Sustainable Use of Bioactive Compounds from Solanum Tuberosum and Brassicaceae Wastes and by-Products for Crop Protection—A Review
Abstract
:1. Introduction
2. Biocompounds in Potato Peel
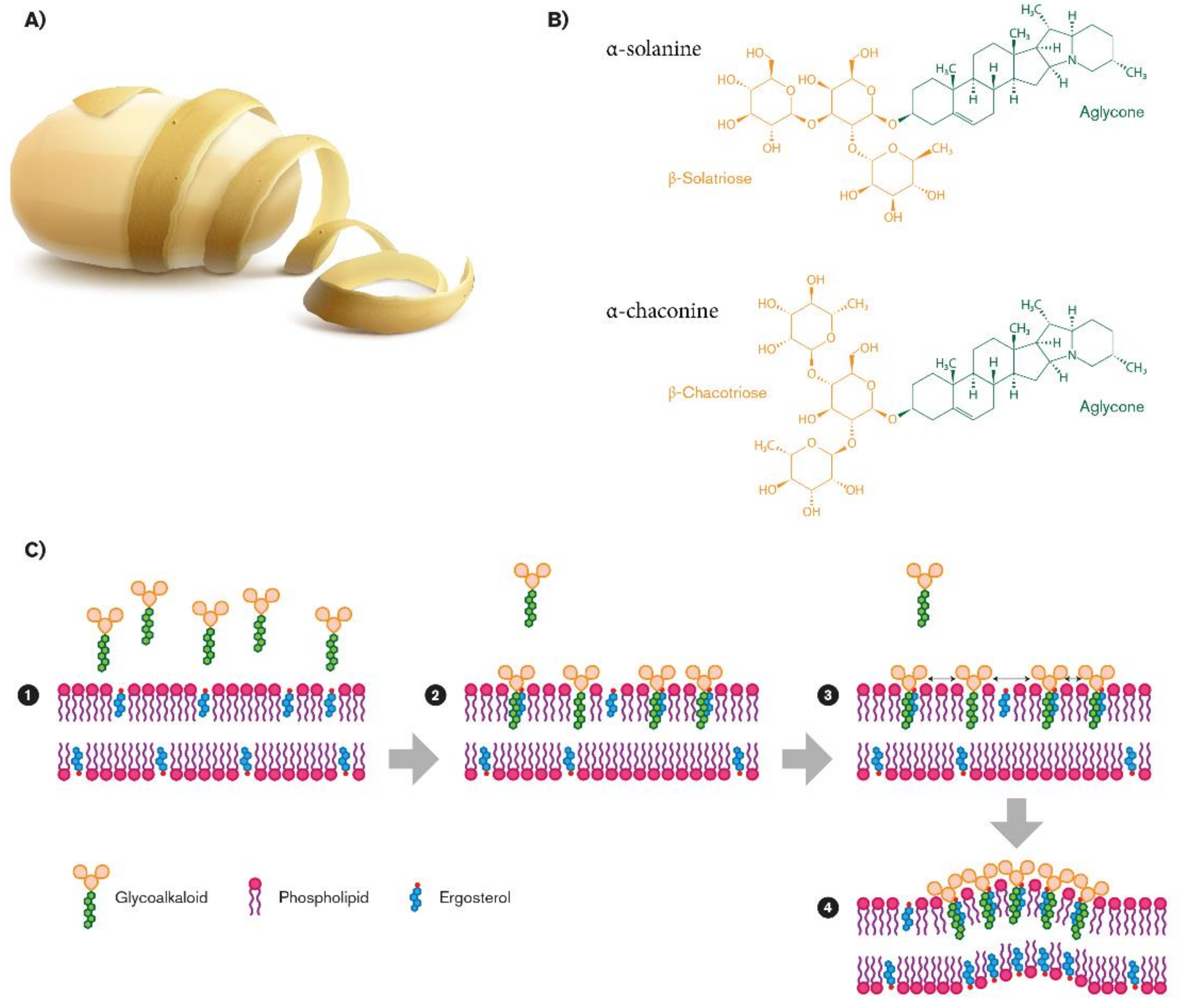
- Potato Glycoalkaloids
- Potato Phenols
2.1. Potato Eco-Friendly Plant Bioprotector Activity Against Biotic Stresses
2.1.1. SGA Activity Against Phytophages
2.1.2. SGA Antifungal Activity
2.1.3. Interaction Between SGAs and Fungal Membrane
2.1.4. Role of Phenols in Plant Protection
2.2. Recovery of Eco-Friendly Bioprotectors from PPW
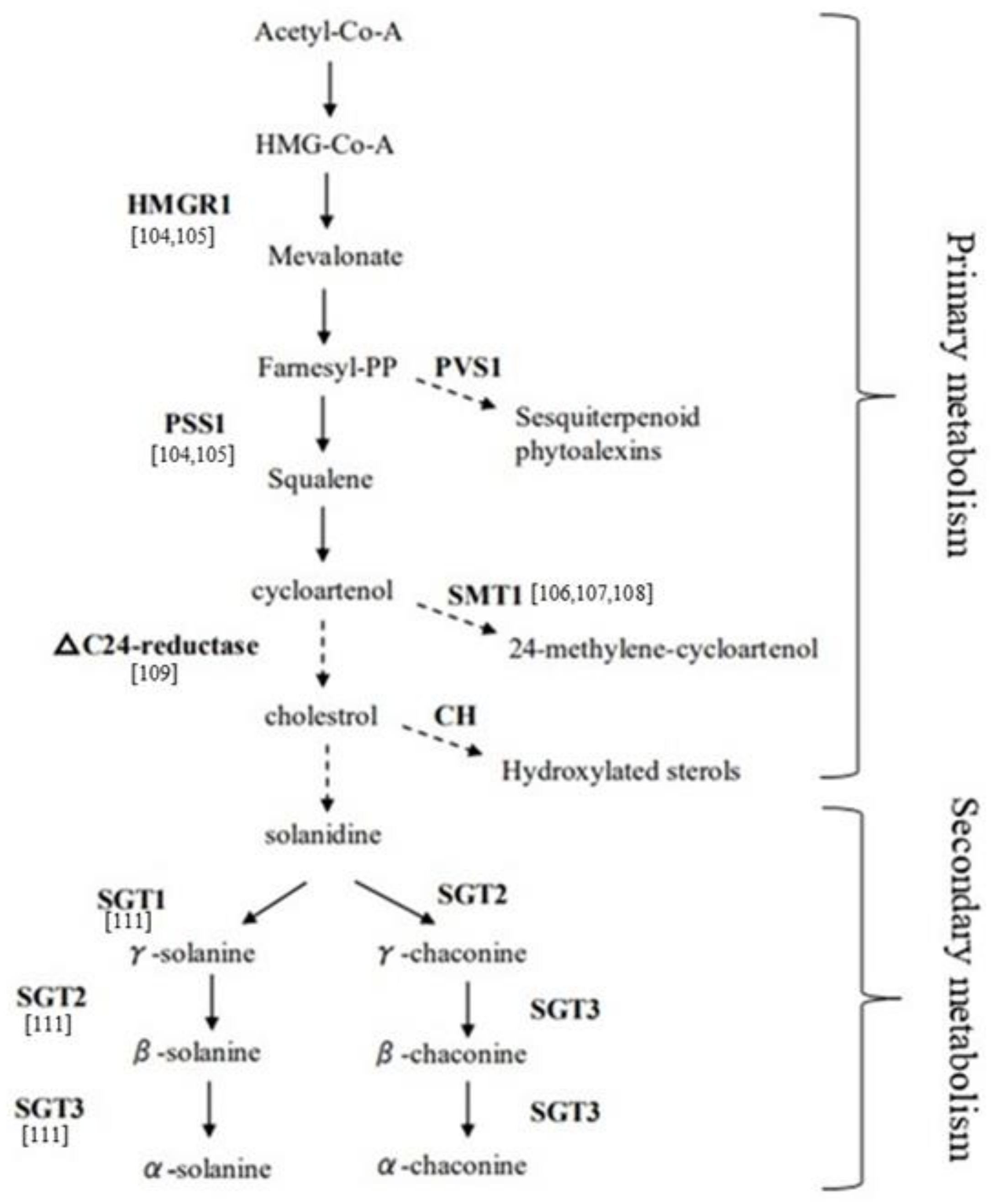
2.3. Molecular Approaches to Modulate Eco-Friendly Bioprotector Production in Potato Peel
3. Biocompounds in Brassicaceae
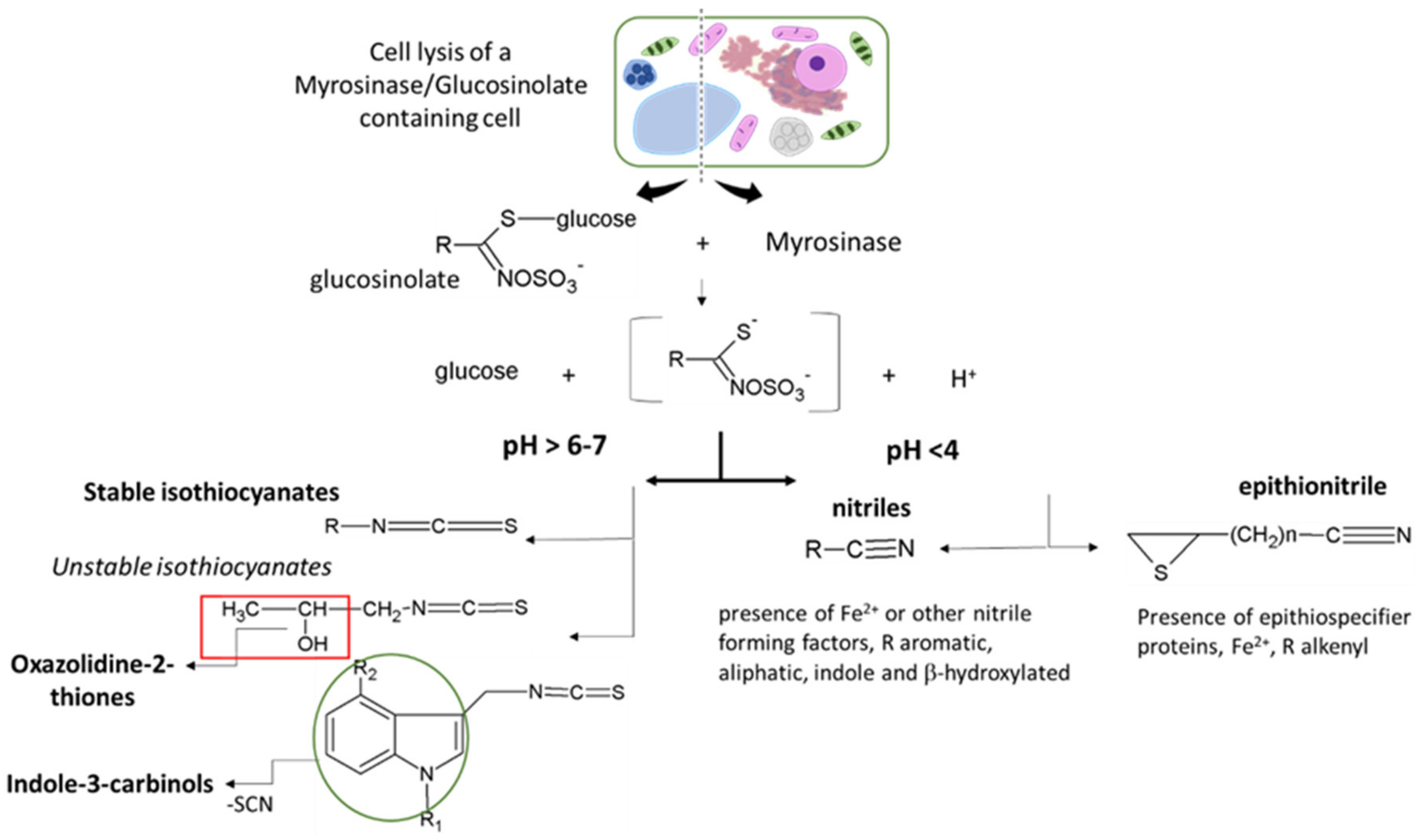
3.1. Glucosinolates, Myrosinases, and Hydrolysis Products
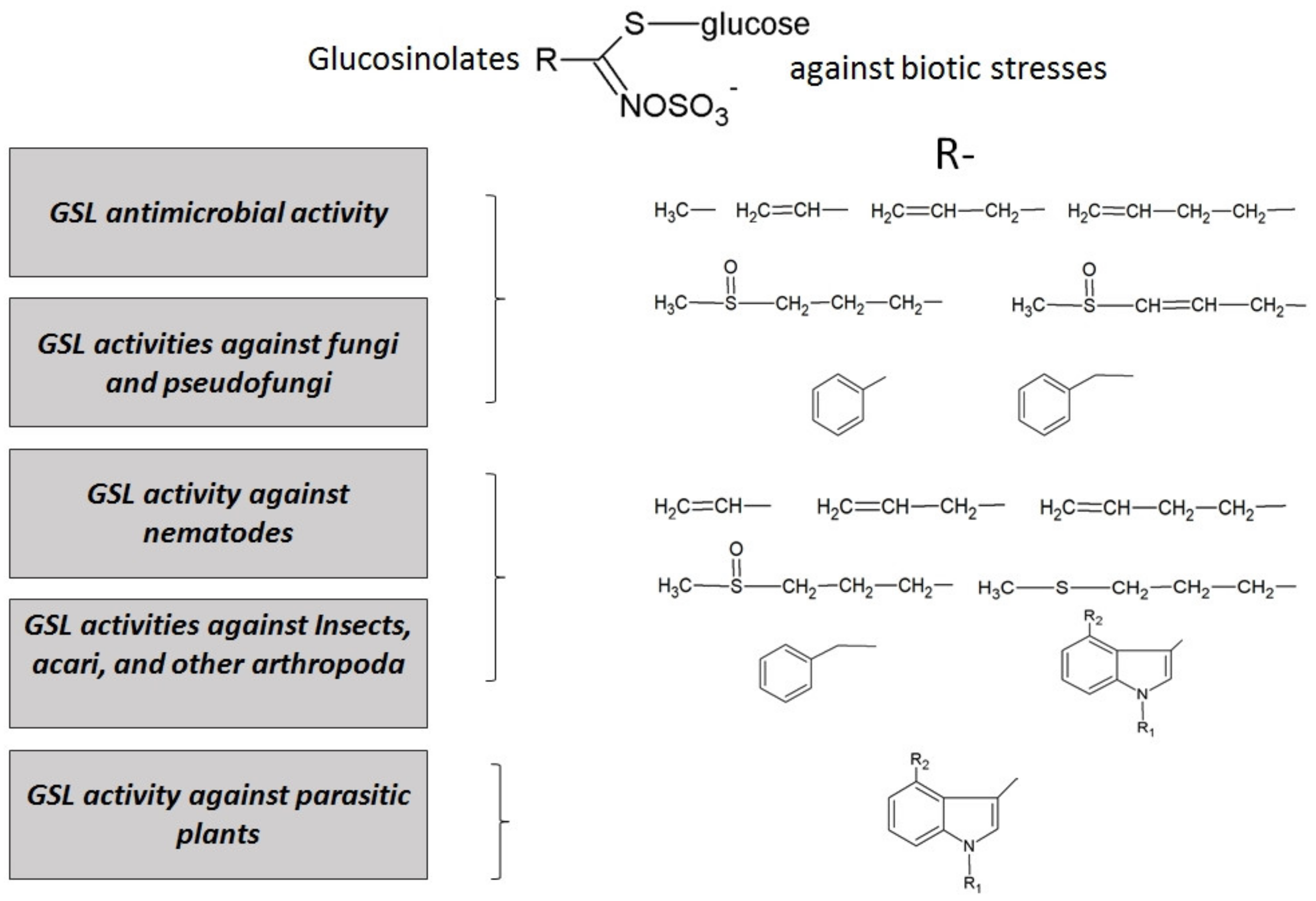
3.2. Brassicaceae Eco-Friendly Plant Bioprotector Activity against Biotic Stresses
3.2.1. GSL Antimicrobial Activity
3.2.2. GSL Antifungal Activity
3.2.3. GSL Activity Against Nematodes and Insects
3.2.4. GSL Activity Against Parasitic Plants
3.2.5. GSLs Role in Biofumigation Crop Protection Management
3.3. Molecular Approaches to Enhance GSL Content in Brassicales
3.3.1. Biotechnology Approaches to Enhance GSL Content
3.3.2. GSL Molecular Markers and Gene Mapping
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AITC | allyl isotiocyanate |
| CA | 3,4-Dihydroxyphenyl)prop-2-enoic acid |
| CQA | 5-O-caffeoylquinic acid |
| CNV | copy number variation |
| CPB | Colorado Potato Beetle |
| DSMs | defatted seed meals |
| DW | dry weight |
| FW | fresh weight |
| GHPs | GSL hydrolysis products |
| GSLs | glucosinolates |
| HPLC | High Performance Liquid Chromatography |
| HPTLC | High Performance Thin Layer Chromatography |
| IPM | integrated pest management |
| ITCs | isotiocyanates |
| MAS | marker assisted selection |
| PPW | Potato Peel Waste |
| PTM | Potato Tuber Moth |
| QTL | quantitative trait locus |
| RIL | recombinant inbred line |
| SGAs | Steroidal glycoalkaloids |
| SA | steroidal alkaloid |
| SNPs | single nucleotide polymorphisms |
| UAE | Ultrasound-assisted extraction |
References
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste-Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Köhler, H.R.; Triebskorn, R. Wildlife Ecotoxicology of Pesticides: Can We Track Effects to the Population Level and Beyond? Science 2013, 759–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keikotlhaile, B.M.; Spanoghe, P.; Steurbaut, W. Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach. Food Chem. Toxicol. 2010, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.N.; He, M.H.; Ouyang, H.B.; Zhu, W.; Pan, Z.C.; Sui, Q.J.; Shang, L.P.; Zhan, J. Cross-resistance of the pathogenic fungus Alternaria alternata to fungicides with different modes of action. BMC Microbiol. 2019, 19, 205. [Google Scholar] [CrossRef] [Green Version]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009, 2009. Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Off. J. Eur. Union. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2018/627113/EPRS_STU(2018)627113_EN.pdf (accessed on 20 February 2021).
- Poveda, J.; Eugui, D.; Velasco, P. Natural control of plant pathogens through glucosinolates: An effective strategy against fungi and oomycetes. Phytochem. Rev. 2020, 19, 1045–1059. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M. Antifungal effect of powdered spices and their extracts on growth and activity of some fungi in relation to damping-off disease control. J. Plant. Prot. Res. 2007, 47, 267–277. [Google Scholar]
- Abdel-Monaim, M.F.; Abo-Elyousr, K.A.M.; Morsy, K.M. Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinus termis Forsik). Crop. Prot. 2011, 30, 185–191. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Abd El-Aziz, G.H.; Abou-Zeid, M.A.; Fahmy, A.H. Environmental impact of the use of some eco-friendly natural fungicides to resist rust disease in wheat. CATRINA 2019, 18, 87–95. [Google Scholar]
- Draz, I.S.; Elkhwaga, A.A.; Elzaawely, A.A.; El-Zahaby, H.M.; Anter Ismai, A.W. Application of plant extracts as inducers to challenge leaf rust of wheat. Egypt J. Biol Pest. Control. 2019, 29, 6. [Google Scholar] [CrossRef] [Green Version]
- Geetha, H.M.; Shetty, H.S. Induction of resistance in pearl millet against mildew disease caused by Sclerospora graminicola using benzothiadiazole, calcium chloride and hydrogen peroxide—A comparative evaluation. Crop. Protection 2012, 21, 601–610. [Google Scholar] [CrossRef]
- Hassan, M.E.M.; Abd El-Rahman, S.S.; El-Abbasi, I.H.; Mikhail, M.S. Change in peroxidase activity due to resistance induced against faba bean chocolate spot disease. Egypt J. Phytopathol. 2007, 35, 35–48. [Google Scholar]
- Radwan, D.E.; Lu, G.; Fayez, K.A.; Mahmoud, S.Y. Protective action of salicylic acid against been yellow mosaic virus infection in Vicia faba leaves. J. Plant. Physiol. 2008, 165, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Benkeblia, N. Potato Glycoalkaloids: Occurrence, biological activities and extraction for biovalorisation—A review. Int. J. Food Sci. Technol. 2020, 55, 2305–2313. [Google Scholar] [CrossRef]
- FAO International Year of Potato. Available online: http://www.fao.org/potato-2008/en/potato/cultivation.html (accessed on 20 February 2020).
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; VerardoInt, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Sepelev, I.; Galoburda, R. Industrial potato peel waste application in food production: A Review. Res. Rural Dev. Int. Sci. Conf. Proc. (Latvia) 2015, 1, 130–136. [Google Scholar]
- Samotyja, U. Potato Peel as a Sustainable Resource of Natural Antioxidants for the Food Industry. Potato Res. 2019, 62, 435–451. [Google Scholar] [CrossRef] [Green Version]
- Di, W. Recycle Technology for Potato Peel Waste Processing: A Review. Procedia Env. Sci. 2016, 31, 103–107. [Google Scholar]
- Arapoglou, D.; Varzakas, T.; Vlyssides, A.; Israilides, C. Ethanol production from potato peel waste (PPW). Waste Manage. 2010, 30, 1898–1902. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods 2020, 9, 1598. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Bartolini, S.; Sanmartin, C.; Orlando, M.; Taglieri, I.; Macaluso, M.; Lucchesini, M.; Trivellini, A.; Zinnai, A.; Mensuali, A. Potato Peels as a Source of Novel Green Extracts Suitable as Antioxidant Additives for Fresh-Cut Fruits. Appl. Sci. 2019, 9, 2431. [Google Scholar] [CrossRef] [Green Version]
- Lazzeri, L.; Riva, G.; D’Avino, L.; Pedretti, E.F. Short introduction to the VALSO and EXTRAVALORE project activities. Ind. Crops Prod. 2015, 75, 1–7. [Google Scholar] [CrossRef]
- Fanigliulo, R.; Pochi, D.; Bondioli, P.; Grilli, R.; Fornaciari, L.; Folegatti, L.; Malaguti, L.; Matteo, R.; Ugolini, L.; Lazzeri, L. Semi-refined Crambe abyssinica (Hochst. EX R.E.Fr.) oil as a biobased hydraulic fluid for agricultural applications. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- D’Avino, L.; Dainelli, R.; Lazzeri, L.; Spugnoli, P. The role of co-products in biorefinery sustainability: Energy allocation versus substitution method in rapeseed and carinata biodiesel chains. J. Clean. Prod. 2015, 94, 108–115. [Google Scholar] [CrossRef]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H. Human, animal and plant health benefits of glucosinolates and strategies for enhanced bioactivity: A systematic review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef]
- Chowa’nski, S.; Adamski, Z.; Marciniak, P.; Rosia’nski, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxin 2016, 8, 1–28. [Google Scholar]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytologist 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, U.; Pocsi, I. Secondary metabolites in fungal plant interaction. Front. Plant. Sci. 2015, 6, 1–23. [Google Scholar]
- De Sotillo, D.R.; Hadley, M.; Wolf-Hall, C. Potato peel extract a nonmutagenic antioxidant with potential antimicrobial activity. J. Food Sci. 1998, 63, 907–910. [Google Scholar] [CrossRef]
