of the reports describing potential anti-tumor properties of Crataegus extracts, we decided to examine the effects of its six species growing in the Podkarpacie region on glioblastoma cells derived from a highly malignant brain tumor. The extracts obtained from berries, leaves, and flowers (CB1–6, CL1–6, and CF1–6 extracts, respectively) were evaluated.
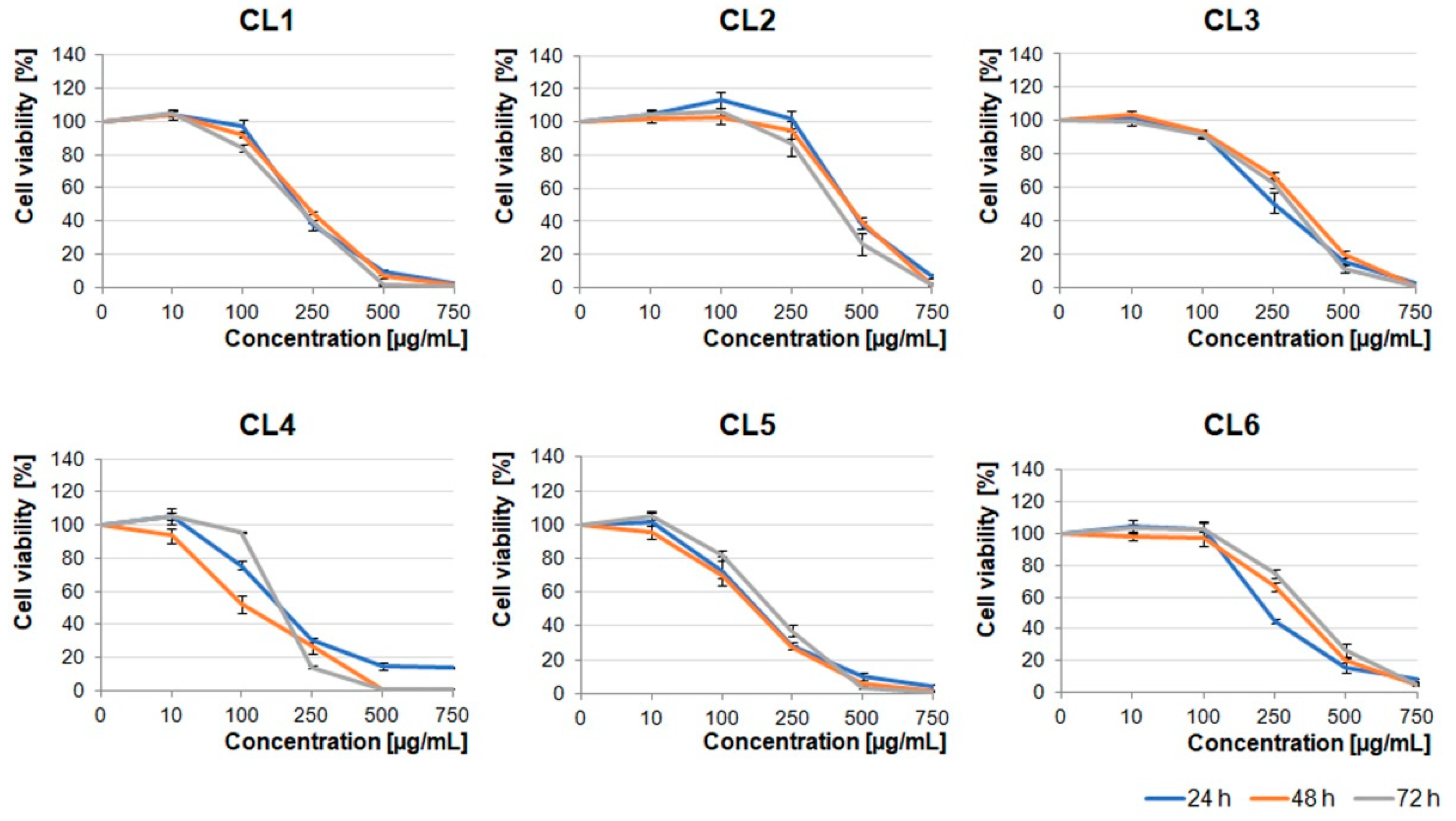
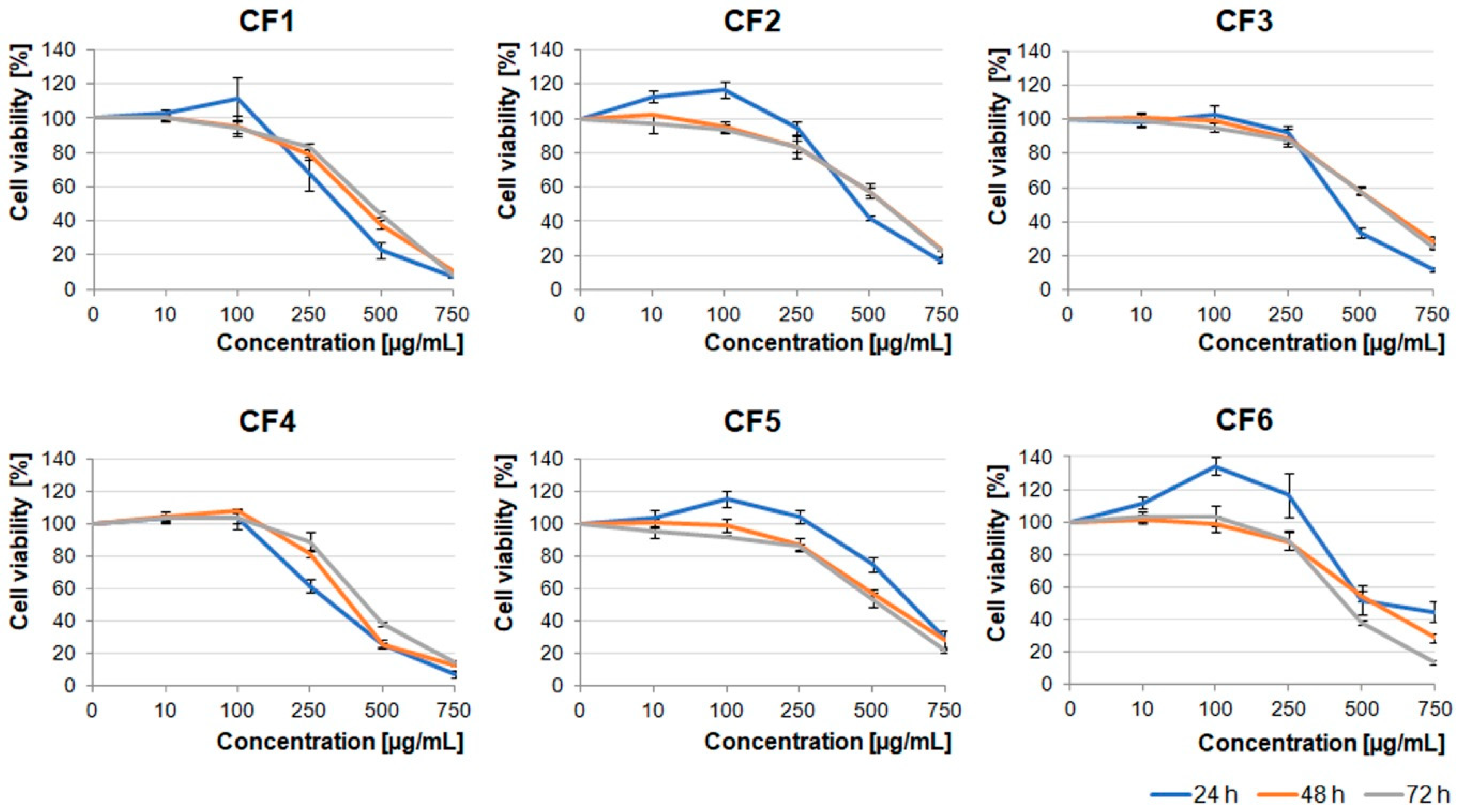
First, we analyzed the cytotoxic effect of increasing concentrations (10–750 µg/mL) of the extracts on the U87MG human glioblastoma cell line, which was characterized by a high invasive potential. As shown in
Figure 1,
Figure 2 and
Figure 3, all the examined types of extracts inhibited glioblastoma cell viability, mainly in the concentration-dependent manner. Moreover, berry extracts showed higher cytotoxic effects on glioblastoma cells than the other extracts (
Figure 1). A dramatic decrease of cell viability was observed under 250 µg/mL concentration of CB1–CB5 extracts. For CB6, a similar effect was evoked by a higher concentration of 500 µg/mL. The EC50 values for each extract type are presented in
Table S1.
