Comparison of Anticoagulation Quality between Acenocoumarol and Warfarin in Patients with Mechanical Prosthetic Heart Valves: Insights from the Nationwide PLECTRUM Study
Abstract
1. Introduction
2. Results
Anticoagulation Quality According to Treatment
3. Material and Methods
Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
References
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Roumieh, M.; Ius, F.; Tudorache, I.; Ismail, I.; Fleissner, F.; Haverich, A.; Cebotari, S. Comparison between biological and mechanical aortic valve prostheses in middle-aged patients matched through propensity score analysis: Long-term results. Eur. J. Cardio-Thorac. Surg. 2014, 48, 129–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menichelli, D.; Ettorre, E.; Pani, A.; Violi, F.; Pignatelli, P.; Pastori, D. Update and Unmet Needs on the Use of Nonvitamin K Oral Anticoagulants for Stroke Prevention in Patients with Atrial Fibrillation. Curr. Probl. Cardiol. 2021, 46, 100410. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Pignatelli, P.; Malato, A.; Poli, D.; Di Minno, M.N.D.; Di Gennaro, L.; Rancan, E.; Pastori, D.; Grifoni, E.; Squizzato, A.; et al. Incidence of thromboembolic complications in patients with atrial fibrillation or mechanical heart valves with a subtherapeutic international normalized ratio: A prospective multicenter cohort study. Am. J. Hematol. 2012, 87, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Pignatelli, P.; Saliola, M.; Carnevale, R.; Vicario, T.; Del Ben, M.; Cangemi, R.; Barillà, F.; Lip, G.Y.; Violi, F. Inadequate anticoagulation by Vitamin K Antagonists is associated with Major Adverse Cardiovascular Events in patients with atrial fibrillation. Int. J. Cardiol. 2015, 201, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Caravaca, J.M.; Roldán, V.; Esteve-Pastor, M.A.; Valdés, M.; Vicente, V.; Marín, F.; Lip, G.Y. Reduced Time in Therapeutic Range and Higher Mortality in Atrial Fibrillation Patients Taking Acenocoumarol. Clin. Ther. 2018, 40, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Le Heuzey, J.; Ammentorp, B.; Darius, H.; de Caterina, R.; Schilling, R.J.; Schmitt, J.; Zamorano, J.L.; Kirchhof, P. Differences among western European countries in anticoagulation management of atrial fibrillation. Data from the PREFER IN AF registry. Thromb. Haemost. 2014, 111, 833–841. [Google Scholar] [PubMed]