- Amanpour, R.; Abbasi-Maleki, S.; Neyriz-Naghadehi, M.; Asadi-Samani, M. Antibacterial effects of Solanum tuberosum peel ethanol extract in vitro. J. HerbMed. Pharmacol. 2015, 4. [Google Scholar]
- Friedman, M.; McDonald, G.M.; Filadelfi-Keszi, M. Potato Glycoalkaloids: Chemistry, Analysis, Safety, and Plant Physiology. CRC Crit. Rev. Plant. Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Kozukue, N.; Yoon, K.S.; Byun, G.I.; Misoo, S.; Levin, C.E.; Friedman, M. Distribution of glycoalkaloids in potato tubers of 59 accessions of two wild and five cultivated Solanum species. J. Agric. Food Chem. 2008, 56, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Carpintero, N.C.; Tokuhisa, J.G.; Ginzberg, I.; Holliday, J.A.; Veilleux, R.E. Sequence diversity in coding regions of candidate genes in the glycoalkaloid biosynthetic pathway of wild potato species. G3 Genes Genomes Genet. 2013, 3, 1467–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mweetwa, A.M.; Hunter, D.; Poe, R.; Harich, K.C.; Ginzberg, I.; Veilleux, R.E.; Tokuhisa, J.G. Steroidal glycoalkaloids in Solanum chacoense. Phytochemistry 2012, 75, 32–40. [Google Scholar] [CrossRef]
- Friedman, M. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Distl, M.; Wink, M. Identification and Quantification of Steroidal Alkaloids from Wild Tuber-Bearing Solanum Species by HPLC and LC-ESI-MS. Potato Res. 2009, 52, 79–104. [Google Scholar] [CrossRef]
- Itkin, M.; Heinig, U.; Tzfadia, O.; Bhide, A.J.; Shinde, B.; Cardenas, P.D.; Bocozeba, S.E.; Unger, T.; Malitsky, S.; Finkers, R.; et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 2013, 341, 175–179. [Google Scholar] [CrossRef]
- Lafta, A.M.; Lorenzen, J.H. Influence of High Temperature and Reduced Irradiance on Glycoalkaloid Levels in Potato Leaves. J. Am. Soc. Hort. Sci. 2000, 25, 563–566. [Google Scholar]
- Dale, M.F.B.; Griffiths, D.W.; Bain, H.; Todd, D. Glycoalkaloid increase in Solanum tuberosum on exposure to light. Ann. Appl. Biol. 1993, 123, 411–418. [Google Scholar] [CrossRef]
- Mekapogu, M.; Sohn, H.-B.; Kim, S.-J.; Lee, Y.-Y.; Park, H.-M.; Jin, Y.-I.; Hong, S.-Y.; Suh, J.-T.; Kweon, K.; Jeong, J.-C.; et al. Effect of Light Quality on the Expression of Glycoalkaloid Biosynthetic Genes Contributing to Steroidal Glycoalkaloid Accumulation in Potato. Am. J. Potato Res. 2016, 93, 264–277. [Google Scholar]
- Choi, D.; Bostock, R.M.; Avdiushko, S.; Hildebrand, O.F. Lipid-derived signals that discriminate wound- and pathogenresponsive isoprenoid pathways in plants: Methyl jasmonate and fungal elicitor arachidonic acid induce different 3-hydroxy3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc. Natl. Acad. Sci. USA 1994, 91, 2329–2333. [Google Scholar]
- Zarins, R.; Kruma, Z. Glycoalkaloids in potatoes: A review. Foodbalt 2017, 7–11. [Google Scholar]
- Friedman, M.; Roitman, J.N.; Kozukue, N. Glycoalkaloid and calystegine contents of eight potato cultivars. J. Agric. Food Chem. 2003, 51, 2964–2973. [Google Scholar] [CrossRef]
- Pacifico, D.; Musmeci, S.; Sanchez del Pulgar, J.; Onofri, C.; Parisi, B.; Sasso, R.; Mandolino, G.; Lombardi-Boccia, G. Caffeic acid and α-chaconine influence the resistance of potato tuber to Phthorimaea operculella (Lepidoptera: Gelechiidae). Am. Potato J. 2019, 96, 403–413. [Google Scholar]
- Friedman, M. Analysis of biologically active compounds in potatoes (Solanum tuberosum L.), tomatoes (Lycopersicon esculentum L.), and jimson weed (Datura stramonium L.) seeds. J. Chromatogr. A 2005, 1054, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Rytel, E.; Czopek, A.T.; Aniolowska, M.; Hamouz, K. The influence of dehydrated potatoes processing on the glycoalkaloids content in coloured-fleshed potato. Food Chem. 2013, 141, 2495–2500. [Google Scholar] [CrossRef] [PubMed]
- OECD. Consensus document on compositional considerations for new varieties of potatoes: Key food and feed nutrients, anti-nutrients and toxicants. In OECD Environmental Health and Safety Publications, Safety of Novel Foods and Feeds No. 4; OECD: Paris, France.
- BfR Opinion No 010/2018 of 23 April 2018. Table Potatoes Should Contain Low Levels of Glycoalkaloids (Solanine). Available online: http://www.bfr.bund.de/cm/349/table-potatoes-should-contain-low-levels-of-glycoalkaloids-solanine.pdf (accessed on 20 February 2021).
- Uluwaduge, D.I. Glycoalkaloids, bitter tasting toxicants in potatoes: A review. Int. J. Food Sci. Nutr. 2018, 3, 188–193. [Google Scholar]
- Hellenäs, K.E.; Branzell, C.; Johnsson, H.; Slanina, P. High levels of glycoalkaloids in the established Swedish potato variety Magnum Bonum. J. Sci. Food Agric. 1995, 68, 249–255. [Google Scholar] [CrossRef]
- Knuthsen, P.; Jensen, U.; Schmidt, B.; Larsen, I.K. Glycoalkaloids in potatoes: Content of glycoalkaloids in potatoes for consumption. J. Food Compos. Anal. 2009, 22, 577–581. [Google Scholar] [CrossRef]
- Wu, Z.G.; Xu, H.Y.; Ma, Q.; Cao, Y.; Ma, J.N.; Ma, C.M. Isolation, identification and quantification of unsaturated fatty acids, amides, phenolic compounds and glycoalkaloids from potato peel. Food Chem. 2012, 135, 2425–2429. [Google Scholar] [CrossRef]
- Kremr, D.; Bajer, T.; Bajerová, P.; Surmová, S.; Ventura, K. Unremitting problems with chlorogenic acid Nomenclature: A review. Quím. Nova 2016, 39, 530–533. [Google Scholar]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Horbury, D.; Baker, L.A.; Quan, W.D.; Greenough, S.E.; Stavros, W.G. Photodynamics of potent antioxidants: Ferulic and caffeic acids. Phys. Chem. Chem. Phys. 2016, 18, 17691–17697. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, M.; Pedulli, G.F. Bond dissociation enthalpy of α-tocopherol and other phenolic antioxidants. J. Org. Chem. 1994, 59, 5063–5070. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Veluri, R.; Weir, T.L.; Bais, H.P.; Stermitz, F.R.; Vivanco, J.M. Phytotoxic and Antimicrobial Activities of Catechin Derivatives. J. Agric. Food Chem. 2004, 52, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, M.L.; Levey, D.J. Antifungal activity of Solanum fruit glycoalkaloids: Implications for frugivoryand seed dispersal. Ecology 1997, 78, 799–809. [Google Scholar] [CrossRef]
- Smith, D.B.; Roddick, J.G.; Jones, J.L. Synergism between the potato glycoalkaloids alpha-chaconine and alpha-solanine in inhibition of snail feeding. Phytochemistry 2001, 57, 229–234. [Google Scholar] [CrossRef]
- Zitnak, A.; Filadelfi, M.A. Estimation of taste thresholds of three potato glycoalkaloids. Can. Inst. Food Technol. J. 1985, 18, 337–339. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G. Interactive antifungal activity of the glycoalkaloids α-solanine and α-chaconine. Phytochemistry 1993, 33, 323–328. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G.; Weissenberg, M.A. Interactions between the glycoalkaloids solasonine and solamargine in relation to inhibition of fungal growth. Phytochemistry 1994, 37, 1007–1011. [Google Scholar] [CrossRef]
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C.; et al. Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads. J. Agric. Food Chem. 2018, 66, 7942–7947. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Hostettmann, K. Plant molluscicides. Phytochemistry 1984, 24, 639–652. [Google Scholar] [CrossRef]
- Nenaah, G. Individual and synergistic toxicity of Solanaceous glycoalkaloids against two coleopteran stored-product insects. J. Pest. Sci. 2011, 84, 77–86. [Google Scholar] [CrossRef]
- Gee, J.M.; Worley, G.M.; Jonhson, I.T.; Price, K.R.; Rutten, A.A.J.J.J.L.; Houben, G.F.; Penninks, A.H. Effects of saponins and glycoalkaloids on the permeability and visibility of mammalian intestinal cells and on the integrity of tissue preparation in vitro. Toxicol. Vitr. 1996, 10, 117–128. [Google Scholar] [CrossRef]
- Ruprich, J.; Rehurkova, I.; Boon, P.E.; Svensson, K.; Moussavian, S.; Van der Voet, H.; Bosgra, S.; Van Klaveren, J.D.; Busk, L. Probabilistic modeling of exposure doses and implications for health risk characterization: Glycoalkaloids from potatoes. Food Chem Toxicol. 2009, 47, 2899–2905. [Google Scholar] [CrossRef]