Crataegus leaf extracts affected glioblastoma cell viability in a pattern similar to that of the berry extracts (
Figure 1 and
Figure 2). It should be noted that CL1, CL4, and CL5 were more toxic than other leaf extracts (
Figure 2).
Extracts obtained from the flowers were less toxic than the ones obtained from berries and leaves. The U87MG cell viability was significantly decreased under concentrations higher than 500 µg/mL (
Figure 3). It should be noted that CF1, CF2, CF5, and CF6 in lower concentrations (≤100 µg/mL) stimulated cell proliferation after 24 h incubation. Cells treated with dimethyl sulfoxide (DMSO), in which these extracts were dissolved (0.02–1.5% dependent on the extract concentration), served as an additional negative control (
Figure S1).
2.2. Do Crataegus Extracts Promote Apoptosis of U87MG Human Glioblastoma Cells?
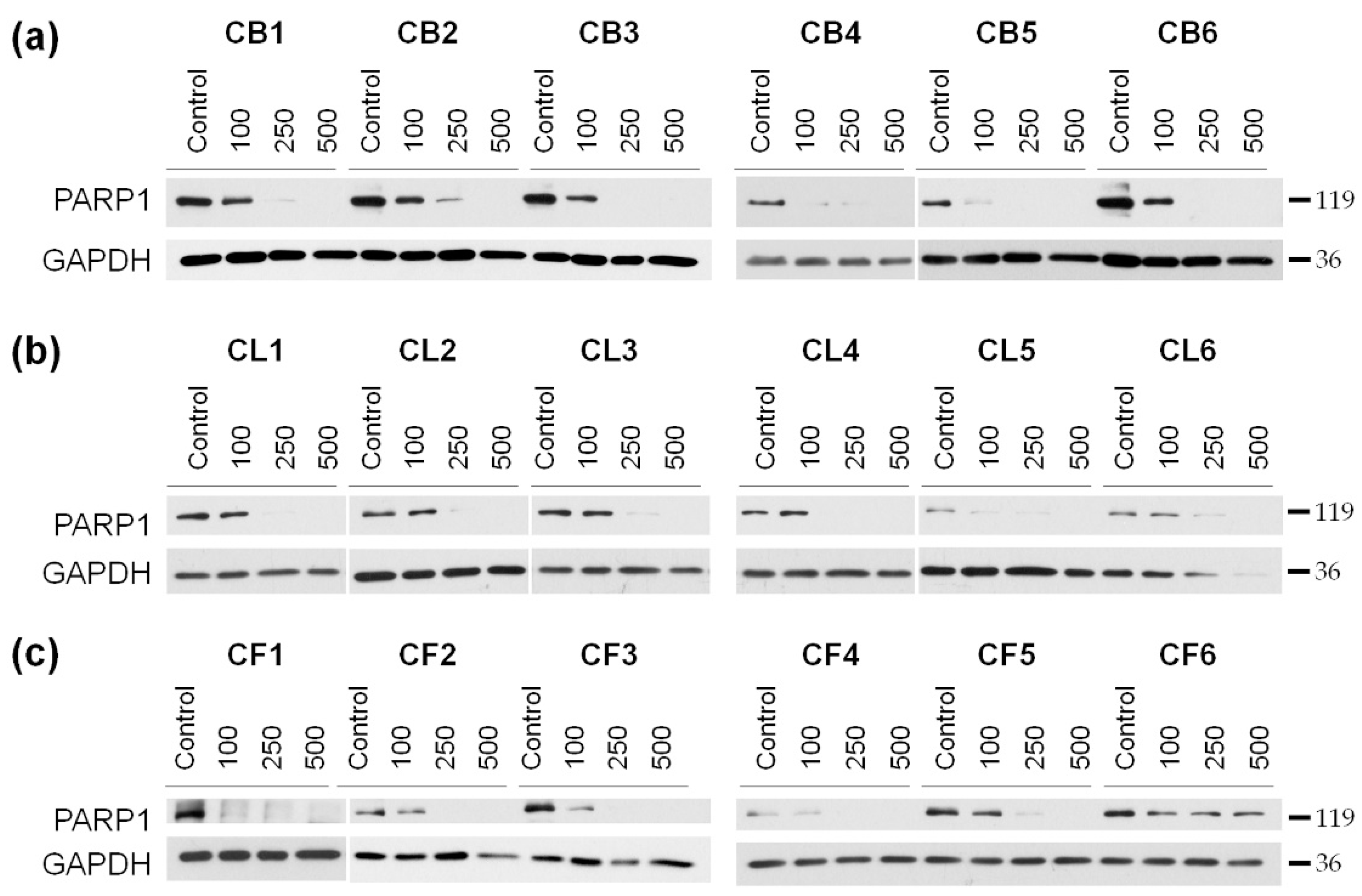
Our next step was to reveal mechanisms of the toxic effects of the extracts on the examined cells. The cells were treated for 48 h with the extract concentrations of 100, 250, and 500 µg/mL, and then the resulted lysates were subjected to the Western blotting to determine the level of poly (ADP-ribose) polymerase 1 (PARP1), which enzymatic cleavage is the hallmark of apoptosis. We showed the concentration-dependent decrease of the full-length PARP1 (
Figure 4 and
Figure S2) during the treatment with
Crataegus extracts, thus, indicating that the extracts could promote apoptotic cell death.
2.3. Crataegus Extracts Promote Apoptosis-Associated Changes in Morphology of U87MG Human Glioblastoma Cells
Numerous studies showed that the changes of cell shape, greatly dependent on the cytoskeleton organization, played an important role in cancer cell invasiveness and the progression of cancer metastasis [
24,
25]. The phenotypic characteristics of glioblastoma cells during a 48 h treatment with
Crataegus extracted at a concentration of 250 µg/mL (with pronounced effects of most of the examined extracts and close to the EC50 value) were analyzed using light microscopy (
Figure 5) and fluorescence staining for the actin cytoskeleton with Alexa Fluor 488-conjugated phalloidin and nuclei with DAPI (
Figure 6).
