Detection of Beta-Glucan Contamination in Nanotechnology-Based Formulations
Abstract
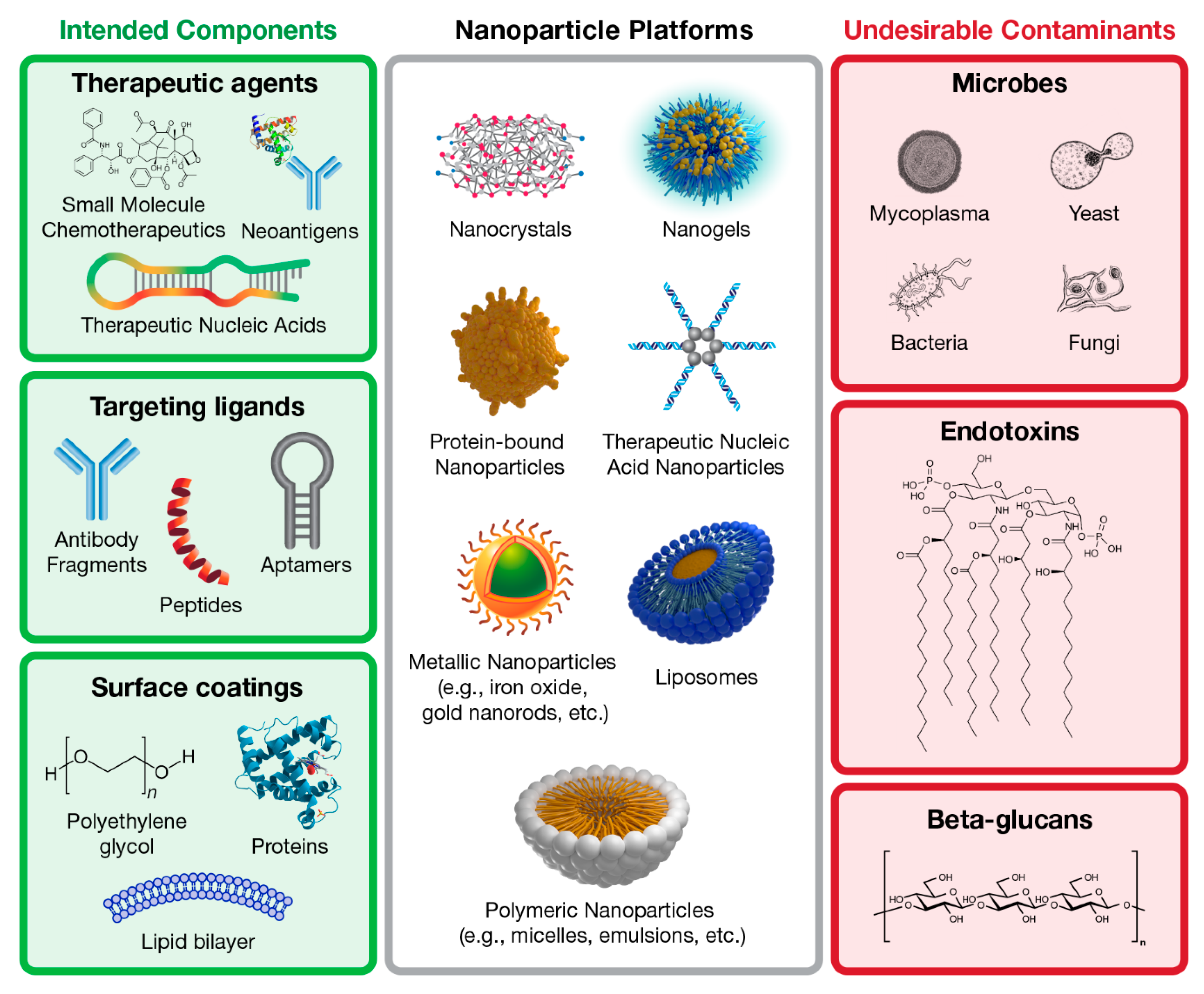
1. Introduction
2. Overview of Beta-Glucans
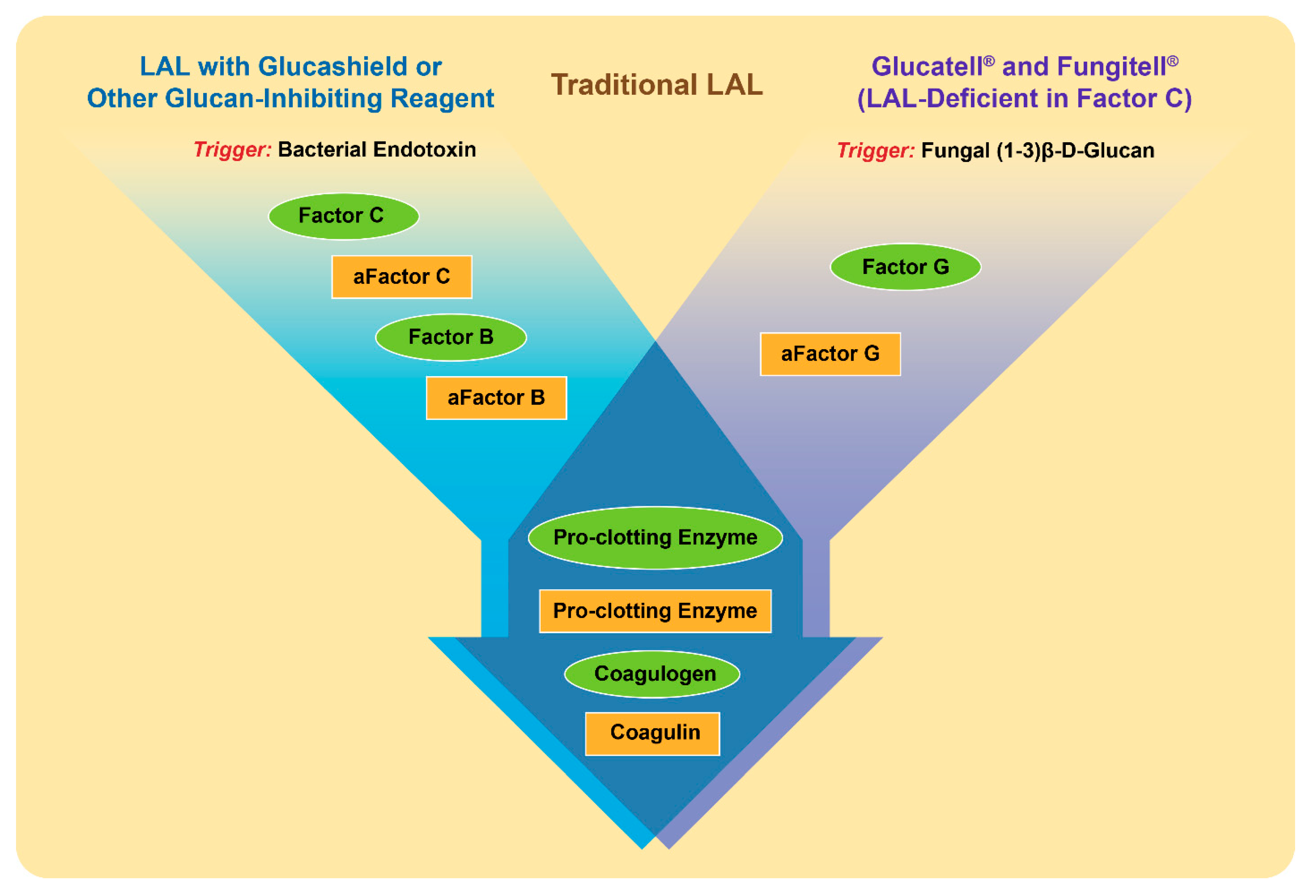
3. Detection of Beta-Glucans
4. Data Interpretation
5. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Haile, L.A.; Puig, M.; Kelley-Baker, L.; Verthelyi, D. Detection of innate immune response modulating impurities in therapeutic proteins. PLoS ONE 2015, 10, e0125078. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Endotoxin and Nanomaterials. In Immunological Properties of Engineered Nanomaterials; Dobrovolskaia, M.A., McNeil, S.E., Eds.; World Scientific Publishing Ltd.: Singapoore, 2016; Volume 1, pp. 143–186. [Google Scholar]
- Das, A.P.; Kumar, P.S.; Swain, S. Recent advances in biosensor based endotoxin detection. Biosens. Bioelectron. 2014, 51, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Dullah, E.C.; Ongkudon, C.M. Current trends in endotoxin detection and analysis of endotoxin-protein interactions. Crit. Rev. Biotechnol. 2017, 37, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.; Lang, P.; Grallert, H.; Motschmann, H. Masking of endotoxin in surfactant samples: Effects on Limulus-based detection systems. Biologicals 2016, 44, 417–422. [Google Scholar] [CrossRef]
- Sharma, S.K. Endotoxin detection and elimination in biotechnology. Biotechnol. Appl. Biochem. 1986, 8, 5–22. [Google Scholar]
- Su, W.; Ding, X. Methods of Endotoxin Detection. J. Lab. Autom. 2015, 20, 354–364. [Google Scholar] [CrossRef]
- US FDA. Guidance for Industry: Pyrogen and Endotoxins Testing: Questions and Answers; US FDA: Montgomery, MD, USA, 2012.