- Patel, B.; Schutte, R.; Sporns, P.; Doyle, J.; Jewel, L.; Fedorak, R.N. Potato glycoalkaloids adversely affect intestinal permeability and aggravate inflammatory bowel disease. Inflamm. Bowel Dis. 2002, 8, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Blankemeyer, J.Y.; Stringer, B.K.; Rayburn, J.R.; Bantle, J.A.; Friedman, M. Effect of potato glycoalkaloids, alpha-chaconine and alpha-solanine on membrane potential of frog embryos. J. Agric. Food Chem. 1992, 40, 2022–2025. [Google Scholar] [CrossRef]
- Roddick, J.G.; Rijnenberg, A.L.; Weissenberg, M. Membrane-disrupting properties of the steroidal glycoalkaloids solasonine and solamargine. Phytochemistry 1990, 29, 1513–1518. [Google Scholar] [CrossRef]
- Sarquis, J.I.; Coria, N.A.; Aguilar, I.; Rivera, A. Glycoalkaloid content in Solanum species and hybrids from a breeding program for resistance to late blight (Phytophthora infestans). Am. J. Potato Res. 2000, 77, 295–302. [Google Scholar] [CrossRef]
- Dahlin, P.; Müller, M.C.; Ekengren, S.; McKee, L.S.; Bulone, V. The Impact of Steroidal Glycoalkaloids on the Physiology of Phytophthora infestans, the Causative Agent of Potato Late Blight. Mol. Plant. Microbe Interact. 2017, 30, 531–542. [Google Scholar] [CrossRef] [Green Version]
- Weete, J.W.; Abril, M.; Blackwell, M. Phylogenic distribution of fungal sterols. PLoS ONE 2010, 5, e10899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roddick, J.G.; Rijnenberg, A.L.; Weissenberg, M. Alterations to the permeability of liposome membranes by the solasodine-based glycoalkaloids solasonine and solamargine. Phytochemistry 1992, 31, 1951–1954. [Google Scholar] [CrossRef]
- Keukens, E.A.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.; de Kruijff, B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta. 1995, 13, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Roddick, J.G.; Rijnenberg, A.L.; Osman, S.F. Synergistic interaction between potato glycoalkaloids α-solanine and α-chaconine in relation to destabilization of cell membranes Ecological. Implic. J. Chem. Ecol. 1988, 14, 889–902. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Antifungal activity of secondary plant metabolites from potatoes (Solanum tuberosum L.): Glycoalkaloids and phenolic acids show synergistic effects. J. Appl. Microbiol. 2016, 120, 955–965. [Google Scholar] [CrossRef]
- Müller, M.M.; Kantola, R.; Kitunen, V. Combining sterol and fatty acid profiles for the characterization of fungi. Mycol. Res. 1994, 98, 593–603. [Google Scholar]
- Mejanelle, L.; Lopez, J.F.; Gunde-Cimerman, N.; Grimalt, J.O. Sterols of melanized fungi from hypersaline environments. Org. Geochem. 2000, 31, 1031–1040. [Google Scholar]
- Kesten, C.; Gámez-Arjona, F.M.; Menna, A.; Scholl, S.; Dora, s.; Huerta, A.I.; Huang, H.Y.; Tintor, N.; Kinoshita, T.; Rep, M.; et al. Pathogen-induced pH changes regulate the growth-defense balance in plants. EMBO J. 2019, 38, e101822. [Google Scholar] [CrossRef]
- Ford, J.E.; McCance, D.J.; Drysdale, R.B. The detoxification of α-tomatine by Fusarium oxysporum f. sp. Lycopersici Phytochemistry 1977, 16, 545–546. [Google Scholar] [CrossRef]
- Oda, Y.; Saito, K.; Ohara-Takada, A.; Mori, M. Hydrolysis of the potato glycoalkaloids alpha-chaconine by filamentous fungi. J. Biosci. Bioeng. 2002, 94, 321–325. [Google Scholar] [CrossRef]
- Hennessy, R.C.; Nielsen, S.D.; Greve-Poulsen, M.; Larsen, L.B.; Sørensen, O.B.; Stougaard, P. Discovery of a Bacterial Gene Cluster for Deglycosylation of Toxic Potato Steroidal Glycoalkaloids α-Chaconine and α-Solanine. J. Agric. Food Chem. 2020, 68, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech. J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, H. Effects of green tea catechins on membrane fluidity. Pharmacology 1999, 59, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar]
- Friedman, M.; Kozukue, N.; Kim, H.J.; Choi, S.H.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J. Food Compos. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Wang, Y.; Naber, M.R.; Crosby, T.W. Effects of Wound-Healing Management on Potato Post-Harvest Storability. Agronomy 2020, 10, 512. [Google Scholar]
- Janjai, S.; Bala, B.K. Solar Drying Technology. Food Eng. Rev. 2012, 4, 16–54. [Google Scholar] [CrossRef]
- Paolo, D.; Bianchi, G.; Morelli, C.F.; Speranza, G.; Campanelli, G.; Kidmose, U.; Lo Scalzo, R. Impact of drying techniques, seasonal variation and organic growing on flavor compounds profiles in two Italian tomato varieties. Food Chem. 2019, 298, 125062. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Tripathi, S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem 2014, 2, 115–119. [Google Scholar]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Jie, D.; Wei, X. Review on the recent progress of non-destructive detection technology for internal quality of watermelon. Comput. Electron. Agric. 2018, 151, 156–164. [Google Scholar] [CrossRef]
- Sørensen, K.K.; Kirk, H.G.; Olsson, K.; Labouriau, R.; Christiansen, J. A major QTL and an SSR marker associated with glycoalkaloid content in potato tubers from Solanum tuberosum × S. sparsipilum located on chromosome I. Theor. Appl. Genet. 2008, 117, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Krits, P.; Fogelman, E.; Ginzberg, I. Potato steroidal glycoalkaloid levels and the expression of key isoprenoid metabolic genes. Planta 2007, 227, 143–150. [Google Scholar] [CrossRef]
- Khan, M.S.; Munir, I.; Khan, I. The potential of unintended effects in potato glycoalkaloids. Afr. J. Biotechnol. 2013, 12, 754–766. [Google Scholar]
- Cui, T.; Bai, J.; Zhang, J.; Zhang, J.; Wang, D. Transcriptional expression of seven key genes involved in steroidal glycoalkaloid biosynthesis in potato microtubers, N.Z.J. Crop. Hortic. Sci. 2014, 42, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Ginzberg, I.; Thippeswamy, M.; Fogelman, E.; Demirel, U.; Mweetwa, A.M.; Tokuhisa, J.; Veilleux, R.E. Induction of potato steroidal glycoalkaloid biosynthetic pathway by overexpression of cDNA encoding primary metabolism HMG-CoA reductase and squalene synthase. Planta 2012, 235, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Arnqvist, L.; Dutta, P.C.; Jonsson, L.; Sitbon, F. Reduction of cholesterol and glycoalkaloid levels in transgenic potato plants by overexpression of a type 1 sterol methyltransferase cDNA. Plant. Physiol. 2003, 131, 1792–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zook, M.N.; Kuc, J.A. Induction of sesquiterpene cyclase and suppression of squalene synthetase activity in elicitor treated or fungal infected potato tuber tissue. Physiol. Mol. Plant. Pathol. 1991, 39, 377–390. [Google Scholar] [CrossRef]
- Shi, J.; Gonzales, R.A.; Madan, J.; Bhattacharyya, K. Identification and Characterization of an S-Adenosyl-l-methionine: D24-Sterol-C-methyltransferase cDNA from Soybean. J. Biol. Chem. 1996, 271, 9384–9389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurun, N. Regulation of Sterol and Glycoalkaloid Biosynthesis in Potato (Solanum tuberosum L.)—Identification of Key Genes and Enzymatic Steps; Acta Universitatis Agriculturae Sueciae: Uppsala, Sweden, 2011; ISSN 1652-6880. [Google Scholar]
- Sawai, S.; Ohyama, K.; Yasumoto, S.; Seki, H.; Sakuma, T.; Yamamoto, T.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Aoki, T.; et al. Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant. Cell 2014, 26, 3763–3774. [Google Scholar]
- Shepherd, L.; Hackett, C.; Alexander, C.; McNicol, J.; Sungurtas, J.; Stewart, D.; McCue, K.; Belknap, W.; Davies, H. Modifying glycoalkaloid content in transgenic potato—Metabolome impacts. Food Chem. 2015, 187, 10–1016. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Carpintero, N.C.; Tokuhisa, J.G.; Ginzberg, I.; Veilleux, R.E. Allelic variation in genes contributing to glycoalkaloid biosynthesis in a diploid interspecific population of potato. Theor. Appl. Genet. 2014, 127, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Hardigan, M.; Laimbeer, P.; Newton, L.; Crisovan, E.; Hamilton, J.; Vaillancourt, B.; Wiegert-Rininger, K.; Wood, J.; Douches, D.; Farré, E.; et al. Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proc. Natl. Acad. Sci. 2017, 114, 14380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárdenas, P.D.; Sonawane, P.; Pollier, J.; Bossche, R.V.; Dewangan, V.; Weithorn, E.; Tal, L.; Meir, S.; Rogachev, I.; Malitsky, S.; et al. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nat. Commun. 2016, 7, 10654. [Google Scholar] [CrossRef] [Green Version]