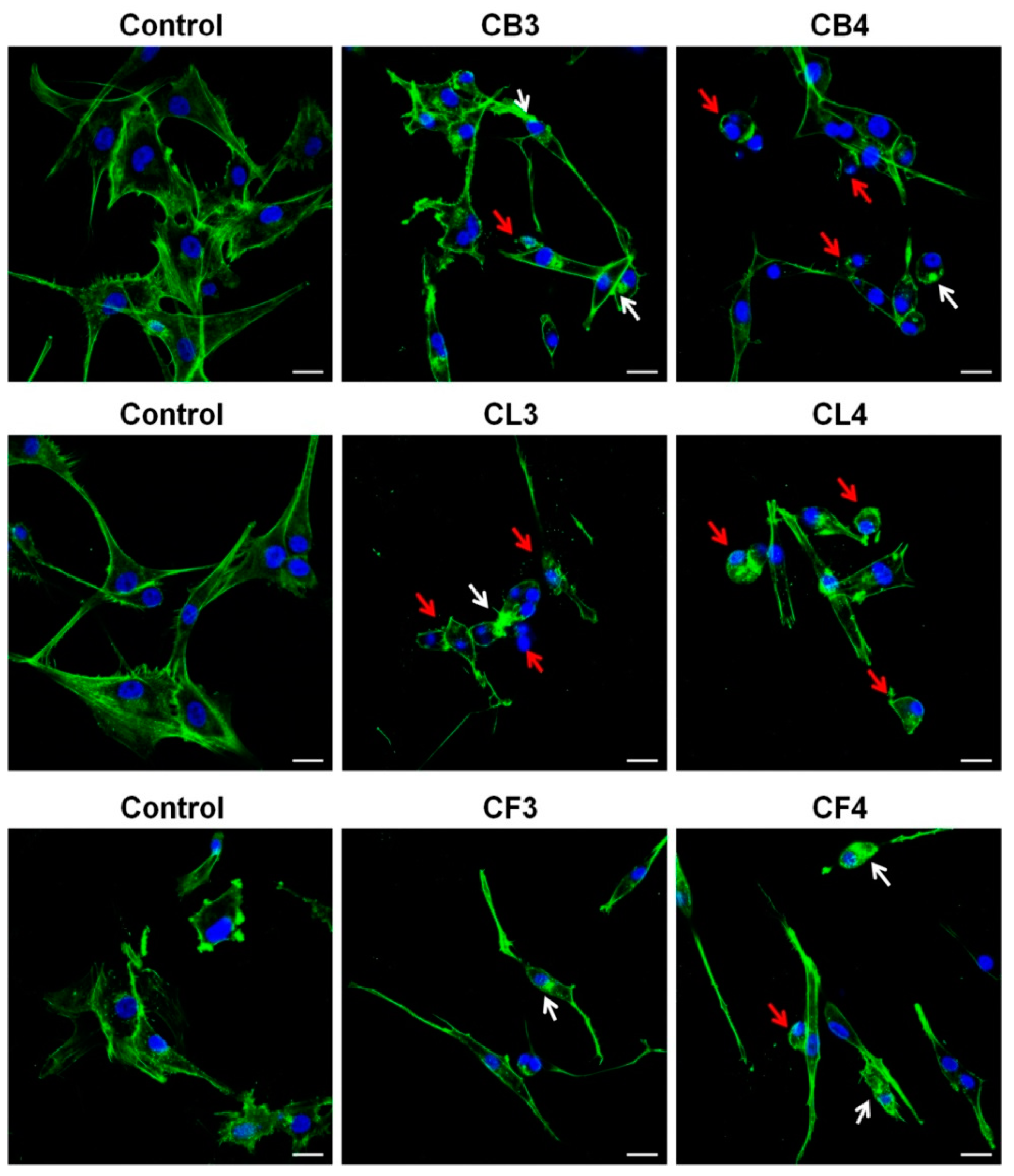
As shown in
Figure 5a, all
Crataegus berry extracts significantly affected the glioblastoma cell morphology. Cells rounded up and did not form lamellipodia, the actin-rich frontal protrusions characteristic of migrating cells. Moreover, the increased number of the cells, with the features of apoptotic cell death such as cell shrinkage and formation of apoptotic bodies (blebs), was observed after the treatment (
Figure 5a, red arrows) [
26]. Similar effects were observed in the cells treated with CL1, CL5, and CL6 leaf extracts (
Figure 5b). In turn, flower extracts had much less pronounced effects on the cell morphology (
Figure 5c). Only CF2 and CF3 extracts evoked more evident changes (see
Figure 5c, red arrows).
Our next step was a detailed analysis of the impact of
Crataegus extracts on the actin cytoskeleton and morphology of the examined U87MG cells. For that, we chose the extracts of two
Crataegus species,
C. x subsphaericea (CB3, CL3, and CF3) and
C. laevigata x rhipidophylla x monogyna (CB4, CL4, and CF4), for which we observed the most pronounced cytotoxic effects (
Figure 6). For a more precise comparison of
Crataegus extracts effects, we chose the concentrations close to their EC50 values (see
Table S1).
Treatment with CB3 and CB4 berry extracts evoked the loss of cell polarity and a significant decrease of lamellipodia formation in glioblastoma cells that could cause the disturbance in cell motility (
Figure 6). Additionally, we observed the formation of actin aggregates, abnormally shaped cell nuclei, and cell rounding, thus, further indicating the progression of apoptotic cell death (
Figure 6, see arrows).
Also, after 48 h incubation with CL3 and CL4 leaf extracts, cells rounded up, and the presence of blebs and actin aggregates was observed (
Figure 6, see arrows).
In turn, treatment with CF3 or CF4 flower extracts caused somewhat different changes in glioblastoma cell morphology (
Figure 6). Cells became spindle-like, elongated, without a clearly pronounced leading edge. It was noteworthy that an increased level of actin stress fibers in the treated cells was observed. Cells with apoptotic features (shrunk, rounded up, and blebbed) were also present, further confirming the observation that the examined extracts induced apoptosis (
Figure 5 and
Figure 6, red arrows).
Thus, treatment with Crataegus extracts caused pronounced morphological changes in glioblastoma cells that could affect the invasiveness and viability of the examined cancer cells.
2.4. Crataegus Extracts Significantly Decrease the Activity of the Prosurvival FAK and Akt Kinases
Cell adhesion and migration are crucial for cell survival and are essential for cancer cell proliferation and invasiveness [
27]. A plethora of proteins are involved in cell attachment to the extracellular matrix (ECM), a three-dimensional network of extracellular macromolecules. Focal adhesion kinase (FAK) is a master regulator of adhesive structure formation and, therefore, is crucial for cell adhesion and motility. It has also been shown that its activity is important for the promotion of cancer cell invasiveness, associated with malignant overgrowth [
27,
28]. These features make FAK a potential target in the development of anti-cancer treatment.