- Van Miert, J.H.A.; Veeger, N.; Ten Cate-Hoek, A.J.; Piersma-Wichers, M.; Meijer, K. Effect of switching from acenocoumarol to phenprocoumon on time in therapeutic range and INR variability: A cohort study. PLoS ONE 2020, 15, e0235639. [Google Scholar]
- Pastori, D.; Lip, G.Y.H.; Poli, D.; Antonucci, E.; Rubino, L.; Menichelli, D.; Saliola, M.; Violi, F.; Palareti, G.; Pignatelli, P.; et al. Determinants of low-quality warfarin anticoagulation in patients with mechanical prosthetic heart valves. The nationwide PLECTRUM study. Br. J. Haematol. 2020, 190, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Antonucci, E.; Pengo, V.; Migliaccio, L.; Testa, S.; Lodigiani, C.; Coffetti, N.; Facchinetti, R.; Serricchio, G.; Falco, P.; et al. Mechanical prosthetic heart valves: Quality of anticoagulation and thromboembolic risk. The observational multicenter PLECTRUM study. Int. J. Cardiol. 2018, 267, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Falk, V.; Baumgartner, H.; Bax, J.J.; de Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef] [PubMed]
- Rosendaal, F.R.; Cannegieter, S.C.; Van Der Meer, F.J.M.; Briët, E. A Method to Determine the Optimal Intensity of Oral Anticoagulant Therapy. Thromb. Haemost. 1993, 69, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Poli, D.; Antonucci, E.; Menichelli, D.; Violi, F.; Palareti, G.; Pignatelli, P.; Testa, S.; Paoletti, O.; Lodigiani, C.; et al. Sex-based difference in anticoagulated patients with mechanical prosthetic heart valves and long-term mortality risk. Int. J. Clin. Pr. 2021, 3, e14064. [Google Scholar] [CrossRef]
- Sawicka-Powierza, J.; Buczkowski, K.; Chlabicz, S.; Gugnowski, Z.; Powierza, K.; Ołtarzewska, A.M. Quality control of oral anticoagulation with vitamin K antagonists in primary care patients in Poland: A multi-centre study. Kardiol. Pol. 2018, 76, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Cieśla-Dul, M.; Żółciński, M.; Tracz, W. Switching from acenocoumarol to warfarin in patients with unstable anticoagulation and its effect on anticoagulation control. Pol. Arch. Intern. Med. 2009, 119, 360–365. [Google Scholar] [CrossRef]