- Manjulatha, M.; Hwang-Bae, S.; Yul-Ho, K.; Su-Jeong, K.; Kwang-Soo, C.; Oh-Keun, K.; Yong-Ik, J.; Su-Young, H.; Jeong-Hwan, N.; Jong-Taek, S.; et al. Comparative Expression of Key Genes Involved in Steroidal Glycoalkaloid Biosynthesis in Tubers of Two Potato Cultivars, Atlantic and Haryoung. Plant. Breed. Biotechnol. 2014, 2, 257–267. [Google Scholar]
- Nahar, N.; Westerberg, E.; Arif, U.; Huchelmann, A.; Guasca, A.O.; Beste, L.; Dalman, K.; Dutta, P.c.; Jonsson, L.; Sitbon, F. Transcript profiling of two potato cultivars during glycoalkaloid-inducing treatments shows differential expression of genes in sterol and glycoalkaloid metabolism. Sci. Rep. 2017, 7, 43268. [Google Scholar] [CrossRef] [PubMed]
- Mariot, R.F.; de Oliveira, L.A.; Voorhuijzen, M.M.; Staats, M.; Hutten, R.C.B.; van Dijk, J.P.; Kok, E.J.; Frazzon, J. Characterization and Transcriptional Profile of Genes Involved in Glycoalkaloid Biosynthesis in New Varieties of Solanum tuberosum L. J. Agric. Food Chem. 2016, 64, 988–996. [Google Scholar]
- Sanford, L.L.; Deahl, K.L.; Sinden, S.L.; Ladd, T.L. Glycoalkaloid contents in tubers from Solanum tuberosum populations selected for potato leafhopper resistance. Am. Potato J. 1992, 69, 693–703. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, C.; Chen, Z.; Kang, Y.; Qin, S. RNA Sequencing Reveals That Both Abiotic and Biotic Stress-Responsive Genes Are Induced during Expression of Steroidal Glycoalkaloid in Potato Tuber Subjected to Light Exposure. Genes 2019, 10, 920. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, T.; Weighill, D.; Proux-Wéra, E.; Levander, F.; Resjö, S.; Burra, D.D.; Moushib, L.I.; Hedley, P.E.; Liljeroth, E.; Jacobson, D.; et al. Proteomics and transcriptomics of the BABA-induced resistance response in potato using a novel functional annotation approach. BMC Genom. 2014, 15, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paudel, J.R.; Davidson, C.; Song, J.; Maxim, I.; Aharoni, A.; Tai, H.H. Pathogen and pest responses are altered due to RNAi-mediated knockdown of Lycoalkaloid Metabolism4 in Solanum tuberosum. Mol. Plant. Microbe Interact. 2017, 30, 876–885. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant. Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Buxdorf, K.; Yaffe, H.; Barda, O.; Levy, M. The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS ONE 2013, 8, e70771. [Google Scholar] [CrossRef] [Green Version]
- Galletti, S.; Sala, E.; Leoni, O.; Cinti, S.; Cerato, C. Aspergillus flavus transformation of glucosinolates to nitriles by an arylsulfatase and a β-thio-glucosidase. Soil Biol. Biochem. 2008, 40, 2170–2173. [Google Scholar]
- Züst, T.; Strickler, S.R.; Powell, A.F.; Mabry, M.E.; An, H.; Mirzaei, M.; York, T.; Holland, C.K.; Kumar, P.; Erb, M.; et al. Independent evolution of ancestral and novel defenses in a genus of toxic plants (Erysimum, Brassicaceae). eLife 2020, 9, e51712. [Google Scholar] [CrossRef] [PubMed]
- Barco, B.; Clay, N.K. Evolution of glucosinolate diversity via whole-genome duplications, gene rearrangements, and substrate promiscuity. Annu. Rev. Plant. Biol. 2019, 70, 585–604. [Google Scholar] [CrossRef]
- Chhajed, S.; Misra, B.B.; Tello, N.; Chen, S. Chemodiversity of the glucosinolate-myrosinase system at the single cell type resolution. Front. Plant. Sci. 2019, 10, 618. [Google Scholar] [CrossRef]
- Zhang, K.X.; Hao, H.Y.; Li, J. Recent research advances in the mustard oil glycoside-black mustard enzyme defense system. J. Plant. Physiol. 2017, 12, 2069–2077. [Google Scholar]
- Jang, M.; Hong, E.; Kim, G.H. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J. Food Sci. 2010, 75, 7. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The Glucosinolates: A sulphur glucoside family of mustard anti-tumour and antimicrobial phytochemicals of potential therapeutic application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pane, C.; Villecco, D.; Roscigno, G.; De Falco, E.; Zaccardelli, M. Screening of plant-derived antifungal substances useful for the control of seedborne pathogens. Arch. Phytopathology Plant. Protect. 2013, 46, 1533–1539. [Google Scholar] [CrossRef]
- Tierens, K.F.J.; Thomma, B.P.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Broekaert, W.F. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant. Physiol. 2001, 125, 1688–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Li, Y.; Bi, Y.; Wang, T.; Dong, Y.; Yang, Q.; Zhang, T. 2-Phenylethyl isothiocyanate exerts antifungal activity against Alternaria alternata by affecting membrane integrity and mycotoxin production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazareth, T.M.; Corrêa, J.A.F.; Pinto, A.C.S.M.; Palma, J.B.; Meca, G.; Bordin, K.; Luciano, F.B. Evaluation of gaseous allyl isothiocyanate against the growth of mycotoxigenic fungi and mycotoxin production in corn stored for 6 months. J. Sci. Food Agric. 2018, 98, 5235–5241. [Google Scholar] [CrossRef]
- Nazareth, T.D.M.; Alonso-Garrido, M.; Stanciu, O.; Mañes, J.; Manyes, L.; Meca, G. Effect of allyl isothiocyanate on transcriptional profile, aflatoxin synthesis, and Aspergillus flavus growth. Int. Food Res. J. 2020, 128, 108786. [Google Scholar] [CrossRef]
- Tracz, B.L.; Bordin, K.; Nazareth, T.D.M.; Costa, L.B.; Macedo, R.E.F.D.; Meca, G.; Luciano, F.B. Assessment of allyl isothiocyanate as a fumigant to avoid mycotoxin production during corn storage. LWT 2017, 75, 692–696. [Google Scholar] [CrossRef]
- Azaiez, I.; Meca, G.; Manyes, L.; Luciano, F.B.; Fernández-Franzón, M. Study of the chemical reduction of the fumonisins toxicity using allyl, benzyl and phenyl isothiocyanate in model solution and in food products. Toxicon 2013, 63, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, I.; Meca, G.; Manyes, L.; Fernández-Franzón, M. Antifungal activity of gaseous allyl, benzyl and phenyl isothiocyanate in vitro and their use for fumonisins reduction in bread. Food Control. 2013, 32, 428–434. [Google Scholar] [CrossRef]
- Quiles, J.M.; Manyes, L.; Luciano, F.B.; Mañes, J.; Meca, G. Effect of the oriental and yellow mustard flours as natural preservative against aflatoxins B1, B2, G1 and G2 production in wheat tortillas. Int. J. Food Sci. Technol. 2015, 52, 8315–8321. [Google Scholar]
- Lazzeri, L.; Curto, G.; Leoni, O.; Dallavalle, E. Effects of glucosinolates and their enzymatic hydrolysis products via myrosinase on the root-knot nematode Meloidogyne incognita (Kofoid et White) Chitw. J. Agric. Food Chem. 2004, 52, 6703–6707. [Google Scholar] [CrossRef]
- Bhushan, S.; Gupta, S.; Sohal, S.K.; Arora, S. Assessment of insecticidal action of 3-Isothiocyanato-1-propene on the growth and development of Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). J. Entomol. Zool. Stud. 2016, 4, 1068–1073. [Google Scholar]
- Furlan, L.; Bonetto, C.; Finotto, A.; Lazzeri, L.; Malaguti, L.; Patalano, G.; Parker, W. The efficacy of biofumigant meals and plants to control wireworm populations. Ind. Crops Prod. 2010, 31, 245–254. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.A.; Zeng, S.; Lin, K.C. Extraction of allyl isothiocyanate from horseradish (Armoracia rusticana) and its fumigant insecticidal activity on four stored-product pests of paddy. Pest. Manag. Sci. 2009, 65, 1003–1008. [Google Scholar] [CrossRef]
- Freitas, R.C.P.; Faroni, L.R.D.; Haddi, K.; Jumbo, V.L.O.; Oliveira, E.E. Allyl isothiocyanate actions on populations of Sitophilus zeamais resistant to phosphine: Toxicity, emergence inhibition and repellency. J. Stored Prod. Res. 2016, 69, 257–264. [Google Scholar] [CrossRef]
- Messiha, N.K.; Ahmed, S.A.; El-Rokiek, K.G.; Dawood, M.G.; El-Masry, R.R. The physiological influence of allelochemicals in two Brassicaceae plant seeds on the growth and propagative capacity of Cyprus rotundus and Zea mays L. World Appl. Sci. J. 2013, 26, 1142–1149. [Google Scholar]