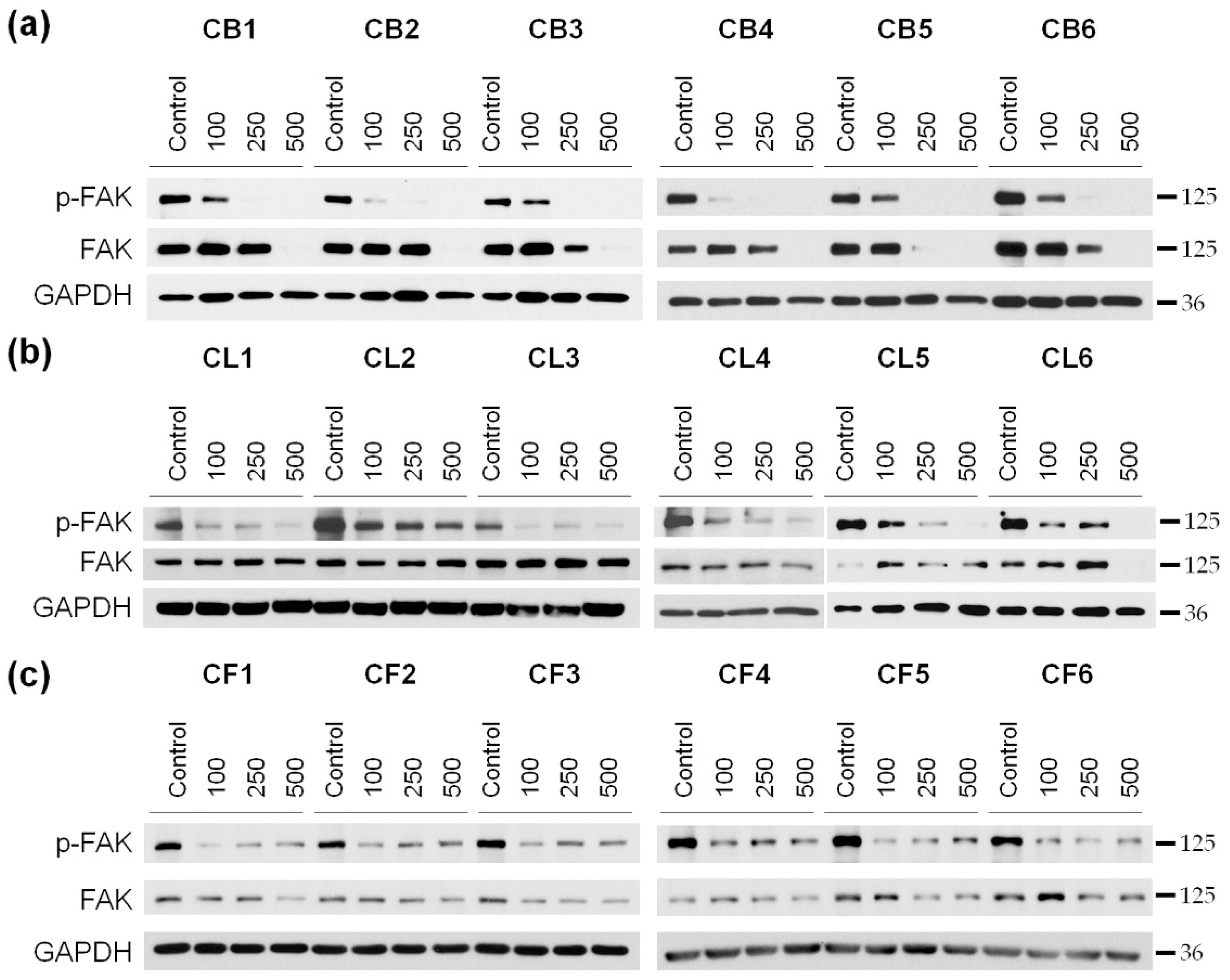
We analyzed the level of FAK kinase and its active phosphorylated form (p-FAK) in glioblastoma cells after 48 h of incubation with
Crataegus extracts (
Figure 7 and
Figure S3). Since the cytotoxic effect of the extracts was concentration-dependent, three different concentrations (100, 250, and 500 µg/mL) of the extracts were used in the analysis.
We observed that the 48 h treatment with
Crataegus berry extracts profoundly inhibited the activity of FAK in U87MG cells in a concentration-dependent manner (
Figure 7a and
Figure 3a). Moreover, incubation of the cells with berry extracts at the concentration of 500 µg/mL completely blocked FAK expression. A similar effect was observed under the treatment with CL5 and CL6 leaf extracts (
Figure 7b and
Figure S3b). The effect of CL1, CL3, and CL4 extracts was very profound already at a concentration of 100 µg/mL and was not further affected by the increased concentration. An analysis of the effect of all six flower extracts also revealed a decrease in the activation level of FAK that was observed as early as at a concentration of 100 µg/mL. It did not seem to be concentration-dependent as similar levels of p-FAK were observed at higher extract concentrations (
Figure 7c and
Figure S3c).
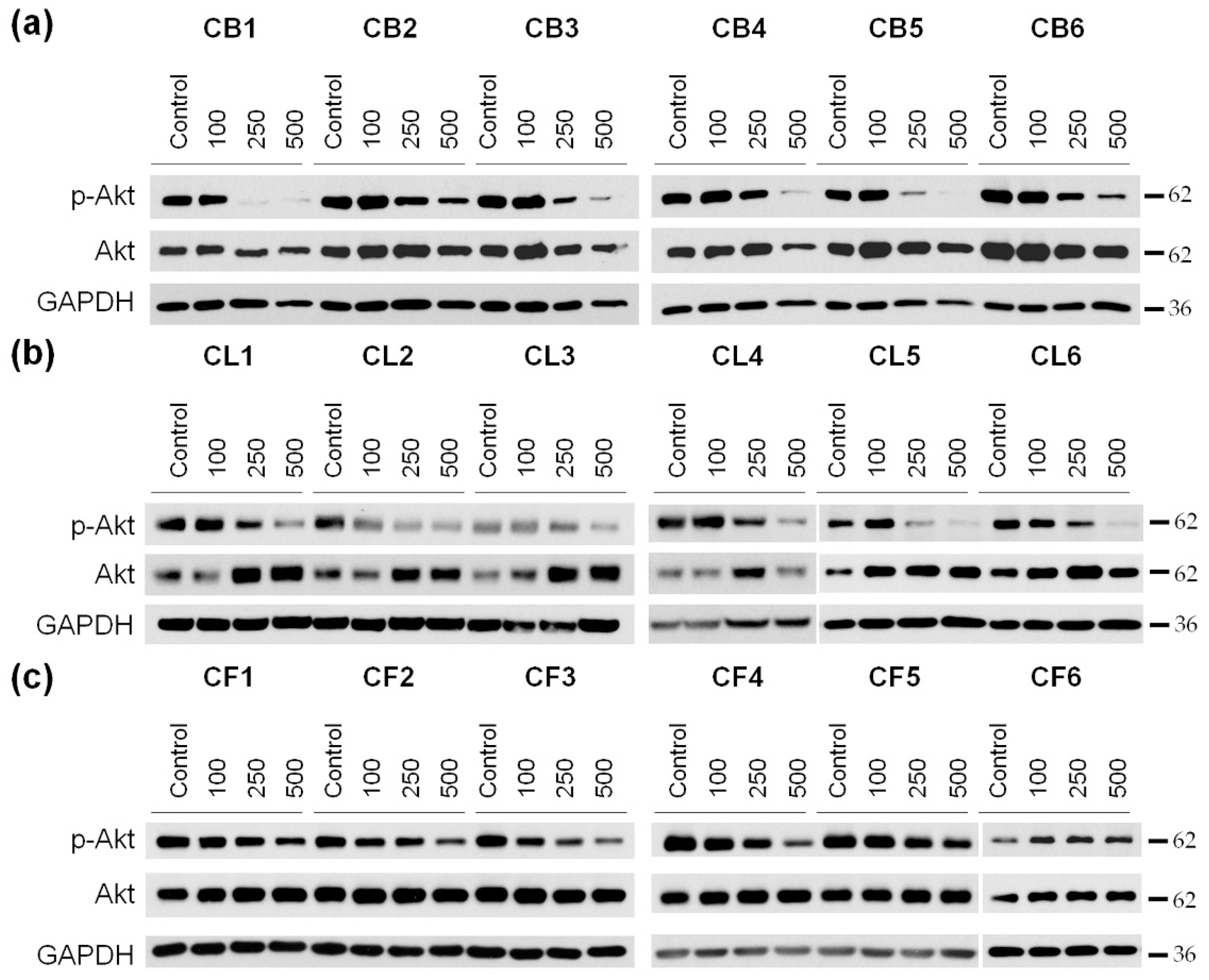
It is known that cell survival, motility, and cell cycle progression are also highly dependent on the activity of protein kinase B (PKB), also known as Akt [
29]. This is why our next step was to examine the phosphorylation (activation) level of Akt in U87MG cells after the incubation with
Crataegus extracts (
Figure 8 and
Figure S4).