- Gebel, M.; Sahin, K.; Lensing, A.W.A.; Meijer, K.; Kooistra, H.A.M. Independent predictors of poor vitamin K antagonist control in venous thromboembolism patients. Thromb. Haemost. 2015, 114, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.C.; Simon, D.N.; Allen, L.A.; Singer, D.E.; Fonarow, G.C.; Kowey, P.R.; Thomas, L.E.; Ezekowitz, M.D.; Mahaffey, K.W.; Chang, P.; et al. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am. Heart J. 2014, 168, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Marusic, S.; Gojo-Tomic, N.; Franić, M.; Božina, N. Therapeutic efficacy of acenocoumarol in a warfarin-resistant patient with deep venous thrombosis: A case report. Eur. J. Clin. Pharmacol. 2009, 65, 1265–1266. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Parrotto, S.; Vicario, T.; Saliola, M.; Mezzaroma, I.; Violi, F.; Pignatelli, P. Antiphospholipid syndrome and anticoagulation quality: A clinical challenge. Atherosclerosis 2016, 244, 48–50. [Google Scholar] [CrossRef] [PubMed]
- El Rouby, S.; Mestres, C.A.; LaDuca, F.M.; Zucker, M.L. Racial and ethnic differences in warfarin response. J. Heart Valve Dis. 2004, 13, 15–21. [Google Scholar] [PubMed]
| Whole Cohort (n = 2111) | Warfarin (n = 1716) | Acenocoumarol (n = 395) | p-Value | |
|---|---|---|---|---|
| Age (years) | 56.8 ± 12.3 | 56.8 ± 12.3 | 56.9 ± 12.2 | 0.869 |
| Age ≥ 65 years (%) | 29.1 | 28.6 | 31.1 | 0.319 |
| Age ≥ 75 years (%) | 4.0 | 4.0 | 4.1 | 0.978 |
| Women (%) | 44.6 | 44.4 | 45.3 | 0.743 |
| Arterial hypertension (%) | 65.9 | 64.7 | 70.9 | 0.020 |
| Diabetes (%) | 13.5 | 13.2 | 14.7 | 0.445 |
| Heart failure (%) | 14.9 | 14.2 | 18.2 | 0.041 |
| Previous thromboembolism * (%) | 7.8 | 7.5 | 9.4 | 0.203 |
| Previous hemorrhage | 3.8 | 4.2 | 2.3 | 0.074 |
| Previous ischemic heart disease (%) | 12.9 | 12.6 | 14.2 | 0.413 |
| Previous clinical outcomes ^ | 5.8 | 6.1 | 4.3 | 0.163 |
| Peripheral artery disease ** (%) | 9.0 | 8.2 | 12.4 | 0.008 |
| Atrial fibrillation (%) | 38.4 | 38.3 | 39.0 | 0.796 |
| Comorbidities § | 1.4 ± 1.0 | 1.4 ± 1.0 | 1.6 ± 1.1 | 0.004 |
| Comorbidities ≥ 3 | 14.5 | 13.6 | 18.5 | 0.013 |
| Concomitant antiplatelet (%) | 17.3 | 16.6 | 20.5 | 0.061 |
| Amiodarone users (%) | 13.2 | 13.3 | 12.4 | 0.618 |
| MPHV site | 0.379 | |||
| Aortic (%) | 60.7 | 60.6 | 61.0 | |
| Mitral (%) | 28.1 | 28.6 | 26.1 | |
| Mitroaortic (%) | 11.2 | 10.8 | 12.9 | |
| INR ranges | 0.898 | |||
| 2.0–3.0 (%) | 27.2 | 27.4 | 26.3 | |
| 2.5–3.5 (%) | 63.6 | 63.4 | 64.5 | |