- Smith, J.D.; Woldemariam, M.G.; Mescher, M.C.; Jander, G.; De Moraes, C.M. Glucosinolates from host plants influence growth of the parasitic plant Cuscuta gronovii and its susceptibility to aphid feeding. Plant. Physiol. 2016, 172, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Lazzeri, L.; Curto, G.; Dallavalle, E.; D’Avino, L.; Malaguti, L.; Santi, R.; Patalano, G. Nematicidal efficacy of biofumigation by defatted brassicaceae meal for control of Meloidogyne incognita (Kofoid et White) Chitw. on a full field zucchini crop. Int. J. Agric. Sustain. 2009, 33, 349–358. [Google Scholar]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Curto, G.; Dallavalle, E.; Matteo, R.; Lazzeri, L. Biofumigant effect of new defatted seed meals against the southern root-knot nematode, Meloidogyne incognita. Ann. Appl. Biol. 2016, 169, 17–26. [Google Scholar] [CrossRef]
- Meyer, S.L.F.; Zasada, I.A.; Orisajo, S.B.; Morra, M.J. Mustard seed meal mixtures: Management of Meloidogyne incognita on pepper and potential phytotoxicity. J. Nematol. 2011, 43, 7–15. [Google Scholar]
- Laquale, S.; Candido, V.; Avato, P.; Argentieri, M.P.; D’addabbo, T. Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Ann. Appl. Biol. 2015, 167, 217–224. [Google Scholar] [CrossRef]
- Ozone Secretariat. 2020. Available online: https://ozone.unep.org/sites/default/files/Handbooks/MP-Handbook-2020-English.pdf (accessed on 27 November 2020).
- Lazzeri, L.; Baruzzi, G.; Malaguti, L.; Antoniacci, L. Replacing methyl bromide in annual strawberry production with glucosinolate-containing green manure crops. Pest. Manag. Sci. 2003, 59, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Curto, G.; Dallavalle, E.; Lazzeri, L. Life cycle duration of Meloidogyne incognita and host status of Brassicaceae and Capparaceae selected for glucosinate content. Nematology 2005, 7, 203–212. [Google Scholar] [CrossRef]
- Lazzeri, L.; Leoni, O.; Manici, L.M.; Palmieri, S.; Patalano, G. Use of Seed Flour as Soil Pesticide. Patent US 7749549, 6 July 2010. [Google Scholar]
- Lazzeri, L.; D’Avino, L.; Ugolini, L.; De Nicola, G.R.; Cinti, S.; Malaguti, L.; Bagatta, M.; Patalano, G.; Leoni, O.; Lazzeri, L. Bio-based products from Brassica carinata A. Braun oils and defatted meals by a second generation biorefinery approach. In Proceedings of the 19th European Biomass Conference, Berlin, Germany, 6–10 June 2011; pp. 1080–1092. [Google Scholar]
- Ntalli, N.; Adamski, Z.; Doula, M.; Monokrousos, N. Nematicidal amendments and soil remediation. Plants 2020, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Paudel, B.R.; Carpenter-Boggs, L.; Higgins, S. Influence of brassicaceous soil amendments on potentially beneficial and pathogenic soil microorganisms and seedling growth in Douglas-fir nurseries. Appl. Soil Ecol. 2016, 105, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Lazzeri, L.; Malaguti, L.; Cinti, S.; Ugolini, L.; De Nicola, G.R.; Bagatta, M.; Casadei, N.; D’avino, L.; Matteo, R.; Patalano, G. The Brassicaceae biofumigation system for plant cultivation and defence. An Italian twenty-year experience of study and application. Acta Hortic. 2013, 1005, 375–382. [Google Scholar]
- Argento, S.; Melilli, M.G.; Branca, F. Enhancing Greenhouse Tomato-Crop Productivity by Using Brassica macrocarpa Guss. Leaves for controlling root-knot nematodes. Agronomy 2019, 9, 820. [Google Scholar] [CrossRef] [Green Version]
- Ngala, B.M.; Haydock, P.P.; Woods, S.; Back, M.A. Biofumigation with Brassica juncea, Raphanus sativus and Eruca sativa for the management of field populations of the potato cyst nematode Globodera pallida. Pest. Manag. Sci. 2015, 71, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Mocali, S.; Landi, S.; Curto, G.; Dallavalle, E.; Infantino, A.; Colzi, C.; D’Errico, G.; Roversi, P.F.; D’Avino, L.; Lazzeri, L. Resilience of soil microbial and nematode communities after biofumigant treatment with defatted seed meals. Ind. Crops Prod. 2015, 75, 79–90. [Google Scholar]
- Rudolph, R.E.; Sams, C.; Steiner, R.; Thomas, S.H.; Walker, S.; Uchanski, M.E. Biofumigation Performance of Four Brassica Crops in a Green Chile Pepper (Capsicum annuum) Rotation System in Southern New Mexico. HortScience 2015, 50, 247–253. [Google Scholar] [CrossRef]
- Argento, S.; Raccuia, S.A.; Melilli, M.G.; Toscano, V.; Branca, F. Brassicas and their glucosinolate content for the biological control of root-knot nematodes in protected cultivation. Acta Hortic. 2013, 1005, 539–544. [Google Scholar]
- Vandicke, J.; De Visschere, K.; Deconinck, S.; Leenknecht, D.; Vermeir, P.; Audenaert, K.; Haesaert, G. Uncovering the biofumigant capacity of allyl isothiocyanate from several Brassicaceae crops against Fusarium pathogens in maize. J. Sci. Food Agric. 2020, 100, 5476–5486. [Google Scholar] [CrossRef]
- Piccinini, E.; Ferrari, V.; Campanelli, G.; Fusari, F.; Righetti, L.; Pagnotta, E.; Lazzeri, L. Effect of two liquid formulations based on Brassica carinata co-products in containing powdery mildew on melon. Ind. Crops Prod. 2015, 75, 48–53. [Google Scholar] [CrossRef]
- Handiseni, M.; Jo, Y.; Lee, K.; Zhou, X. Screening brassicaceous plants as biofumigants for management of Rhizoctonia solani AG1-IA. Plant. Dis. 2016, 100, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Koron, D.; Sonjak, S.; Regvar, M. Effects of non-chemical soil fumigant treatments on root colonisation with arbuscular mycorrhizal fungi and strawberry fruit production. Crop. Prot. 2014, 55, 35–41. [Google Scholar] [CrossRef]
- Neubauer, C.; Heitmann, B.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant. Pathol. 2004, 140, 341–352. [Google Scholar]
- Ugolini, L.; Martini, C.; Lazzeri, L.; D’Avino, L.; Mari, M. Control of postharvest grey mould (Botrytis cinerea Per.: Fr.) on strawberries by glucosinolate-derived allyl-isothiocyanate treatments. Postharvest Biol. Technol. 2014, 90, 34–39. [Google Scholar] [CrossRef]
- Ríos, P.; González, M.; Obregón, S.; Carbonero, M.; Leal, J.; Fernández, P.; De Haro, A.; Sánchez, M. Brassica-based seedmeal biofumigation to control Phytophthora cinnamomi in the Spanish “dehesa” oak trees. Phytopathol. Mediterr. 2017, 56, 392–399. [Google Scholar]
- Serrano- Pérez, P.; Palo, C.; Rodríguez -Molina, M.D.C. Efficacy of Brassica carinata pellets to inhibit mycelial growth and chlamydospores germination of Phytophthora nicotianae at different temperature regimes. Sci. Hortic. 2017, 216, 126–133. [Google Scholar] [CrossRef]
- Ríos, P.; Obregón, S.; De Haro, A.; Fernández -Rebollo, P.; Serrano, M.; Sánchez, M. Effect of Brassica Biofumigant Amendments on Different Stages of the Life Cycle of Phytophthora cinnamomi. J. Phytopathol. (1986) 2016, 164, 582–594. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Vettraino, A.M.; Vannini, A. Efficacy of biofumigation with Brassica carinata commercial pellets (BioFence) to control vegetative and reproductive structures of Phytophthora cinnamomi. Plant. Dis. 2016, 100, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukovata, L.; Jaworski, T.; Kolk, A. Efficacy of Brassica juncea granulated seed meal against Melolontha grubs. Ind. Crops Prod. 2015, 70, 260–265. [Google Scholar] [CrossRef]
- Piccinini, E.; Ferrari, V.; Campanelli, G.; Fusari, F.; Righetti, L.; Matteo, R.; Lazzeri, L. Effect of two bio-based liquid formulations from Brassica carinata in containing red spider mite (Tetranychus urticae) on eggplant. Ind. Crops Prod. 2015, 75, 36–41. [Google Scholar] [CrossRef]
- Zuluaga, D.L.; Van Ommen Kloeke, A.E.E.; Verkerk, R.; Röling, W.F.M.; Ellers, J.; Roelofs, D.; Aarts, M.G.M. Biofumigation using a wild Brassica oleracea accession with high glucosinolate content affects beneficial soil invertebrates. Plant. Soil 2015, 394, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Kirkegaard, J.A.; Matthiessen, J. Developing and refining the biofumigation concept. Agroindustria 2004, 3, 233–239. [Google Scholar]
- Lu, P.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Biofumigation with Brassica plants and its effect on the inoculum potential of Fusarium yellows of Brassica crops. Eur. J. Plant. Pathol. 2010, 126, 387–402. [Google Scholar]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1907 (accessed on 9 April 2021).