- US-Pharmacopoeia. Bacterial Endotoxins; US-Pharmacopoeia: Washington, WA, USA, 2017. [Google Scholar]
- International Standards Organization. ISO 29701:2010. Nanotechnologies—Endotoxin Test on Nanomaterial Samples for In Vitro Systems—Limulus Amebocyte Lysate (LAL) Test. 2010. Available online: https://www.iso.org/obp/ui/#iso:std:iso:29701:ed-1:v1:en (accessed on 23 July 2020).
- Kuriakose, A.; Chirmule, N.; Nair, P. Immunogenicity of Biotherapeutics: Causes and Association with Posttranslational Modifications. J. Immunol. Res. 2016, 2016, 1298473. [Google Scholar] [CrossRef]
- Fisher, A.C.; Lee, S.L.; Harris, D.P.; Buhse, L.; Kozlowski, S.; Yu, L.; Kopcha, M.; Woodcock, J. Advancing pharmaceutical quality: An overview of science and research in the U.S. FDA’s Office of Pharmaceutical Quality. Int. J. Pharm. 2016, 515, 390–402. [Google Scholar] [CrossRef]
- US FDA. Considerations in Demonstrating Interchangeability with A Reference Product; US FDA: Montgomery, MD, USA, 2019.
- Han, B.; Baruah, K.; Nguyen, D.V.; Williams, D.L.; Devriendt, B.; Cox, E.; Bossier, P. Beta-glucan’s varying structure characteristics modulate survival and immune-related genes expression from Vibrio harveyi-infected Artemia franciscana in gnotobiotic conditions. Fish Shellfish Immunol. 2020, 102, 307–315. [Google Scholar] [CrossRef]
- Odabasi, Z.; Mattiuzzi, G.; Estey, E.; Kantarjian, H.; Saeki, F.; Ridge, R.J.; Ketchum, P.A.; Finkelman, M.A.; Rex, J.H.; Ostrosky-Zeichner, L. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: Validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 2004, 39, 199–205. [Google Scholar] [CrossRef]
- Zeković, D.B.; Kwiatkowski, S.; Vrvić, M.M.; Jakovljević, D.; Moran, C.A. Natural and modified (1-->3)-beta-d-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.W.; Lindermuth, J.; Fish, P.A.; Palace, G.P.; Stevenson, T.T.; DeMong, D.E. A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998, 273, 22014–22020. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Passarelli, V.; Evangelista, V.; Frassanito, A.M.; Gualtieri, P. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat. Prod. Rep. 2011, 28, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.; Wouters, I.M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate. Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef]
- Akhapkina, I.G.; Antropova, A.B.; Akhmatov, E.A.; Zheltikova, T.M. Effects of the Linear Fragments of Beta-(1→3)-Glucans on Cytokine Production in vitro. Biochemistry (Mosc) 2018, 83, 1002–1006. [Google Scholar] [CrossRef]
- Mourits, V.P.; Arts, R.J.W.; Novakovic, B.; Matzaraki, V.; de Bree, L.C.J.; Koeken, V.; Moorlag, S.; van Puffelen, J.H.; Groh, L.; van der Heijden, C.; et al. The role of Toll-like receptor 10 in modulation of trained immunity. Immunology 2020, 159, 289–297. [Google Scholar] [CrossRef]
- Rubin-Bejerano, I.; Abeijon, C.; Magnelli, P.; Grisafi, P.; Fink, G.R. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe 2007, 2, 55–67. [Google Scholar] [CrossRef]
- Sonck, E.; Stuyven, E.; Goddeeris, B.; Cox, E. The effect of beta-glucans on porcine leukocytes. Vet. Immunol. Immunopathol. 2010, 135, 199–207. [Google Scholar] [CrossRef]
- Higashi, T.; Hashimoto, K.; Takagi, R.; Mizuno, Y.; Okazaki, Y.; Tanaka, Y.; Matsushita, S. Curdlan induces DC-mediated Th17 polarization via Jagged1 activation in human dendritic cells. Allergol. Int. 2010, 59, 161–166. [Google Scholar] [CrossRef]
- Baram, L.; Cohen-Kedar, S.; Spektor, L.; