Type 2 Diabetes Alters Vascular Cannabinoid Receptor 1 Expression, Phosphorylation Status, and Vasorelaxation in Rat Aorta
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Serum Glucose
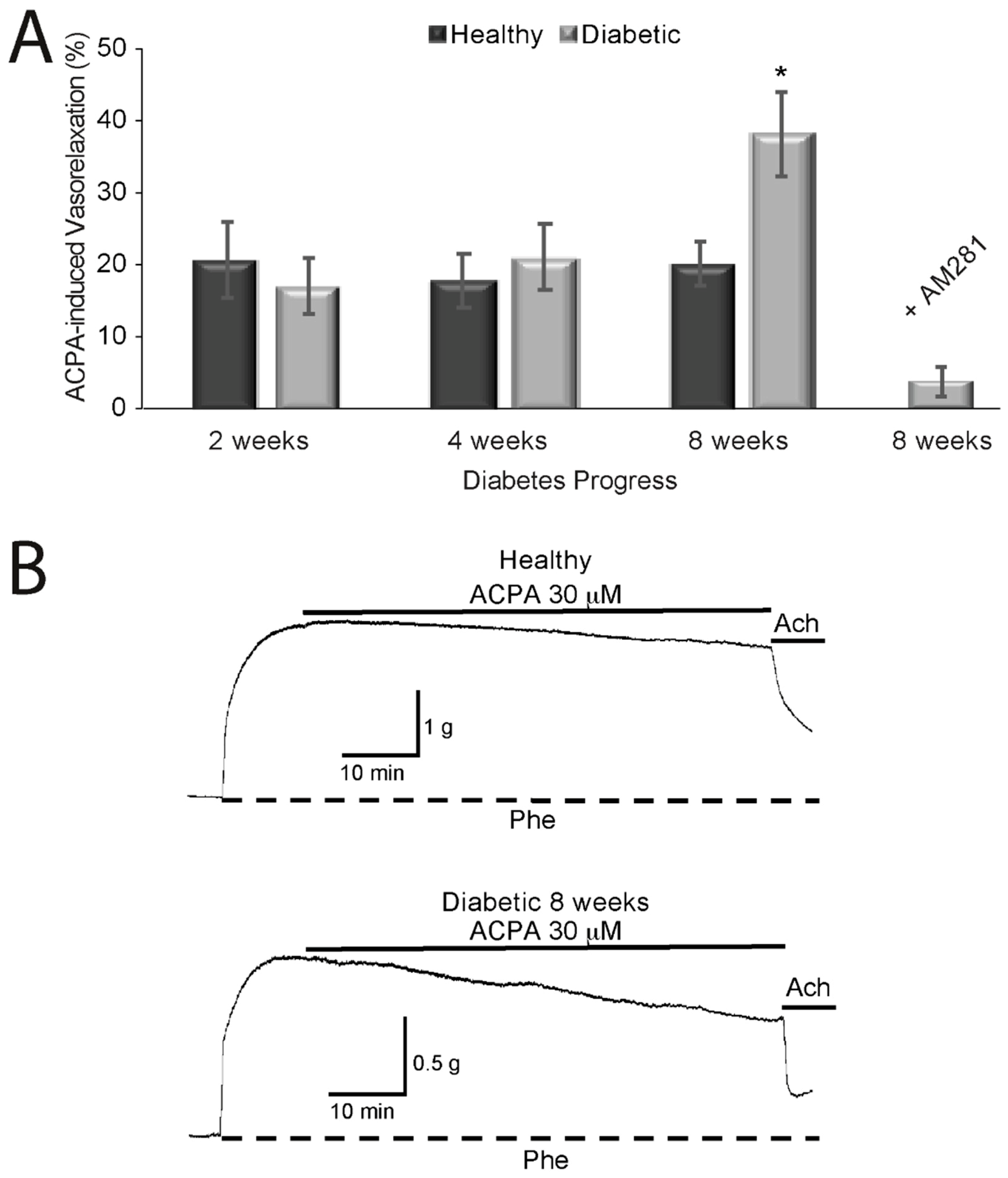
2.2. Effects of Cannabinoids on Vasorelaxation of Aortic Rings from Diabetic and Healthy Rats
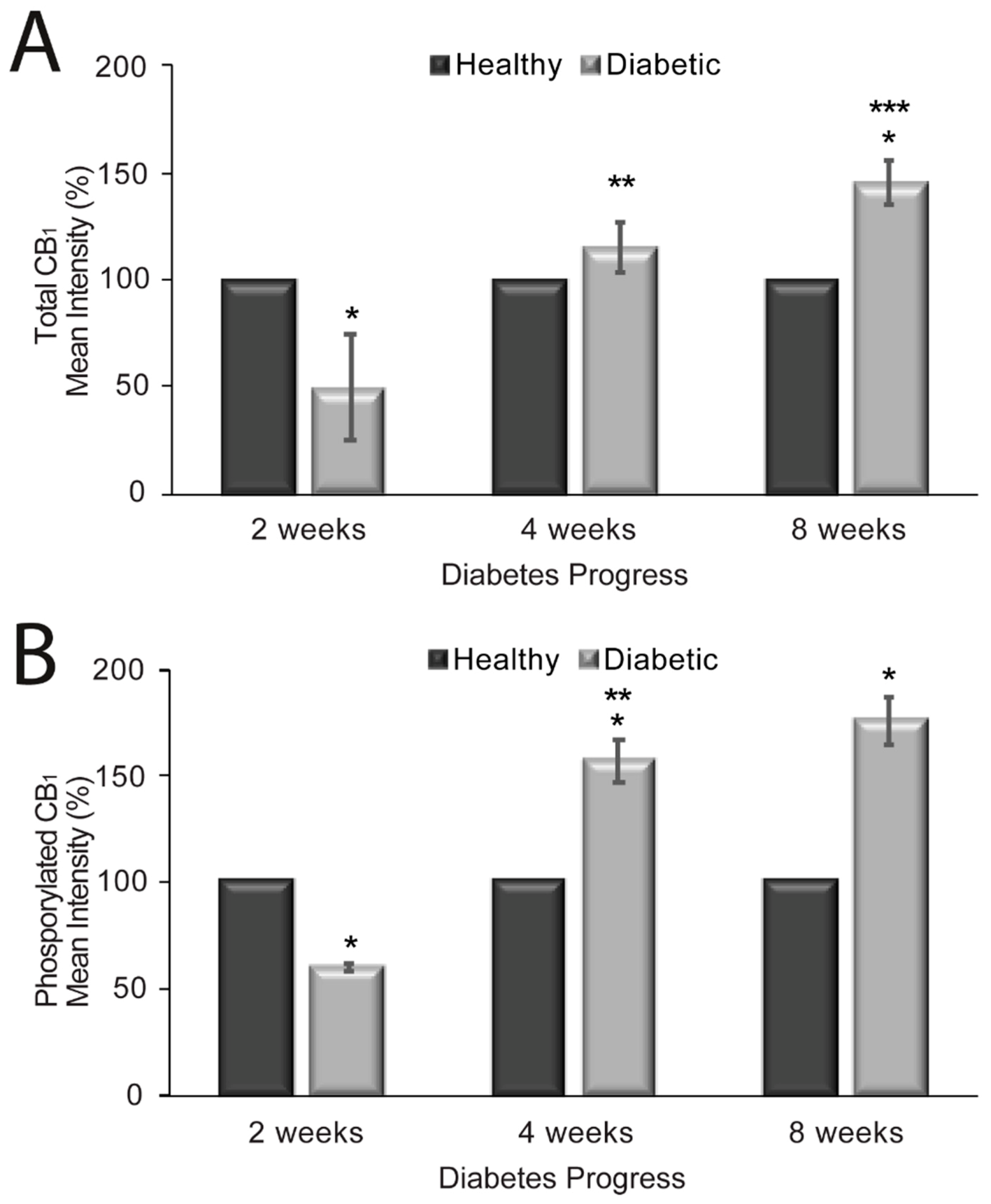
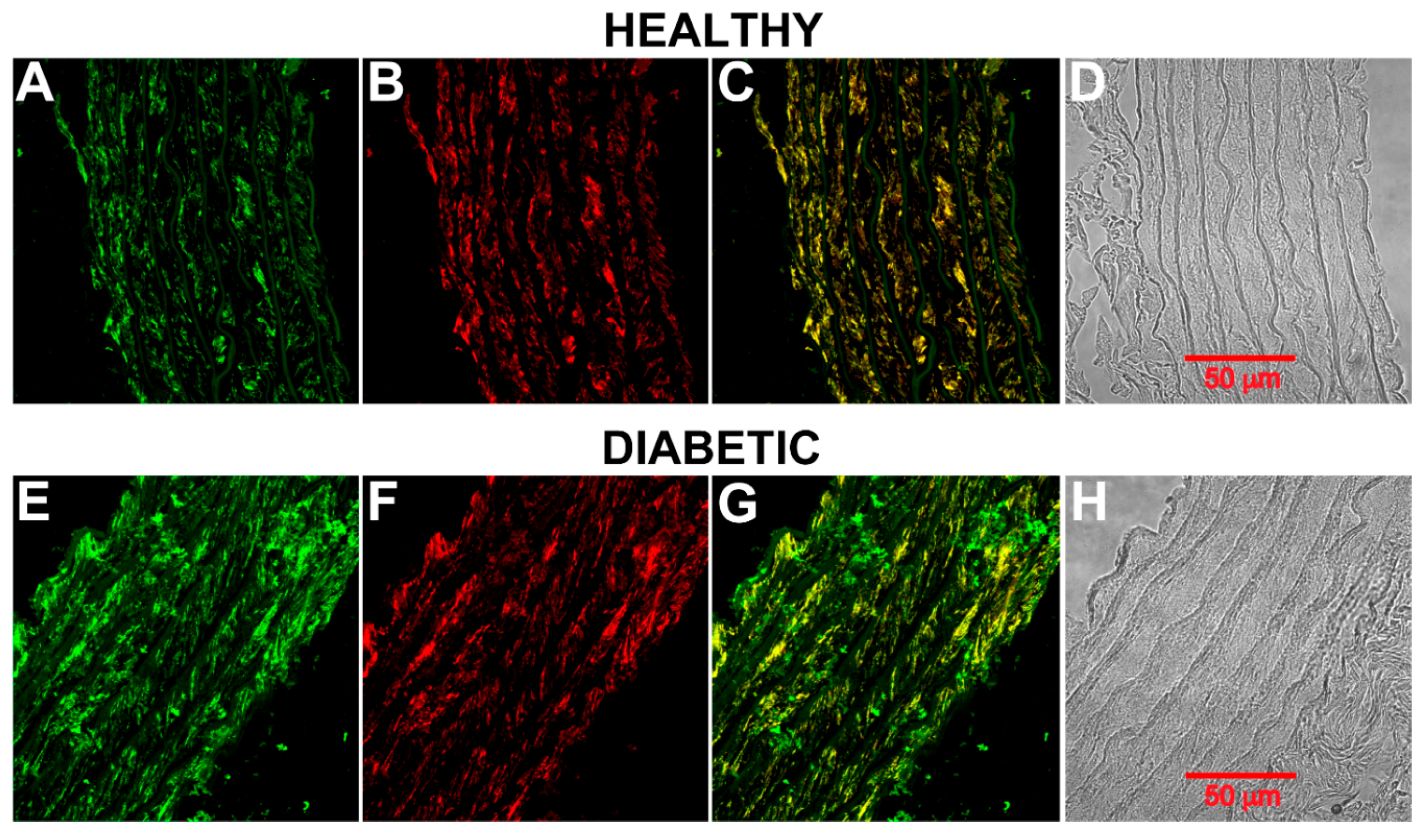
2.3. CB1 Receptor Expression, Phosphorylation Status and Localization in Diabetic Rat Aorta
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Induction of Diabetes
4.3. Cannabinoid Administration
4.4. Glucose Measurements
4.5. Removal and Preparation of Aortas
4.6. Aortic Ring Tension Recordings
4.7. Immunohistochemical Analysis of Aortic Rings
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Cristino, L.; Becker, T.; Di Marzo, V. Endocannabinoids and energy homeostasis: An update. BioFactors 2014, 40, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Vilches-Flores, A.; Delgado-Buenrostro, N.L.; Navarrete-Vázquez, G.; Villalobos-Molina, R. CB1 cannabinoid receptor expression is regulated by glucose and feeding in rat pancreatic islets. Regul. Pept. 2010, 163, 81–87. [Google Scholar] [CrossRef]
- Sidibeh, C.O.; Pereira, M.J.; Lau Börjesson, J.; Kamble, P.G.; Skrtic, S.; Katsogiannos, P.; Sundbom, M.; Svensson, M.K.; Eriksson, J.W. Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance. Endocrine 2017, 55, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, K.A.; Mcainch, A.J.; Zhang, Y.; Kelly, D.J.; Hryciw, D.H. Elevated cannabinoid receptor 1 and G protein-coupled receptor 55 expression in proximal tubule cells and whole kidney exposed to diabetic conditions. Clin. Exp. Pharmacol. Physiol. 2015, 42, 256–262. [Google Scholar] [CrossRef]