- Matteo, R.; Back, M.A.; Reade, J.P.H.; Ugolini, L.; Pagnotta, E.; Lazzeri, L. Effectiveness of defatted seed meals from Brassicaceae with or without crude glycerin against black grass (Alopecurus myosuroides Huds.). Ind. Crops Prod. 2018, 111, 506–512. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soil-borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Schnug, E.; Haneklaus, S. Glucosinolates—the agricultural story. In: Kopriva, S., ed. Glucosinolates. Adv. Bot. Res. 2016, 80, 281–302. [Google Scholar]
- Gupta, S.K. Brassicas. In Breeding Oilseed Crops for Sustainable Production, 1st ed; Elsevier: Amsterdam, Netherlands, 2016; pp. 33–53. [Google Scholar]
- Faulkner, K.; Mithen, R.; Williamson, G. Selective increase of the potential anticarcinogen 4-methylsulphinylbutyl glucosinolate in broccoli. Carcinogenesis 1998, 19, 605–609. [Google Scholar]
- Mithen, R.; Faulkner, K.; Magrath, R.; Rose, P.; Williamson, G.; Marquez, J. Development of isothiocyanate enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor. Appl. Genet. 2003, 106, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Riaz, A.; Goyal, S.; Abel, S.; Quiros, C.F. Inheritance of Three Major Genes Involved in the Synthesis of Aliphatic Glucosinolates in Brassica oleracea. J. Am. Soc. Hort. Sci. 2001, 126, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Hirani, A.H.; McVetty, P.B.E.; Daayf, F.; Quiros, C.F.; Li, G. Reducing progoitrin and enriching glucoraphanin in Brassica napus seeds through silencing of the GSL-ALK gene family. Plant. Mol. Biol. 2012, 79, 179–189. [Google Scholar] [CrossRef]
- Petersen, A.; Wang, C.; Crocoll, C.; Halkier, B.A. Biotechnological approaches in glucosinolate production. J. Integr. Plant. Biol. 2018, 60, 1231–1248. [Google Scholar] [CrossRef]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering glucosinolate profiles modulates disease resistance in plants. Plant. J. 2006, 46, 758–767. [Google Scholar] [CrossRef]
- Wentzell, A.M.; Rowe, H.C.; Hansen, B.G.; Ticconi, C.; Halkier, B.A.; Kliebenstein, D.J. Linking metabolic QTL with network and cis-eQTLs controlling biosynthetic pathways. PLoS Genet. 2007, 3, e162. [Google Scholar] [CrossRef] [Green Version]
- Neal, C.S.; Fredericks, D.P.; Griffiths, C.A.; Neale, A.D. The characterisation of AOP2: A gene associated with the biosynthesis of aliphatic alkenyl glucosinolates in Arabidopsis thaliana. BMC Plant. Biol. 2010, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Chadwick, M.; Puranik, M.; Tudor, R.; Methven, L.; Kennedy, S.; Wagstaff, C. The Eruca sativa Genome and Transcriptome: A Targeted Analysis of Sulfur Metabolism and Glucosinolate Biosynthesis Pre and Postharvest. Front. Plant. Sci. 2020, 11, 525102. [Google Scholar]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The effect of sulfur nutrition on plant glucosinolate content: Physiology and molecular mechanisms. Plant. Biol. (Stuttg) 2007, 9, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Mithen, R.; Ho, E. Isothiocyanates for Human Health. Mol. Nutr Food Res. 2018, 62, e1870079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traka, M.H.; Saha, S.; Huseby, S.; Kopriva, S.; Walley, P.G.; Barker, G.C.; Moore, J.; Mero, G.; van den Bosch, F.; Constant, H.; et al. Genetic regulation of glucoraphanin accumulation in Benefortè broccoli. New Phytol. 2013, 198, 1085–1095. [Google Scholar]
- Ruffoni, B.; Pistelli, L.; Bertoli, A.; Pistelli, L. Plant cell cultures: Bioreactors for industrial production. Adv. Exp. Med. Biol. 2010, 698, 203–218. [Google Scholar]
- Kastell, A.; Zrenner, R.; Schreiner, M.; Kroh, L.; Ulrichs, C.; Smetanska, I.; Mewis, I. Metabolic engineering of aliphatic glucosinolates in hairy root cultures of Arabidopsis thaliana. Plant. Mol. Biol. Rep. 2015, 33, 598–608. [Google Scholar] [CrossRef]
- Wielanek, M.; Urbanek, H. Enhanced glucotropaeolin production in hairy root cultures of Tropaeolum majus L. by combining elicitation and precursor feeding. Plant. Cell Tiss. Organ. Cult. 2006, 86, 177–186. [Google Scholar] [CrossRef]
- Hirani, A.H.; Li, G.; Zelmer, C.D.; McVetty, P.B.E.; Asif, M.; Goyal, A. Molecular genetics of glucosinolate biosynthesis in Brassicas: Genetic manipulation and application aspects. In Crop Plant; Goyal, A., Ed.; IntechOpen, Inc.: Palm Gardens, AB, Canada, 2012. [Google Scholar]
- Neequaye, M.; Stavnstrup, S.; Lawrenson, T.; Hundleby, P.; Troncoso-Rey, P.; Saha, S.; Harwood, W.; Traka, H.M.; Mithen, R.; Østergaard, L. CRISPR-Cas9-mediated editing of myb28 impairs glucoraphanin accumulation of Brassica oleracea in the field. bioRxiv Preprint 2020. [Google Scholar] [CrossRef]
- Gao, M.; Li, G.; Yang, B.; Qiu, D.; Farnham, M.; Quiros, C. High-density Brassica oleracea linkage map: Identification of useful new linkages. Theor. Appl. Genet. 2007, 115, 277–287. [Google Scholar]
- Issa, R.A. Identification of glucosinolate profile in Brassica oleracea for quantitative trait locus mapping. Ph.D. Thesis, University of Warwick, Coventry, UK, 2010. [Google Scholar]
- Hirani, A.H. QTL mapping, gene identification and genetic manipulation of glucosinolates in Brassica rapa L. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2011. [Google Scholar]
- Lou, P.; Zhao, J.; He, H.; Hanhart, C.; Del Carpio, D.P.; Verkerk, R.; Custers, J.; Koornneef, M.; Bonnema, G. Quantitative trait loci for glucosinolate accumulation in Brassica rapa leaves. New Phytol. 2008, 179, 1017–1032. [Google Scholar]
- Ramchiary, N.; Padmaja, K.L.; Sharma, S.; Gupta, V.; Sodhi, Y.S.; Mukhopadhyay, A.; Arumugam, N.; Pental, D.; Pradhan, A.K. Mapping of yield influencing QTL in Brassica juncea: Implications for breeding of a major oilseed crop of dryland areas. Theor. Appl. Genet. 2007, 115, 807–817. [Google Scholar] [CrossRef]
- Hirani, A.H.; Geng, J.; Zhang, J.; Zelmer, C.D.; McVetty, P.B.E.; Daayf, F.; Li, G. Quantitative Trait Loci Mapping and Candidate Gene Identification for Seed Glucosinolates in Brassica rapa L. Crop. Sci. 2016, 56, 942–956. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Liu, Z.; Liang, J.; Wu, J.; Cheng, F.; Mei, S.; Wang, X. A naturally occurring variation in the BrMAM-3 gene is associated with aliphatic glucosinolate accumulation in Brassica rapa leaves. Hortic. Res. 2018, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Quijada, P.A.; Udall, J.A.; Lambert, B.; Osborn, T.C. Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): Identification of genomic regions from winter germplasm. Theor. Appl. Genet. 2006, 113, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Friedt, W.; Pons-Kuhnemann, J.; Freitag, N.M.; Link, K.; Snowdon, R.J. Association of gene-linked SSR markers to seed glucosinolate content in oilseed rape (Brassica napus ssp. napus). Theor. Appl. Genet. 2008, 116, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Lionneton, E.; Aubert, G.; Ochatt, S.; Merah, O. Genetic analysis of agronomic and quality traits in mustard (Brassica juncea). Theor. Appl. Genet 2004, 109, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Ripley, V.L.; Roslinsky, V. Identification of an ISSR marker for 2-propenyl glucosinolate content in Brassica juncea L. and conversion to a SCAR marker. Mol. Breed. 2005, 16, 57–66. [Google Scholar] [CrossRef]
- Howell, P.M.; Sharpe, A.G.; Lydiate, D.J. Homoeologous loci control the accumulation of seed glucosinolates in oilseed rape (Brassica napus). Genome 2003, 46, 454–460. [Google Scholar] [CrossRef]
- Mahmood, T.; Ekuere, U.; Yeh, F.; Good, A.G.; Stringam, G.R. Molecular mapping of seed aliphatic glucosinolates in Brassica juncea. Genome 2003, 46, 753–760. [Google Scholar] [CrossRef]
- Zou, Z.; Ishida, M.; Li, F.; Kakizaki, T.; Suzuki, S.; Kitashiba, H.; Nishio, T. QTL analysis using SNP markers developed by next-generation sequencing for identification of candidate genes controlling 4-methylthio-3-butenyl glucosinolate contents in roots of radish, Raphanus sativus L. PLoS ONE 2013, 8, e53541. [Google Scholar]
- Javidfar, F.; Cheng, B. Construction of a genetic linkage map and QTL analysis of erucic acid content and glucosinolate components in yellow mustard (Sinapis alba L.). BMC Plant. Biol. 2013, 13, 142. [Google Scholar]
- Cardellina, J.H. Biologically natural products in the search for new agrochemicals. In Biologically active natural products: Potential use in agriculture; Gulter, H.G., Ed.; American Chemical Society: Washington, WA, USA, 1988; pp. 305–311. [Google Scholar]
- Gulter, H.G. Natural products and their potential in agriculture: A personal overview. In Biologically active natural products: Potential use in agriculture; Gulter, H.G., Ed.; American Chemical Society: Washington, WA, USA, 1998; pp. 1–2. [Google Scholar]
- Emosairue, S.O.; Ukeh, D.A. Field trial of neem products for the control of okra flea beetles (Podagrica spp) in South Eastern Nigeria. Afr. J. Plant. Prot. 1996, 6, 27–33. [Google Scholar]
- Tewari, S.N.; Nayak, M. Activity of four-plant leaf extracts against three fungal pathogens of rice. Trop. Agric. (Trinidad) 1991, 68, 373–375. [Google Scholar]
- Al-Abed, A.S.; Quasem, J.R.; Abu-Blan, H.A. Antifungal effects of some common wild plant species on certain plant pathogenic fungi. Dirasat (Pure Appl. Sci.) 1993, 20, 149–158. [Google Scholar]
- Amadioha, A.C. Control of powdery mildew of pepper (Capsicum annum L) by leaf extracts of papaya (Asimina triloba). J. Herbs Spices Med. Plants 1998, 6, 41–47. [Google Scholar] [CrossRef]
- Amadioha, A.C. Controlling rice blast in vitro and in vivo with extracts of Azadirachta indica. Crop. Prot. 2000, 19, 287–290. [Google Scholar] [CrossRef]
- Amadioha, A.C.; Obi, V.I. Fungitoxic activity of extracts from Azadirachta indica and Xylopia aethiopica on Colletotrichum lindemuthianum in Cowpea. J. Herbs Spices Med. Plants 1998, 6, 33–40. [Google Scholar] [CrossRef]
- Mason, J.R.; Mathew, D.N. Evaluation of neem as a bird repellent chemical. Int. J. Pest Manag 1996, 42, 47–49. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Metclaf, R.A.; Rhodes, A.N. Cucurbitacins as Kairomones for diabroticide beetles. Proc. Natl. Acad. Sci. USA 1980, 77, 3769–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neilson, J.K.; Larsen, L.M.; Sorenson, H.J. Cucurbitacins E and I in Iberis amara, feeding inhibitors for Phyllotreta nemorum. Phytochemistry 1977, 16, 1519–1522. [Google Scholar]
- Singh, D.C. Scope of medicinal and aromatic plants in pest management. In Proceedings of the International Symposium, Allelopathy in sustainable Agriculture, Forestry and Environment, New Delhi, India, 6–8 September 1994; p. 68. [Google Scholar]
- Fandohan, P.; Gbenou, J.D.; Gnonlonfin, B.; Hell, K.; Marasas, F.O.; Wingfield, M.G. Effect of essential oils on the growth of Fusarium verticillioides and fumonisin contamination in corn. J. Agric. Food Chem. 2004, 52, 6824–6829. [Google Scholar] [CrossRef]
- Osbourn, A.E. Antimicrobial phytoprotectants and fungal pathogens: A commentary. Fungal Genet. Biol. 1999, 26, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, K.; Balconi, C.; Sherwood, J.E.; Giroux, M. Increased tolerance to fungal diseases of rice plants transformed with puroindoline genes. Mol. Plant. Microbe Interact. 2001, 14, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.B.; Monteiro, S.; Freitas, R.; Santos, C.N.; Chen, Z.; Batista, L.M.; Duarte, J.; Borges, A.; Teixeira, A.R. The role of plant defence proteins in fungal pathogenesis. Mol. Plant. Pathol. 2007, 8, 677–700. [Google Scholar] [CrossRef]
- Balconi, C.; Lanzanova, C.; Motto, M. Ribosome-inactivating proteins in cereals. In Toxic Plant Proteins; Martin, R.J., Ed.; Hartley, Springer series Plant Cell Monographs 18; Springer: Verlag: Berlin/Heidelberg, Germany, 2010; pp. 149–166. [Google Scholar]
- Punja, Z. Genetic engineering of plants to enhance resistance to fungal pathogens—A review of progress and future prospects. Can. J. Plant. Pathol. 2001, 23, 216–235. [Google Scholar] [CrossRef]
- Tarchevsky, I.A. Pathogen-Induced Plant Proteins (Review). Appl. Biochem. Microbiol. 2001, 37, 441–455. [Google Scholar] [CrossRef]
- Keen, N.T. Plant disease resistance: Progress in basic understanding and practical application. Adv. Bot Res. 1999, 30, 291–328. [Google Scholar]
- Balconi, C.; Stevanato, P.; Motto, M.; Biancardi, E. Breeding for biotic stress resistance/tolerance in plants In Crop. Production for Agricultural Improvement; Ashraf, M., Ozturk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 2012; pp. 57–114. [Google Scholar]
- Mesterházy, A.; Oláh, J.; Popp, J. Losses in the Grain Supply Chain: Causes and Solutions. Sustainability 2020, 12, 2342. [Google Scholar] [CrossRef] [Green Version]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef]
- Balconi, C.; Berardo, N.; Locatelli, S.; Lanzanova, C.; Torri, A.; Redaelli, R. Evaluation of ear rot (Fusarium verticillioides) resistance and fumonisin accumulation in Italian maize inbred lines. Phytopathol. Mediterr. 2014, 53, 14–26. [Google Scholar]
- Balconi, C.; Motto, M.; Mazzinelli, G.; Berardo, N. Ear secondary traits related to aflatoxin accumulation in commercial maize hybrids under artificial field inoculation. World Mycotoxin J. 2010, 3, 239–250. [Google Scholar] [CrossRef]
- Castegnaro, M.; McGregor, D. Carcinogenic risk assessment of mycotoxins. Rev. Med. Vet. 1998, 149, 671–678. [Google Scholar]
- CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems; Task Force Report; Council for Agricultural Science and Technology: Ames, IA, USA, 2003; Volume 139. [Google Scholar]
- Lanzanova, C.; Torri, A.; Motto, M.; Balconi, C. Characterization and interaction between b-32 maize ribosome-inactivating protein and fungal pathogens development in vivo and in vitro. Maydica 2011, 56, 83–93. [Google Scholar]
- Balazs, E.; Schepers, J.S. The mycotoxin threat to world safety. Int. J. Food Microbiol. 2007, 119, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berardo, N.; Lanzanova, C.; Locatelli, S.; Laganà, P.; Verderio, A.; Motto, M. Levels of total fumonisins in maize samples from Italy during 2006–2008. Food Addit. Contam. Part. B 2011, 4, 116–124. [Google Scholar] [CrossRef] [PubMed]
- European Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R1881 (accessed on 9 April 2021).