A significant decrease in Akt phosphorylation in glioblastoma cells was observed under the cell incubation with nearly all the extracts with the most profound effect of berry extracts, in particular of CB1 (
Figure 8a–c and
Figure S4a–c). In turn, the 48 h treatment with CF1–CF5
Crataegus flower extracts just slightly reduced the level of p-Akt (
Figure 8c and
Figure S4c). Interestingly, we did not observe significant changes in the p-Akt level during the treatment with CF6, regardless of the increase in its concentration.
FAK inhibition, together with a decrease in Akt activity upon treatment with the examined extracts, suggested that the extracted compound could profoundly reduce cell growth and migration, and thus, the invasiveness of U87MG glioblastoma cells.
A better understanding of the mechanisms of the observed effects of Crataegus extracts and their individual components should be beneficial for the development of this plant extract-based potential treatment of highly invasive human glioblastomas. Therefore, we aimed at qualitative and quantitative analyses of the content of the examined Crataegus extracts.
2.5. Identification of Polyphenolic Compounds
It was shown that the health-promoting properties of numerous plant materials, including anti-oxidant, anti-inflammatory, and anti-cancer effects, depended mainly on the content of polyphenols [
30]. Therefore, our next step was the analysis for the content of these compounds in the extracts obtained from berries, leaves, and flowers of the examined six species of
Crataegus. The identification of polyphenolic compounds was performed in the UPLC-PDA-MS/MS system in positive and negative ion mode. The results are summarized in
Table 1. The UPLC chromatograms of berries, leaves, and flowers first
Crataegus species (C1) are presented in
Figures S5–S7. A total number of 37 compounds was detected in morphological parts of the examined
Crataegus species. We found 29 compounds in berries, 14 in leaves, and 13 compounds in flowers. Among all the compounds, four belonged to the anthocyanin category, six to flavan-3-ols, five to hydrolyzable tannins, nine to phenolic acids, and 13 to flavonols.
Anthocyanins are one of the most important flavonoid pigments found in many plant organs [
31]. The anthocyanin profile in
Crataegus was obtained by detection with the typical absorption maximum for anthocyanins of 520 nm. In total, four compounds were assigned, but only in berries (see
Table 1). Their identification was based on fragmentation patterns. Peaks 1 and 3 showed the fragment ion at 287
m/z that corresponded to cyanidin. These compounds were identified as cyanidin 3-
O-glucoside (
m/z 449) and cyanidin 3-
O-arabinoside (
m/z 419), respectively. Peak 2 was assigned as pelargonidin 3-
O-rutinoside based on the mass spectrum detected at 579
m/z and the characteristic fragment ions with
m/z 271 and 433 (loss of rhamnose and rutinose). The last anthocyanin (peak no. 4) with the molecular ion
m/z 463 and the fragment ion 301
m/z was identified as peonidin 3-
O-glucoside.
Another group of polyphenolic compounds id flavan-3-ols, a structurally complex subclass of flavonoids, showing a high pro-health potential [
32,
33]. Six flavan-3-ols were detected in berries, three in leaves, and two in flowers of
Crataegus. Compounds 7 and 8 were identified as (+)-catechin and (−)-epicatechin (
m/z 289), based on commercially available compound standards. Peaks 5, 6, and 9 showed an MS/MS fragment ion at
m/z 289 (epicatechin derived from a cleavage of the link between procyanidin monomers) [
34]. These compounds were assigned as B-type procyanidin trimer (
m/z 865), dimer (
m/z 577), and tetramer (
m/z 1442), respectively. In turn, compound no. 10, representing the parent ion at
m/z 451 belonging to flavalignan isomers groups, was identified as a cinchonain [
35].
Hydrolyzable tannins form another group that we identified in
Crataegus extracts, including five compounds in the berries and one in leaves. Tannins possess well-characterized but opposite activities, ranging from favorable anti-oxidants to harmful pro-oxidants [
36]. Peak no. 11 was assigned to the group of the glycosylated form of ellagic acid [
37]. Thus, this compound at
m/z 433 and fragment of
m/z 301 (loss of a hexose) was identified as ellagic acid pentoside. Compounds 12 and 13 possessed the same molecular ion
m/z 781 and fragment ion
m/z 299, that corresponded to the punicalin isomer (α/
β-isomer) [
38]. Since the
β-isomer eluted before the α-isomer, peak 12 was identified as
β-isomer and peak 13 as α-isomer (isopunicalin). The last two hydrolyzable tannins (peak no. 14 and 15) with
m/z 721, 739, and
m/z 301 ion fragments assigned to bis-HHDP-glucose, belonging to galloyl substituents, were detected as 2-
O-galloylpunicalin (
m/z 933) and eucalbanin A (
m/z 1085), respectively [
39].
Phenolic acids belong to the next main group of polyphenolic compounds detected in all food products and present both in free and conjugated forms. Among the analyzed parts of Crataegus, six phenolic acids were identified in berries, five in leaves, and five in flowers. Compound 16 detected at m/z 191 was identified as quinic acid according to the standards. Two peaks, no. 17 and 21, had fragment ions m/z 163 typical for p-coumaric and m/z 119, corresponding to decarboxylated form coumaric acid. Therefore, these two peaks were tentatively annotated as coumaroylquinic acid and 3-O-p-coumaroylquinic acid, respectively. Peaks no. 19 and 22 represented acylated quinic acid derivatives, including chlorogenic acids, with the ion at m/z 353 and a fragment m/z 191 (quinic acid) typical of caffeoylquinic acid were identified as 4-O-caffeoylquinic acid and 3-O-caffeoylquinic acid. Compound no. 24 identified as 3,4-O-dicaffeoylquinic acid (515 m/z), with the fragmentation ion at m/z 353 also belonging to this category. Compound 18 with m/z of 315 and the produced ion at m/z 153, corresponding to protocatechuic acid after the neutral loss of the hexoside group, was identified as protocatechuic acid glucoside. The other two phenolic acids compounds 20 (m/z 297) and 23 (m/z 281), were assigned to unidentified caffeic and cumaric derivatives, respectively.
Flavonols are a large class of flavonoids, widely distributed within the plant kingdom and present in high concentrations in the epidermis of leaves and fruit skin. Eight compounds of this category were detected in berries, four in leaves, and six in flowers of
Crataegus. Among flavonols, three compounds (no. 25, 26, 27) with the same molecular weight occurred in berries. These compounds were tentatively identified based on the primary ion at
m/z 787 and MS/MS fragmentation, giving the ion at
m/z 421 through the loss of galloyl moiety and gallic acid, respectively; thus, were identified as tetra-
O-galloyl-glucosides. Peak no. 29 (
m/z 609) produced a fragmentation ion at
m/z 285, belonging to luteolin derivatives, was identified as luteolin 6,8-C-diglucoside. Analyzed compounds 28, 36, and 37 with ions
m/z of 431, 563, and 619, characteristic for apigenin derivatives, were tentatively identified as apigenin 8-C-glucoside (vitexin), apigenin 6-C-glucoside-8-C-arabinoside, and cratenacin, respectively. Based on the standards, four quercetin derivatives (fragment ion
m/z 301) were detected such as: quercetin, then 3-
O-rutinoside (no. 30,
m/z 609), quercetin 3-
O-glucoside (no. 32,
m/z 463), quercetin 3-
O-galactoside (no. 33,
m/z 463), and quercetin 3-
O-acetyl hexoside (no. 34,
m/z 505). The next two peaks, 31 (
m/z 433) and 35 (
m/z 463), were assigned as naringenin 7-
O-glucoside and myricetin 3-
O-rhamnoside, respectively. The assignment was based on the presence of the characteristic fragment ions at
m/z 271 for naringenin and
m/z 317 for myricetin [
40].