- Díaz-Asensio, C.; Setién, R.; Echevarría, E.; Casis, L.; Casis, E.; Garrido, A.; Casis, O. Type 1 diabetes alters brain cannabinoid receptor expression and phosphorylation status in rats. Horm. Metab. Res. 2008, 40, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Dannert, M.T.; Alsasua, A.; Herradon, E.; Martín, M.I.; López-Miranda, V. Vasorelaxant effect of Win 55,212-2 in rat aorta: New mechanisms involved. Vascul. Pharmacol. 2007, 46, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pastor, E.; Andrade, F.; Sánchez-Pastor, J.M.; Elizalde, A.; Huerta, M.; Virgen-Ortiz, A.; Trujillo, X.; Rodríguez-Hernández, A. Cannabinoid receptor type 1 activation by arachidonylcyclopropylamide in rat aortic rings causes vasorelaxation involving calcium-activated potassium channel subunit alpha-1 and calcium channel, voltage-dependent, L type, alpha 1C subunit. Eur. J. Pharmacol. 2014, 729, 100–106. [Google Scholar] [CrossRef]
- Hillard, C.J. Endocannabinoids and vascular function. J. Pharmacol. Exp. Ther. 2000, 294, 27–32. [Google Scholar] [PubMed]
- Randall, M.D.; Kendall, D.A.; O’Sullivan, S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004, 142, 20–26. [Google Scholar] [CrossRef]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the endocannabinoid system in diabetes and diabetic complications. Br. J. Pharmacol. 2016, 173, 1116–1127. [Google Scholar] [CrossRef]
- Horvth, B.; Mukhopadhyay, P.; Hask, G.; Pacher, P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am. J. Pathol. 2012, 180, 432–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altinok, A.; Coşkun, Z.M.; Karaoʇlu, K.; Bolkent, S.; Akkan, A.G.; Özyazgan, S. Δ9-tetrahydrocannabinol treatment improved endothelium-dependent relaxation on streptozotocin/nicotinamide-induced diabetic rat aorta. Acta Physiol. Hung. 2015, 102, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bátkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kaminski, N.E.; Wang, D.H. VR1-mediated depressor effects during high-salt intake: Role of anandamide. Hypertension 2005, 46, 986–991. [Google Scholar] [CrossRef] [Green Version]
- Stanley, C.P.; Wheal, A.J.; Randall, M.D.; O’Sullivan, S.E. Cannabinoids alter endothelial function in the Zucker rat model of type 2 diabetes. Eur. J. Pharmacol. 2013, 720, 376–382. [Google Scholar] [CrossRef]