- European Commission Regulation (EC) No 1126/2007 of 28 September 2007 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Fusarium Toxins in Maize and Maize Products. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32007R1126 (accessed on 9 April 2021).
- Hartings, H.; Lanzanova, C.; Lazzaroni, N.; Balconi, C. How maize trackles Diabrotica v. virgifera attack. Maydica 2016, 61, 1–8. [Google Scholar]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Crossa, J.; Yuen, J.; Djurle, A. Effect of leaf rust on grain yield and yield traits of durum wheats with race-specific and slow-rusting resistance to leaf rust. Plant. Dis. 2006, 90, 1065–1072. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
| Metabolite/Extract: Glycoalkaloids | Target: Molluscs | Effect | References |
|---|---|---|---|
| α-chaconine; α-solanine | Helix aspersa L. | Feeding deterrence | [64] |
| Target: Protoza | |||
| α-chaconine; α-solanine | Trichomonas vaginalis | Growth inhibition | [68] |
| Peel extracts | Salmonella typhimurium; Eschrichia coli | Mutagenic activity | [30] |
| Peel extracts | S.aureus (Gram +); P.aeruginosa (Gram -) | Growth inhibition | [31] |
| Target: Insects | |||
| α-chaconine | Phorimaea operculella | Strongly related with PTM larval mortality | [45] |
| α-chaconine; α-solanine | Tribolium castaneum, Sitophilus oryzae L. | Toxic against adults | [70] |
| α-chaconine; α-solanine | Trogoderma granarium | Antifeedant activity | [25] |
| α-solanine | Galleria mallonella L. | Increased mortality of larvae; pupae and adults, decreased fertility and fecundity | [27] |
| α-solanine | Myzus persicae | Antifeedant activity, decreased fecundity, increase mortality of pupae | [27] |
| α-chaconine; α-solanine | Zophabas atratus | Decrease heart activity in pupae and adults | [27] |
| vegetable waste extract | Culex quinquefasciatus, Nopheles stephensi | Larvicidal activity | [27] |
| leaf extract | Zophobas atratus, Tenebrio molitor | In vivo cardioinhibitory activity in pupae and adults, increased mortality | [27] |
| leaf extract | Leptinotarsa decemlineata, Spadoptera exigua | Increased mortality of larvae, pupae and adults, disturbance in fertility and fecondity | [27] |
| Target: Amphibia | |||
| α-chaconine; α-solanine | Frog embryos | Impact on membrane | [74] |
| Target: Fungi | |||
| α-chaconine; α-solanine | Ascobus crenulatus, Alternaria brassicicola, Phoma medicaginis, Rhizoctonia solani | Effect on the fungal growth | [66] |
| α-chaconine; α-solanine | Ascobus crenulatus, Alternaria brassicicola, Phoma medicaginis, Rhizoctonia solani | Spore germination inhibition Fungal growth inhibition | [67] |
| α-chaconine; α-solanine | Alternaria alternata, Pyrenophora teres f. teres, Pyrenophora tritici-repentis | Effect on the fungal growth | [82] |
| solanidine | Phythophtora infestans | Inhibition of mycelial growth | [77] |
| Metabolites/Extract: Phenols | Target: Protozoa | Effect | References |
| CA; CGA | Trichomonas vaginalis | Growth inhibition | [68] |
| Target: Insects | |||
| CA; CGA | Phorimaea operculella | Strongly related with potato PTM larval mortality | [45] |
| Glucosinolate/isothiocyanate | Target: Nematodes | Effect | References |
| Allyl GSL from leaf flour of Brassica macrocarpa Guss. | Meloidogyne spp. root-knot nematodes, target crop: tomato in greenhouse | B. macrocarpa leaf flour inserted in the soil at a GSL dose of 300 µmol m−2 showed similar effect to Vydate 5G® on root disease index, root weight, and marketable yield | [160] |
| GSL from the DSMs of 13 Brassicaceae species: Barbarea verna, B. carinata, B. nigra, B. rapa B. tournefortii, B. oleracea var. acephala Crambe abyssinica, Eruca sativa, Lepidium densiflorum, Lepidium sativum, Raphanus sativus, Rapistrum rugosum, and Sinapis alba. | Meloidogyne incognita in pots in glasshouse-controlled conditions, with Solanumlycopersicum L. cv. UC82 as host plant. | Among the tested DSMs, the best results for all inoculations were achieved by Eruca sativa, Barbarea verna (300 µmol m−2 GSLs) and Brassica nigra (370 µmol m−2 GSLs), whereas the other species gave either alternate results or results not different from untreated or sunflower DSM controls. All the DSMs, including sunflower, determined a clear positive effect on tomato vigour. | [149] |
| Allyl GSL (98% of total GSLs) from B. juncea; 4-(Methylsulfinyl)butyl GSL (72 % of total GSLs) from R. sativus; and 4-pentenyl GSL (38% of total GSLs) from E. sativa | cyst nematode Globodera pallida, target crop: potato | 10 kg ha−1 E. sativa, 8 kg ha−1 B. juncea, and 20 kg ha−1 R. sativus were sown, cultivated, and incorporated in soil, in open field trials, both in summer and winter. The incorporation of green materials was done at complete flowering for summer trials or one week prior the potato planting for winter trials. A positive linear regression of GSL concentration (mol m−2) in incorporated biomasses and G. pallida mortality was determined for all brassicaceous species cultivated during summer; only R. sativus and E. sativa demonstrated a significant relationship between GSL concentration and G. pallida mortality. | [161] |
| GSL from Brassica carinata DSM | Meloidogyne incognita root-knot nematodes, target crop: tomato in greenhouse. | A pot trial was conducted on tomato plants grown in a soil naturally infested with M. incognita, amended with B. carinata DSM (3 t ha−1 or 40 mmol m−2 GSL), and finally compared to a soil fumigated with Vapam (Sodium methyldithiocarbamate) and to an untreated control. Both B. carinata DSM and Vapam treatments were effective in protecting tomato plants against M. incognita but they exhibited different effects on soil biota. In general, nematode populations strongly responded to B. carinata DSM amendments both in terms of abundance and structure. Although the free-living nematode structure was negatively influenced by the two treatments, B. carinata DMS proved to be the best compromise between efficiency to control M. incognita and environmental impact. | [162] |
| GSL from four cultivars: three mustards (Brassica juncea ‘Caliente 61′, ‘Caliente 199′, and ‘Pacific Gold’) and one broccoli (Brassica oleracea var. botrytis ‘Arcadia’) | 2-year open field study of biofumigant of the four Brassicales in a chile pepper Capsicum annuum ‘AZ-20′, rotation system in southern New Mexico. | Broccoli produced lower biomass and lower GSL concentrations than the mustard treatments but may be a valuable crop for growers with nematode issues because Meloidogyne incognita populations decreased in its presence. Based on high biomass production and high GSL concentration, B. juncea ‘Caliente 199′ showed the most potential as a biofumigant crop for southern New Mexico | [163] |
| Leaf flour of dry plants of B. juncea, E. sativa, R. sativus and B. macrocarpa, characterized for sinigrin content. | Meloidogyne spp. root-knot nematodes, target crop: tomato in greenhouse | Leaf flours were distributed before planting (60 and 90 g m−2), with the mean dose corresponding to allyl GSL content in the commercial formulate (Nemathorin) applied at 3 g m−2. Disease index detected on the tomato roots at the end of the growing cycle resulted in all thesis lower than the control and Nemathorin, whereas it was lower with 60 g m−2 E. sativa and 90 g m−2 R. sativus, in comparison to 90 g m−2 B. juncea. | [164] |
| Glucosinolate/isothiocyanate | Target: Fungi | Effect | References |
| Pure AITC, and macerated plant tissues from 18 different cultivars amongst Raphanus sativus, Sinapis alba, Brassica carinata, Brassica juncea | Fusarium gramineaum and Fusarium poae in vitro and pot experiment | B. carinata and B. junceae (AITC containing tissues) performed a better mycelial growth reduction in Petri dish. Fusarium poae resulted more tolerant to AITC than F. graminearum. In general, all pots added with Brassicaceae plant material presented a reduced fungal infection, but only B. juncea plant material alleviated F. graminearum negative effect on maize growth. | [165] |
| Pure Allyl isothiocanate | maize grains contaminated with Aspergillus flavus in glass jars | maize grains contaminated with A. flavus in glass jars of 1 L and treated with 0.125, 0.25, 0.5, 1 and 5 μL of AITC. After 7 days of storage, the mycelial growth was significantly reduced in doses higher than 0.125 μL/L of AITC. All doses of AITC significantly reduced the fungal growth and Aflatoxin B1 production in maize after 30 d, regardless of moisture content. | [135] |
| GSL derived from bio-based experimental formulations con-taining either B. carinata oil 1.5% and B. carinata DSM 3 g L−1 (270 µmol GSL L−1), or 2% B. carinata oil and B. carinata DSM 4.5 g L−1 (405 µmol GSL L−1). | Podosphorea xanthii control on melon in open field | The field trials carried out over two years demonstrated the efficacy of the two bio-based experimental formulations based on B. carinata biomasses. In particular, the formulation with the highest concentration of oil and DSM gave results statistically not different from those of penconazole (Topas). | [166] |
| AITC from six B. juncea (L.) Czern cultivars, two B. rapa cultivars and one B. oleracea, as macerated of frozen plant tissues | Rhizoctonia solani AG1-1A, in vitro assays. Target plant: Oryza sativa | 3 g of macerated frozen tissues, amended or not with 3 different soils, were confined to the lid of an upside-down Petri dish, containing potato dextran agar (PDA) medium with a disc of agar inoculated with R. solani AG1-1A. The dishes were incubated for 72 h at 25 °C. All six B. juncea cultivars consistently inhibited mycelium growth (90% inhibition) in all soils tested; E. sativa which was not considered as a brassicaceous, achieved 60% R. solani AG1-1A inhibition if amended into soils with the lowest levels of pH, organic matter, proteins, calcium and magnesium. | [167] |
| GSLs from above-ground parts of Brassica juncea (L.) Czern. & Coss, ‘Negro Caballo’, Eruca sativa Miller, and Sinapis alba L., ‘Asta’ | Arbuscular mycorrhizal fungi (AMF) colonization, and Fragaria x ananassa Duch. var. ‘Marmolada’ strawberry yield | 91 g of each biofumigant plant per kg of soil were added to AMF inoculated or not inoculated soil, corresponding to 78.9 mg GSL per kg of soil to the B. juncea treatment, 75.2 mg GSL per kg of soil to the E. sativa treatment and 28.6 mg GSL per kg of soil to the S. alba treatment. The soil treatments with biofumigant plants revealed moderate inhibitory effects on strawberry plant AMF colonization, whereas they increased the plant growth and fruit production, especially for the B. juncea and S. alba treatments. Effects of solarization were also investigated. | [168] |