2.6. Quantification of Polyphenolic Compounds
The quantification of polyphenolic compounds was performed with the use of the UPLC-PDA-MS/MS method and quantitative data calculated from the calibration curves. The profile and content of polyphenols differing in the individual morphological parts of the
Crataegus species are presented in
Figure 9 and
Table S2. The highest content of polyphenolic compounds was determined in the berries, where the estimated value ranged from 8980.77 (in CB1) to 14,038.21 (in CB4) mg/100 g dry basis (d.b.). In turn, the total content of polyphenolic compounds in flowers and leaves was about twice lower and ranged from 3028.82 to 7718.00 mg/100 g d.b., and 3963.15 to 6263.56 mg/100 g d.b. for CF2 and CF1, and CL3, and CL1, respectively.
Among the examined morphological parts of the six species of Crataegus, CB4 berry extracts were characterized by the highest content of polyphenolic compounds, in which approximately 55.1% of all phenols were flavan-3-ols. Their quantity was 7671.94 mg/100 g d.b. and was about 14 and 9 times higher, respectively, with respect to the flowers and leaves of the same Crataegus species. The dominant compounds were procyanidin dimer (2578.00 mg/100 g d.b.), procyanidin trimer (2000.45 mg/100 g d.b.), and (−)-epicatechin (1800.44 mg/100 g d.b.). The other categories of polyphenolic compounds were present in CB4 berries in the following order: phenolic acids (25.1%) > flavonols (15.2%) > hydrolyzable tannins (4.5%) > anthocyanins (0.1%).
As indicated above, the flower and leaf extracts with the highest total content of polyphenolic compounds were derived from the first species of Crataegus (C1). In these morphological parts, the most abundant compounds were phenolic acids, which constituted 52.5% and 41.4% of all CF1 flower and CL1 leaf phenols, respectively. Their amount was 4052.36 and 2591.63 mg/100 g d.b. and was about 4 and 2 times higher with respect to the CB1 berries. The most dominant compounds in CF1 flower extract were 3-O-caffeoylquinic acid (2588.40 mg/100 g d.b.), 3-O-p-coumaroylquinic acid (551.02 mg/100 g d.b.), and 3,4-O-di-caffeoyl-quinic acid (458.56 mg/100 g d.b.). In the CL1 leaf extract, the content of particular compounds was: 3-O-caffeoylquinic acid (1463.94 mg/100 g d.b.), unidentified caffeic derivative (441.64 mg/100 g d.b.), and 3-O-p-coumaroylquinic acid (357.04 mg/100 g d.b.). The quantitative profile of the other categories of polyphenolic compounds present in CF1 flowers and CL1 leaves were as follows: flavonols (42.1% and 39.1%, respectively) > flavan-3-ols (5.4% and 18.4%) > hydrolyzable tannins (0% and 1.2%).
3. Discussion
Despite the fact that in recent years progress in the treatment of glioblastomas has been made, these malignant brain tumors are still one of the most deadly and most difficult to treat in all oncology [
41]. These most common primary brain tumors account for nearly 70% of oligodendroglial, and astrocytic tumors are characterized by a rapid clinical course, poor response rates, high invasiveness, and short survival time, on average from 12 to 15 months since diagnosis [
42]. Thus there is the need to search for new, alternative, and/or complementary therapies that could improve the prevention and/or treatment of glioblastomas.
Crataegus is one of the best known medicinal plants with a high content of biologically active ingredients, the most important of which are polyphenols [
30]. These compounds have gained importance in recent years due to their high cytotoxic potential against several cancer cells. It was shown that polyphenol-rich extracts from various anatomical parts of
Crataegus promoted apoptosis of breast adenocarcinoma (MCF-7) [
43], cervical cancer (HeLa), liver cancer (HepG2), neuroblastoma (IMR-32), colorectal adenocarcinoma (SW480, Coco-2) [
44,
45], and melanoma (B16F10) [
21]. At the same time, these extracts showed no cytotoxic effects on healthy colon epithelial cells [
45] and normal human peripheral mononuclear cells [
46]. Additionally, the anti-oxidant activity of the phenolic composition and individual compounds from
Crataegus pinnatifida berries [
47],
Crataegus monogyna leaves [
48], and
Crataegus oxyacantha flowers and berries [
49] were described. The recent reports showed mild cytotoxic effects of phenolic acids from
Petroselinum crispum L. on human U87MG cells [
22], flavonoids from
Lippa graveolens on human U251MG cells [
23], and anthocyanins from
Rubus liebmannii and
Rubus palmeri on C6 and RG2 rat and murine, respectively, glioma cell lines [
50]. However, there were no studies performed at the cellular level, and all the morphological parts of
Crataegus were tested in the same set of experiments.
In this work, we performed, for the first time, a thorough contemporaneous screening of the anti-cancer potential of six Crataegus species, i.e., we examined whether polyphenolic extracts from their berries, leaves, and flowers could affect the viability, morphology, and invasive potential of the well-characterized human glioblastoma U87MG cell line. Moreover, we determined both qualitatively and quantitatively the content of these extracts to identify the species and/or plant organs with the highest anti-cancer potential. It was noteworthy that our polyphenolic extracts were processed with the use of the adsorbing resin (RP-18) that enabled obtaining polyphenol-rich preparations devoid of sugars, proteins, and minerals.
Our results showed that all the examined polyphenolic Crataegus extracts inhibited the viability of the examined cells in a dose-dependent manner. However, our data indicated that the level of the observed inhibition did not depend on the incubation time, as prolonged incubation (up to 72 h) had little or any effect on the treated cells, indicating that the extract compounds could target the vital processes relatively fast. We also demonstrated that the berry extracts evoked the strongest cytotoxic activity in comparison with the leaf and flower extracts.
This differential effect of the extracts on U87MG cells was most likely related to the distinctive classes of polyphenolic components found in anatomical parts of the examined
Crataegus species. Phytochemical analysis revealed that, among the identified and quantified polyphenolic compounds, the highest number (29 out of 37 in total) was found in berries, mainly in the CB4 sample, which also showed the strongest inhibitory effect on U87MG cell viability. Noticeably, the berries were the only organs containing anthocyanins, though in relatively small amounts (<0.1%). The most abundant group within the berry extracts was flavan-3-ols, including flavonoid oligomers such as procyanidin dimer and procyanidin trimer. Reports published so far by other groups showed that purified procyanidins inhibited cell viability and/or induced cell apoptosis of breast cancer cells (MCF-7 and MDA-MB-468 lines), lung cancer (A427 lines), prostate cancer (DU145 line), colorectal cancer (HCT-8, HT29, and Caco-2 lines) and bladder cancer (BIU87 lines) without a cytotoxic effect on untransformed counterparts [
51]. It was also shown that the chemical structure of polyphenols was a decisive factor in their anti-cancer effectiveness, and, in the case of oligomeric flavan-3-ols, their degree of polymerization positively correlated with the reduced viability of neoplastic cells [
52].
Our UPLC analysis also showed that the CB4 sample, derived from berries of
C. laevigata x rhipidophylla x monogyna, compared to the berry extracts from other species, contained approximately 3 times more phenolic acids, in particular of 4-
O-caffeoylquinic acid and 3-
O-caffeoylquinic acid. This group of compounds was shown to have cytotoxic effects on breast adenocarcinoma cells (MCF-7) [
43], melanoma cells (HCT15), colon adenocarcinoma (HT29) [
53], and lung carcinoma (NCI-H23) [
52]. Presumably, the relationship between the structure and anti-tumor activity was also applied to these compounds. It was also proposed that esterification of the carboxyl group of caffeic acid with quinic acid could affect the biological effectiveness of these derivatives [
54].