- Li, J.; Kaminski, N.E.; Wang, D.H. Anandamide-induced depressor effect in spontaneously hypertensive rats: Role of the vanilloid receptor. Hypertension 2003, 41, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Wheal, A.J.; Bennett, T.; Randall, M.D.; Gardiner, S.M. Cardiovascular effects of cannabinoids in conscious spontaneously hypertensive rats. Br. J. Pharmacol. 2007, 152, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Wheal, A.J.; Randall, M.D. Effects of hypertension on vasorelaxation to endocannabinoids in vitro. Eur. J. Pharmacol. 2009, 603, 79–85. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.X.; Yuan, F.; Ma, H.J.; Maslov, L.; Zhang, Y. Enhanced vasorelaxation effect of endogenous anandamide on thoracic aorta in renal vascular hypertension rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 950–955. [Google Scholar] [CrossRef]
- Matias, I.; Gonthier, M.P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Petrosino, S.; Hoareau, L.; Festy, F.; Pasquali, R.; et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and β-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef]
- Hopps, J.J.; Dunn, W.R.; Randall, M.D. Enhanced vasorelaxant effects of the endocannabinoid-like mediator, oleamide, in hypertension. Eur. J. Pharmacol. 2012, 684, 102–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, L.I.F.; Lemos, C.; Ledent, C.; Carvalho, E.; Köfalvi, A. Chronic insulinopenia/hyperglycemia decreases cannabinoid CB 1 receptor density and impairs glucose uptake in the mouse forebrain. Brain Res. Bull. 2019, 47, 101–109. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Rajesh, M.; Mukhopadhyay, P.; Horváth, B.; Patel, V.; Al-Gayyar, M.M.H.; Pillai, B.A.; Pacher, P. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia. 2011, 54, 1567–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gestrich, C.; Duerr, G.D.; Heinemann, J.C.; Meertz, A.; Probst, C.; Roell, W.; Schiller, W.; Zimmer, A.; Bindila, L.; Lutz, B.; et al. Activation of endocannabinoid system is associated