| 3.0–4.0 (%) | 9.2 | 9.2 | 9.2 | |
| TiTR (%) | 60.6 ± 19.5 | 61.6 ± 19.4 | 56.1 ± 19.2 | <0.001 |
| Low-quality anticoagulation | ||||
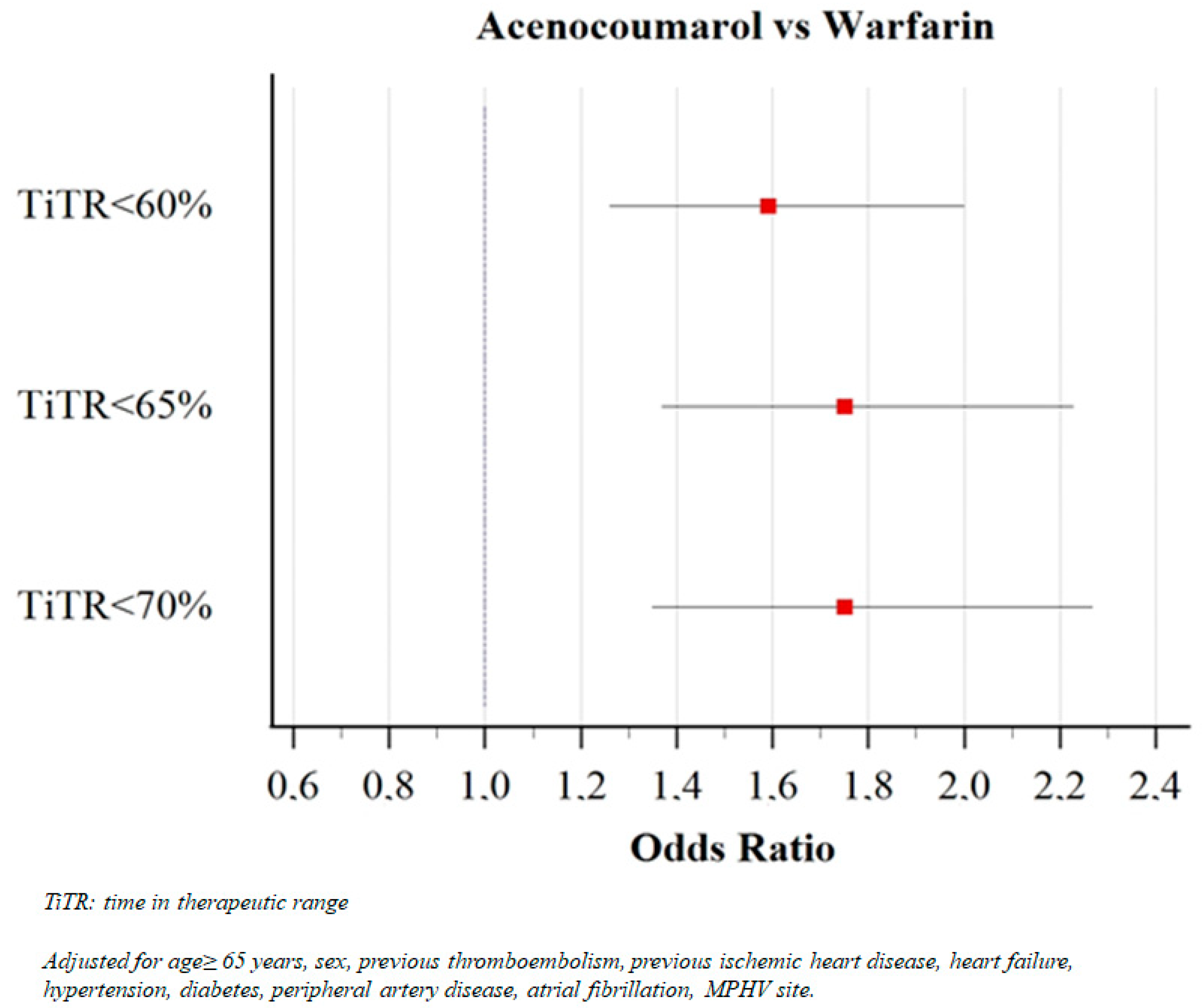
| TiTR < 60% (%) | 48.5 | 46.3 | 58.0 | <0.001 |
| TiTR < 65% (%) | 60.1 | 57.8 | 70.4 | <0.001 |
| TiTR < 70% (%) | 66.9 | 64.8 | 76.2 | <0.001 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Female sex | 0.964 | 0.774–1.201 | 0.743 |
| Age ≥ 65 years | 0.886 | 0.669–1.124 | 0.319 |
| Atrial fibrillation | 1.030 | 0.823–1.289 | 0.796 |
| Hypertension | 1.326 | 1.044–1.683 | 0.021 |
| Diabetes | 1.129 | 0.827–1.542 | 0.446 |
| PAD | 1.594 | 1.128–2.252 | 0.008 |
| Heart failure | 1.351 | 1.012–1.804 | 0.041 |
| Previous TE | 1.282 | 0.874–1.881 | 0.204 |
| Previous ischemic heart disease | 1.141 | 0.831–1.566 | 0.414 |
| Previous hemorrhage | 0.532 | 0.264–1.074 | 0.078 |
| Comorbidities ≥ 3 | 1.443 | 1.081–1.927 | 0.013 |
| Previous clinical outcomes | 0.690 | 0.408–1.166 | 0.166 |
| Mitral vs. Aortic | 0.907 | 0.703–1.170 | 0.453 |
| Mitroaortic vs. Aortic | 1.183 | 0.842–1.662 | 0.332 |
| Concomitant antiplatelet | 1.301 | 0.988–1.713 | 0.061 |
| Amiodarone | 0.920 | 0.661–1.279 | 0.619 |
| OAC Type | Mean TiTR | p | TiTR < 60% (%) | p | TiTR < 65% (%) | p | TiTR < 70% (%) | p | |
|---|---|---|---|---|---|---|---|---|---|
| Women | Warfarin | 58.9 ± 19.1 | <0.001 | 52.4 | 0.001 | 63.4 | <0.001 | 70.6 | 0.003 |
| Acenocoumarol | 51.9 ± 18.6 | 65.9 | 77.7 | 81.6 | |||||
| Men | Warfarin | 63.8 ± 19.4 | 0.004 | 41.5 | 0.008 | 53.2 | 0.003 | 60.2 | 0.002 |
| Acenocoumarol | 59.6 ± 19.0 | 51.4 | 64.4 | 71.8 | |||||
| Arterial hypertension | Warfarin | 60.6 ± 19.8 | <0.001 | 49.7 | <0.001 | 60.6 | <0.001 | 67.2 | <0.001 |
| Acenocoumarol | 54.8 ± 19.1 | 62.9 | 73.9 | 79.3 | |||||
| Diabetes | Warfarin | 57.8 ± 19.3 | 0.019 | 56.8 | 0.016 | 66.1 | 0.052 | 70.5 | 0.060 |
| Acenocoumarol | 51.1 ± 19.9 | 74.1 | 79.3 | 82.8 | |||||
| Age (≥65 years) | Warfarin | 60.2 ± 18.9 | 0.001 | 48.9 | 0.006 | 61.3 | 0.001 | 68.2 | 0.004 |
| Acenocoumarol | 53.6 ± 18.4 | 62.6 | 77.2 | 81.3 | |||||
| Aortic MPHV | Warfarin | 65.0 ± 19.3 | <0.001 | 37.8 | <0.001 | 49.6 | <0.001 | 57.2 | <0.001 |
| Acenocoumarol | 59.5 ± 18.9 | 50.2 | 63.9 | 69.7 | |||||
| Mitral/Mitroaortic MPHV | Warfarin | 56.4 ± 18.5 | 0.001 | 59.5 | 0.014 | 70.3 | 0.010 | 76.5 | 0.007 |