| GSLs from Brassica juncea, Raphanus sativus, and Sinapis alba | Verticillium dahlia in vitro and in soils | Commercial standards of methyl ITC, propenyl AITC, 4-(methylsulfinyl)but-3-enyl4- methyl s u l f i n yl-3-butenyl ITC, benzyl ITC and 2-phenylethyl ITC were tested in vitro against V. dahliain sand at ITC concentrations of 1, 5, 25, 125, 625 nmol g−1 sand. Furthermore, the effect of propenyl ITC with a dose of 150 nmol g−1 on V. dahliae in natural infested soil samples from 22 sites with different crop rotation history and infestation levels was tested. All ITCs tested suppressed microsclerotia of V. dahlia in vitro in sterile sand, and the ITCs containing an aromatic moiety were considerably more toxic than the aliphatic ITCs. In natural soils the ITC toxicities seem negatively correlated to organic carbon content in the soils. In experiments with biomass incorporation in soil, B. juncea reduced the infection significatively (69-80% efficacy), while S. alba and R. sativus gave mortalities between 9-37%. Overall, the study demonstrates that brassicaceous green manures are hardly able to release ITCs at levels necessary for an adequate suppression of V. dahlia microsclerotia in natural soils and because organic matter can reduce the availability of ITCs and their effect. The authors conclude that more promising is the incorporation of high GSL-containing seed meal formulations, which should generate more effective ITC concentrations. | [169] |
| GSL-derived AITC released from B. carinata DSM | Botrytis cinerea, in vitro and in vivo with strawberries as plant host. | In in vitro trial AITC had a fungistatic effect against the pathogen. In in vitro trials two varieties of organic grown strawberries, infected with B. cinerea were exposed for 4 h in an atmosphere enriched either with synthetic AITC or ITC derived from DSM (0.1 mg L−1). The AITC treatment (pure or GSL-derived ITC) reduced the decay caused by the pathogen significantly different from the untreated fruit. Residue analysis performed on fruit at the end of storage showed values lower than 1 mg kg−1. Total phenolic content and antioxidant capacity estimated in treated and untreated strawberries showed no significant difference between control and AITC treated fruit. | [170] |
| Glucosinolate/isothiocyanate | Target: Pseudofungi | Effect | References |
| GSLs from DSMs of Brassica napus, Brassica carinata and Brassica juncea genotypes | Phytophthora cinnamomi, in vitro and in planta on Lupinus luteus | DSMs with high levels of allyl GSL inhibited mycelial growth and effectively inhibited the viability of chlamydospores in treated soils. Roots symptoms were less when plants grew in soils biofumigated with B. carinata and B. juncea DSMs with highest allyl GSL contents in comparison with plants in control soils. In particular B. juncea DSM (3 ÷ 30 µmol allyl GSL per gram of soil) had the largest effect on decreasing root necrosis by P. cinnamomi in Lupinus. | [171] |
| GSL from Brassica carinata pellets (Biofence) | Phytophthora nicotianae in vitro, in vivo with pepper plant as host. | Sensitivity of the vegetative structures of P. nicotianae to Brassica carinata pellets (Biofence) was evaluated in vitro at different doses and temperatures. The effectiveness of the pellets varied depending on the dose. The highest dose of pellets tested (24 mg) was fungitoxic to mycelium regardless of temperature for all the isolates. Moreover, biofumigation was effective in suppressing chlamydospores germination when the pellets were incorporated into the soil (1.5 and 3 g L−1 of soil) under different temperature regimes. In bioassays with pepper plants, both rates of B. carinata pellets (1.5 and 3 g L−1 of soil) reduced populations of P. nicotianae totally controlled the disease after a 4-week biofumigation treatment. | [172] |
| GSLs from above-ground parts of B. napus, B. carinata and B. juncea genotypes at different phenological stages | Phytophthora cinnamomi, in vitro and in planta on Lupinus luteus | Genotypes of Brassica with high levels of allyl GSL inhibited mycelial growth, decreased sporangial production, and effectively inhibited the viability of chlamydospores in soil, but only B. carinata (10 g/75 mL soil) decreased disease symptoms in L. luteus roots. | [173] |
| GSL from B. carinata pellets (Biofence) | Phytophthora cinnamomi in vitro and in vivo on Quercus cerris | Maximum inhibition of vegetative or reproductive structure in vitro occurred at 15 °C and decreased as temperature increased. In vivo assays confirmed efficacy of pellets (3 g L−1) in reducing the pathogen, but a total inhibition was not reached even if at high doses in comparison to the maximum dose tested in vitro assays (0.4 g L−1). | [174] |
| Glucosinolate/isothiocyanate | Target: Insects, acari, and other arthropoda | Effect | References |
| GSL from Brassica juncea granulated seed meal (Kosmalski Herbs & Spices) | Melolontha melolontha grubs | In dose–response experiments the mortality of the grubs at each instar was significantly dependent on the GSL concentration applied with the granulate. The mortality reached 100% in the smallest grubs at 320 µmol L−1, whereas at the same GSL concentration 95% of the bigger grubs (4.5÷7 mm) died. In field tests the mortality was 67.4%. | [175] |
| GSL derived from bio-based experimental formulations con-taining either B. carinata oil 1.5% and B. carinata DSM 3 g L−1 (270 µmol GSL L−1), or 2% B. carinata oil and B. carinata DSM 4.5 g L−1 (405 µmol GSL L−1). | red spider mite Tetranychus urticae on eggplant in open field trials. | The 2-year results indicated that the application of both formulations have a clear effect in containing mites, statistically different from the untreated control. Moreover, the ability of pest control of the formulation with the higher concentrations of oil and DSM was not different from the commercial chemical acaricide (fenazaquin), | [176] |
| GSL from leaf material of purple sprouting broccoli ‘Santee’, Savoy cabbage ‘Wintessa’, and the wild B. oleracea accession Winspit | Folsomia candida (springtail), Eisenia andrei (earthworm) and the soil bacterial community. | Biofumigation experiments were performed using the springtail Folsomia candida and the earthworm Eisenia andrei, each representing a functional soil invertebrate group with important effects on soil processes. Biofumigation was performed using freeze-dried leaves of the three different B. oleracea genotypes: One percent of freeze-dried leaf material relative to total soil (that is about 200 µmol kg−1 soil in GSL for Winspit accession; 125 µmol kg−1 soil in GSL for Santee accession, and 10 µmol kg−1 soil in GSL for Wintessa accession) was used for biofumigation. After 28 days, Winspit (but-3-enyl GSL as dominant GSL) was the genotype displaying highest toxicity to soil invertebrates. Earthworm survival was not affected by the B. oleracea plant material, and overall, the bacterial community was quite resilient to biofumigation. | [177] |
| GSLs derived by chopped fresh plants from Brassica juncea, sel. ISCI 99 and biofumigant meals derived from defatted seeds of Brassica carinata sel. ISCI 7 | Wireworm populations (Agriotes brevis Candeze, Agriotes sordidus Illiger, and Agriotes ustulatus Schäller) was evaluated under both pot assays (on maize and lettuce) and field conditions on maize and potato. | In pot assays a clear rate effect was demonstrated, with sufficient seed meal to supply approximately 160 moles of GSL L−1 of soil resulting in significant wireworm mortality. At field level the protection of maize and potato crops comparable to that provided by Regent®. | [142] |
| Brassicaceae species | Biotechnological approach to enhance GSL content | Results | References |
|---|---|---|---|
| Brassica oleracea var. Italica | Introgression of three QTLs from B. villosa to commercial variety via classical breeding via MAS | Broccoli variety, Beneforté, with high level of ITC | [186] |
| Brassica oleracea var. Italica (broccoli) x Brassica oleracea var. botrytis (cauliflower) Brassica oleracea var. Italica x Brassica oleracea var. Lacinato Brassica oleracea var. Lacinato (collard) x Brassica oleracea var. botrytis | Classical breeding via MAS | RILs with high content of of 4-(methylsulfinyl)butyl and low (R)-2-hydroxybut-3-enyl GSL | [187] |
| Brassica napus | iRNA to target the GSL-ALK gene | B. napus iRNA lines with high content of of 4-(methylsulfinyl)butyl and low (R)-2-hydroxybut-3-enyl GSL | [188] |
| A. thaliana | Overexpression of CYP79D2 and CYP79A1/A2 genes | p35S: CYP79D2 plants with enhanced isopropyl and methylpropyl GSLs and resistant to Erwinia carotovorap35S: CYP79A1/A2 plants were less susceptible to P. syringe but more susceptible to A. brassiciola | [190] |
| A. thaliana | Overexpression of AOP2 gene | Upregulation of aliphatic alkenyl GSL biosynthetic pathway | [192] |
| A. thaliana | Overexpression of CYP79F1 and CYP79F2 genes | Hairy roots overexpressing the transgenes showed an increased aliphatic GSL rate compared to wild type roots but lower than leaf extract | [198] |
| B. rapa x B. oleracea | Conventional breeding via MAS | Cabbage lines with increased GSL content | [200] |
| B. oleracea | Kncock-out of MYB28 gene via CRISPR/Cas9 mediated editing | downregulation of aliphatic GSL biosynthetic genes and reduction in methionine-derived GSL content in myb28 mutant broccoli plants | [201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacifico, D.; Lanzanova, C.; Pagnotta, E.; Bassolino, L.; Mastrangelo, A.M.; Marone, D.; Matteo, R.; Lo Scalzo, R.; Balconi, C. Sustainable Use of Bioactive Compounds from Solanum Tuberosum and Brassicaceae Wastes and by-Products for Crop Protection—A Review. Molecules 2021, 26, 2174. https://doi.org/10.3390/molecules26082174
Pacifico D, Lanzanova C, Pagnotta E, Bassolino L, Mastrangelo AM, Marone D, Matteo R, Lo Scalzo R, Balconi C. Sustainable Use of Bioactive Compounds from Solanum Tuberosum and Brassicaceae Wastes and by-Products for Crop Protection—A Review. Molecules. 2021; 26(8):2174. https://doi.org/10.3390/molecules26082174
Chicago/Turabian StylePacifico, Daniela, Chiara Lanzanova, Eleonora Pagnotta, Laura Bassolino, Anna Maria Mastrangelo, Daniela Marone, Roberto Matteo, Roberto Lo Scalzo, and Carlotta Balconi. 2021. "Sustainable Use of Bioactive Compounds from Solanum Tuberosum and Brassicaceae Wastes and by-Products for Crop Protection—A Review" Molecules 26, no. 8: 2174. https://doi.org/10.3390/molecules26082174