with persistent inflammation in human aortic aneurysm. BioMed Res. Int. 2015, 2015, 456582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; MacKie, K.; Pacher, P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010, 160, 688–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillamat-Prats, R.; Rami, M.; Herzig, S.; Steffens, S. Endocannabinoid signalling in atherosclerosis and related metabolic complications. Thromb. Haemost. 2019, 119, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.E.; Brown, S.; Hille, B.; Mackie, K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J. Neurosci. 1998. [Google Scholar] [CrossRef] [Green Version]
- Saleh, D.O.; Bayoumi, A.R.; El-Eraky, W.I.; El-Khatib, A.S. Streptozotocin-induced vascular and biochemical changes in rats: Effects of rosiglitazone vs. metformin. Bull. Fac. Pharm. 2013, 51, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Bucci, M.; Roviezzo, F.; Brancaleone, V.; Lin, M.I.; Di Lorenzo, A.; Cicala, C.; Pinto, A.; Sessa, W.C.; Farneti, S.; Fiorucci, S.; et al. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Vella, R.K.; Jackson, D.J.; Fenning, A.S. Δ9-tetrahydrocannabinol prevents cardiovascular dysfunction in STZ-diabetic Wistar-Kyoto rats. BioMed Res. Int. 2017, 2027, 7974149. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, A.; Shin, J.; Jung, M.H. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int. J. Mol. Sci. 2019, 20, 2109. [Google Scholar] [CrossRef] [Green Version]
- Barutta, F.; Grimaldi, S.; Gambino, R.; Vemuri, K.; Makriyannis, A.; Annaratone, L.; Di Marzo, V.; Bruno, G.; Gruden, G. Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy. Nephrol. Dial. Transplant. 2017, 32, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ibarra, A.; Huerta, M.; Villalpando-Hernández, S.; Ríos-Silva, M.; Díz-Reval, M.I.; Cruzblanca, H.; Mancilla, E.; Trujillo, X. The effects of dietary iron and capsaicin on hemoglobin, blood glucose, insulin tolerance, cholesterol, and triglycerides, in Healthy and diabetic Wistar rats. PLoS ONE 2016, 1, e0152625. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.R.; Bauermann, L.; Morsch, A.L.B.; Zanin, R.F.; Corrêa, M.; Silva, A.C.; Mazzanti, C.; Morsch, V.M.; Lunkes, G.I.; Schetinger, M.R.C. Enhanced NTPDase and 5′-nucleotidase activities in diabetes mellitus and iron-overload model. Mol. Cell. Biochem. 2007, 298, 101–107. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
| 2 Weeks | 4 Weeks | 8 Weeks | ||||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| Body Weight (g) | ||||||
| Healthy | 279 ± 3.84 | 322 ± 8.23 * | 319 ± 14.4 | 338 ± 4.85 | 321 ± 6.69 | 342 ± 8.71 * |
| Diabetes | 235 ± 9.3 | 263 ± 10.8 * | 280 ± 8.16 | 243 ± 9.9 | 308 ± 13.33 | 275 ± 14.65 |
| Fasting Glucose (mg/dL) | ||||||
| Healthy | 81 ± 1.78 | 85 ± 2.56 | 76.80 ± 2.2 | 78 ± 2.21 | 83 ± 4.49 | 77 ± 1.5 |
| Diabetes | 319 ± 19.28 | 405 ± 19.83 * | 288 ± 18.46 | 478 ± 34.7 * | 310 ± 13.13 | 441 ± 13.71 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Pastor, E.; Trujillo, X.; Ramos-Flores, C.; Ríos-Silva, M.; Andrade, F.; Cárdenas, Y.; Castro, E.; Urzúa, Z.; Newton-Sánchez, O.; Huerta, M. Type 2 Diabetes Alters Vascular Cannabinoid Receptor 1 Expression, Phosphorylation Status, and Vasorelaxation in Rat Aorta. Molecules 2020, 25, 4948. https://doi.org/10.3390/molecules25214948
Sánchez-Pastor E, Trujillo X, Ramos-Flores C, Ríos-Silva M, Andrade F, Cárdenas Y, Castro E, Urzúa Z, Newton-Sánchez O, Huerta M. Type 2 Diabetes Alters Vascular Cannabinoid Receptor 1 Expression, Phosphorylation Status, and Vasorelaxation in Rat Aorta. Molecules. 2020; 25(21):4948. https://doi.org/10.3390/molecules25214948
Chicago/Turabian StyleSánchez-Pastor, Enrique, Xóchitl Trujillo, Christian Ramos-Flores, Mónica Ríos-Silva, Felipa Andrade, Yolitzy Cárdenas, Elena Castro, Zorayda Urzúa, Oscar Newton-Sánchez, and Miguel Huerta. 2020. "Type 2 Diabetes Alters Vascular Cannabinoid Receptor 1 Expression, Phosphorylation Status, and Vasorelaxation in Rat Aorta" Molecules 25, no. 21: 4948. https://doi.org/10.3390/molecules25214948