Therefore, the high content of phenolic acids and flavan-3-ols in the examined
Crataegus berries could explain their strongest effect on U87MG cells. However, it cannot be ruled out that the high biological activity of the
Crataegus berry extracts could also result from the synergistic effect of polyphenols present in this plant organ. This notion seemed to be confirmed by the studies demonstrating that the effect of the combination of two polyphenolic acids on the anti-proliferative activity of melanoma cells was more pronounced than the mono treatment [
55]. It is noteworthy that combination therapy is known to be more effective in treating cancers as it reduces the chances of developing drug resistance. It was shown, among others, that polyphenolic compounds apart from the mentioned mutual combinations could also be combined with other natural compounds such as sugar derivatives, glycosides, peptides, and amino acids [
51]. These studies seemed to be highly relevant in the context of our recent report showing that plant-derived amino acid canavanine (an arginine analog) had a strong cytotoxic effect on two human glioblastoma cell lines, U87MG and U251MG [
56].
To better understand the mechanisms of the observed cytotoxicity triggered by the extracts, we examined the level of the apoptosis-related PARP1 protein. The cleavage of PARP1, mainly by the executive caspases, led to irreversible DNA damage and played an important role in the regulation of cell death as well as was considered to be a hallmark of ongoing apoptosis. In our study, we observed that Crataegus extracts promoted PARP1 cleavage, thus indicating induction of processes leading to apoptotic cell death. PARP1 cleavage was accompanied by morphological aberrations and changes in the cytoskeleton organization characteristic for apoptosis. In particular, treatment of the cells with Crataegus berry extracts led to cell shrinkage, blebbing, and changes in the nuclei shape. The treatment also affected the FAK and Akt signaling pathways that were known to control cell proliferation, invasiveness, and cell cycle progression. The observed concentration-dependent decrease in the activity of FAK and Akt kinases upon the treatment were most pronounced for berry extracts, further confirming their potential of being used in the development of a potential anti-glioblastoma treatment.
Summarizing, we compared for first time effects of the extracts of berries, leaves, and flowers of six species of Crataegus on human glioblastoma U87MG cells and showed that the berry extracts, in particular, that of C. laevigata x rhipidophylla x monogyna, had the strongest cytotoxic effects. Namely, the substantial decrease of cell viability, the induction of the processes leading to apoptotic cell death, and inhibition of the prosurvival signaling pathways that were observed. We suggest that these effects could be associated with the high content of flavan-3-ols and phenolic acids in the berry extracts. The data presented herein could serve in the future as the ground for the development of a potential anti-glioblastoma therapeutical strategy based either on a single compound or the multi-compound combination.
4. Materials and Methods
4.1. Materials and Reagents
CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay was purchased from Promega (G5421; Madison, WI, USA). Alexa Fluor 488-conjugated phalloidin was from Invitrogen (A12379; Waltham, MA, USA). Vectashield anti-fade reagent with DAPI was obtained from Vector Laboratories (H-1200; Burlingame, CA, USA). The following antibodies were used: against PARP1 (#9532), FAK (#3285) and p-FAK (#8556), Akt (#9272) and p-Akt (#9271; Cell Signaling Technologies; Danvers, MA, USA), and GAPDH (MAB374; Millipore, St. Louis, MO, USA). Secondary antibodies were from Millipore (USA): HRP-conjugated anti-mouse (AP308P) and anti-rabbit IgG (AP307P). Methanol (64860), acetonitrile (64851), dimethyl sulfoxide (DMSO, D2438), LiChroprep RP-18 (40–63 µm) (1.13900) were purchased from Sigma-Aldrich (Steinheim, Germany). Chlorogenic acid (#4991S), caffeic acid (#6034S), kaempferol 3-O-glucoside (#1243S), quercetin 3-O-diglucoside (#1347S), quercetin-3-O-galactoside (#1027S), quercetin 3-O-rutinoside (#1139S), naringenin 7-O-glucoside (#1160S), myricetin 3-O-rhamnoside (#1029S), pelargonidin 3-O-rutinoside (#0943), peonidin 3-O-glucoside (#0929S), apigenin 8-O-glucoside (#1232S), quinic acid (#0762A), (+)-catechin (#0796S), and (−)-epicatechin (#0977S) were obtained from Extrasynthese (Lyon, France). Cyanidin 3-O-glucoside chloride (1151935USP) and punicalin (A + B mixture) (PHL83532) were purchased from Sigma-Aldrich (Steinheim, Germany).
4.2. Plant Material
The material derived from six hawthorn species (
Crataegus), namely C.
monogyna,
C. rhipidophylla,
C. x subsphaericea,
C. laevigata x rhipidophylla x monogyna,
C. macrocarpa, and
C. laevigata, collected by Dr. Mateusz Wolanin from the Department of Botany of the Institute of Biology and Biotechnology, University of Rzeszów, was used in the study. Flower (F) and leaf (L) samples were collected in May, and berries (B) in October at the Błażowa and Piątkowa near Rzeszow (Poland). Characteristics of the selected plants are presented in
Table 2. The collected material was lyophilized (ALPHA 1-2 LD plus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany), then ground with a coffee grinder, and stored at −20 °C until preparation of the extracts.
4.3. Extracts Preparation
Powdered berries, leaves, and flowers (30 g of each) of six
Crataegus species were suspended in 300 mL of acetone (50%;
v/
v) and left in a shaded place at room temperature (RT) for 24 h. After that, each obtained suspension was centrifuged at 12,000×
g for 10 min (Centrifuge 5430, Eppendorf, Hamburg, Germany), decanted, and the pellets were re-extracted with acetone (70%;
v/
v) using ultrasound (Sonic 10 ultrasonic bath, Polsonic, Warsaw, Poland) at 30 °C for 30 min. After centrifugation (12,000×
g for 10 min), the supernatants were combined and pre-concentrated using a rotary evaporator at 40 °C (R-215 Rotavapor System, Buchi, Switzerland). The obtained samples were then applied to a column containing adsorber LiChroprep RP-18 (40–63 µm; previously preconditioned with absolute methanol and equilibrated with distilled water). The column was first washed with water to remove polar compounds, and then polyphenols compounds were eluted with methanol (99.8%). The eluates were collected, evaporated, lyophilized, and stored at −20 °C until further analysis. For biological assays,
Crataegus berries and leaf extracts were dissolved in water, and
Crataegus flower extracts were dissolved in DMSO and diluted in the cell culture medium prior to the experiments. The yield of the particular samples is presented in
Table S3.
4.4. Cell Culture
U87MG human glioblastoma cell line was obtained from the Cell Lines Service (Eppelheim, Germany). Cells were cultured at 37 °C in the atmosphere with 5% CO2, in Dulbecco’s Modified Eagle Medium-GlutaMAX-1 (DMEM; Gibco 31966021, Gibco, Waltham, MA, USA), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco 10500064, Gibco, Waltham, MA, USA), and antibiotics (100 U/mL penicillin, 100 U/mL streptomycins; Gibco 15140122, Gibco, Waltham, MA, USA). For the passaging, cells were washed by phosphate-buffered saline (PBS) and trypsinized (0.25% trypsin-EDTA; Gibco 25200056, Gibco, Waltham, MA, USA).
4.5. MTS Cell Viability Assay
Cells were seeded into 96-well microplates (Greiner, Kremsmünster, Austria) at a density of 8 × 103 cells/well and then incubated at 37 °C. After 18 h incubation, the medium was changed into the experimental conditions. Five concentrations of Crataegus extracts were used: 10, 100, 250, 500, and 750 µg/mL. After 24, 48, and 72 h of incubation, the MTS test was performed according to the manufacturer protocol (Promega, Madison, WI, USA). The absorbance was measured at 490 nm using a microplate reader (Sunrise Microplate Reader Remote-Elisa Assays, Tecan Trading AG, Männedorf, Switzerland), and cell viability was estimated as a percentage relative to control (untreated cells, 100%).