| Acenocoumarol | 50.8 ± 18.4 | 70.1 | 80.5 | 86.4 | |||||
| INR range 2.0–3.0 | Warfarin | 70.3 ± 19,0 | 0.026 | 26.5 | 0.125 | 37.4 | 0.014 | 43.6 | 0.012 |
| Acenocoumarol | 65.7 ± 19.1 | 34.0 | 50.5 | 57.3 | |||||
| INR range above 2.0–3.0 | Warfarin | 58.4 ± 18.6 | <0.001 | 53.8 | <0.001 | 65.4 | <0.001 | 72.8 | <0.001 |
| Acenocoumarol | 52.7 ± 18.1 | 66.4 | 77.4 | 82.9 | |||||
| Atrial fibrillation | Warfarin | 58.5 ± 19.4 | 0.002 | 41.9 | 0.002 | 53.4 | <0.001 | 61.1 | 0.001 |
| Acenocoumarol | 53.1 ± 19.0 | 53.1 | 67.2 | 72.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menichelli, D.; Poli, D.; Antonucci, E.; Cammisotto, V.; Testa, S.; Pignatelli, P.; Palareti, G.; Pastori, D.; the Italian Federation of Anticoagulation Clinics (FCSA). Comparison of Anticoagulation Quality between Acenocoumarol and Warfarin in Patients with Mechanical Prosthetic Heart Valves: Insights from the Nationwide PLECTRUM Study. Molecules 2021, 26, 1425. https://doi.org/10.3390/molecules26051425
Menichelli D, Poli D, Antonucci E, Cammisotto V, Testa S, Pignatelli P, Palareti G, Pastori D, the Italian Federation of Anticoagulation Clinics (FCSA). Comparison of Anticoagulation Quality between Acenocoumarol and Warfarin in Patients with Mechanical Prosthetic Heart Valves: Insights from the Nationwide PLECTRUM Study. Molecules. 2021; 26(5):1425. https://doi.org/10.3390/molecules26051425
Chicago/Turabian StyleMenichelli, Danilo, Daniela Poli, Emilia Antonucci, Vittoria Cammisotto, Sophie Testa, Pasquale Pignatelli, Gualtiero Palareti, Daniele Pastori, and the Italian Federation of Anticoagulation Clinics (FCSA). 2021. "Comparison of Anticoagulation Quality between Acenocoumarol and Warfarin in Patients with Mechanical Prosthetic Heart Valves: Insights from the Nationwide PLECTRUM Study" Molecules 26, no. 5: 1425. https://doi.org/10.3390/molecules26051425
APA StyleMenichelli, D., Poli, D., Antonucci, E., Cammisotto, V., Testa, S., Pignatelli, P., Palareti, G., Pastori, D., & the Italian Federation of Anticoagulation Clinics (FCSA). (2021). Comparison of Anticoagulation Quality between Acenocoumarol and Warfarin in Patients with Mechanical Prosthetic Heart Valves: Insights from the Nationwide PLECTRUM Study. Molecules, 26(5), 1425. https://doi.org/10.3390/molecules26051425