4.6. Western Blot Analysis of the Cell Lysates
Cells were seeded at a density of 3 × 105 cells per 6-cm culture dish (Sarstedt, Nümbrecht, Germany). After 18 h of culture, cells were treated with Crataegus extracts at three concentrations of 100, 250, and 500 µg/mL for 48 h. After incubation, cells were washed twice with ice-cold PBS and lysed in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% IGEPAL, 50 mM NaF) supplemented with 2 mM Na3VO4, 1 mM PMSF, phosphatase, and protease inhibitors (04906837001 and 04693116001, respectively; Roche, Mannheim, Germany) in 4 °C for 20 min. Cell lysates were received after centrifugation at 13,500× g at 4 °C for 20 min, and supernatants were collected. The protein concentration was determined according to the Bradford assay (Bio-Rad, Hercules, CA, USA, USA). Next, cell lysates were mixed with the Laemmli buffer (0.25 M Tris-HCl, pH 6.8, 50% glycerol, 5% SDS, 5% β-mercaptoethanol, 0.05% bromophenol blue) and incubated for 5 min at 98 °C. Cell proteins were separated in 10 or 12% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) and transferred to a nitrocellulose membrane (Amersham 10600002, Freiburg, Germany). Subsequently, membranes were blocked by 5% bovine serum albumin (BSA) solution or 3–5% fat-free milk solution in 0.2% TBS-T (Tris-buffered saline containing 0.2% Triton X-100) for 1 h, and incubated with primary antibodies with PARP (1:750), FAK (1:1000), p-FAK (1:1000), Akt (1:1000), and p-Akt (1:1000) overnight at 4 °C; with GAPDH (1:20,000) for 30 min at the room temperature (RT), and then with secondary antibodies (1:10,000) for 1 h at RT. GAPDH was used as a protein loading control. Protein bands were detected using ECL substrate (Millipore, St. Louis, MO, USA or Termo Fisher Scientific, Waltham, MA, USA). Then, band densitometry was quantified using the Fiji distribution of the ImageJ 1.52a software (National Institutes of Health, Bethesda, MD, USA, and the University of Wisconsin, Madison, WI, USA). The level of GAPDH was used as a protein loading control.
4.7. Light Microscopy Analysis
Cells were seeded at a density of 3 × 10
5 cells per 6-cm culture dish (Sarstedt, Nümbrecht, Germany). After 18 h, cells were treated with
Crataegus extracts (250 µg/mL) for 48 h. Non-treated cells were used as a control. Cells incubated with DMSO at concentrations of 0.02–1.5% dependent on the flower extract concentrations (see
Figure S1) served as an additional control for the flower extract analysis. Cell morphology was analyzed by light microscopy (Nikon Eclipse Ti-U fluorescent microscope equipped with a 20× objective and DS-Qi2 digital camera, Tokyo, Japan).
4.8. Fluorescent Stainings and Confocal Microscopy Analysis
Cells were seeded in 6-well plates (Sarstedt, Nümbrecht, Germany) with glass coverslips (VWR 631-0153, VWR, Gdańsk, Poland) at a density of 4 × 104 cells/well. After 48 h treatment with Crataegus extracts (at concentrations close to the EC50 values), the cells were washed twice with PBS and incubated with 4% paraformaldehyde solution (PFA) at RT for 20 min. Then, the cells were washed with PBS, incubated with a 50 mM NH4Cl solution for 30 min, and permeabilized with 0.2% Triton X-100 in PBS for 10 min. Filamentous actin was visualized by staining with Alexa Fluor 488-conjugated phalloidin (1:40 in PBS) for 20 min. Next, the cells were washed with PBS/0.02% Triton X-100 and mounted by Vectashield anti-fade reagent containing DAPI to stain the nuclei. Microphotographs were collected using Zeiss LSM780, Inverted Axio Observer Z.1 with Plan Apochromat 40×/1.4 Oil DIC objective (Carl Zeiss AG, Jena, Germany). Images were prepared using the Zen Blue 2.1 software (Carl Zeiss Microscopy, Jena, Germany).
4.9. Determination of Polyphenols Profile
Determination of polyphenolic compounds was carried out using the Ultra Performance Liquid Chromatography (UPLC) Waters ACQUITY system (Waters, Milford, MA, USA). UPLC was equipped with a binary pump manager, column manager, sample manager, photodiode array (PDA) detector, tandem quadrupole mass spectrometer (TQD) with electrospray ionization (ESI) source. Separation of polyphenols was performed using a 1.7 µm, 100 mm × 2.1 mm UPLC BEH RP C18 column (Waters, Milford, MA, USA). For the anthocyanin investigation, the mobile phase consisted of 2% formic acid in water, v/v (solvent A), and 2% formic acid, in 40% acetonitrile, v/v (solvent B). However, in the case of other polyphenolic compounds, water (solvent A) and 40% acetonitrile, v/v (solvent B) were used. The flow rate was kept constant at 0.35 mL/min for a total run time of 8 min. The system was run with the following gradient program: from 0 min 5% B, from 0 to 8 min linear to 100% B, and from 8 to 9.5 min for washing and back to initial conditions. The injection volume of the samples was 5 µL, and the column was supported at 50 °C. The following TQD parameters were used: cone voltage of 30 V, capillary voltage of 3500 V, source and desolvation temperature 120 °C and 350 °C, respectively, and desolvation gas flow rate of 800 L/h. Characterization of the individual polyphenolic compounds was performed on the basis of the retention time, mass-to-charge ratio, fragment ions, and comparison of data obtained with commercial standards and literature findings. Obtained data were processed in Waters MassLynx v.4.1 software (Waters, Milford, MA, USA). Extracts (10 mg of each) of Crataegus berries and leaves were dissolved in water, while the flower was extracted in acetonitrile (50%; v/v),and then diluted in water in 1:5 ratio. All experiments were done in triplicates, and the results were expressed as mg/100 g of dry basis (d.b.).
4.10. Quantification of Polyphenolics and Method Validation
The quantification of polyphenolic compounds was performed by the use of different internal standards (
Table S4). Stock solutions of phenolic standards were prepared at 1 mg/mL after dissolving in 50% acetonitrile in water. For quantification of phenolic compounds, the working solutions of mixed analytes at the concentrations of 25, 50, 100, 150, 250 µg/mL were obtained by dilution of the appropriate volume of stock solutions. Concentrations of polyphenolics were calculated by preparing a calibration curve of mass concentration vs. peak area. The slope of the regression line and values of correlation coefficient (R
2) for each standard curve were obtained using MS Excel 2019 software. Limits of detection (LOD) and limits of quantification (LOQ) were calculated for each sample in triplicates. Calibration curves were obtained consecutively by plotting concentration against the peak area. The mean of the slope (S) and standard deviation of intercept (δ) were calculated from the standard curve of three replicates. LOD and LOQ were calculated with the following Equations:
The intra- and interday variations were determined using relative standard deviation (RSD) values, which were <3.5% for all the analyzed compounds.
4.11. Statistics
All experiments were performed at least three times. The results were shown as means ± SD. Statistical significance for polyphenolic compounds was analyzed by one-way analysis of variance (ANOVA) using Duncan’s test. Values marked with different letters indicated statistically significant differences (p < 0.05). Values marked with the same letter within the same group did not differ statistically. All calculations were made in Statistica v. 13.3 software (StatSoft, Krakow, Poland). Statistical analyses of the levels of proteins were performed using a one-way ANOVA test in the GraphPad Prism 8.4.3 software (San Diego, CA, USA). Statistical significance was defined as p < 0.05.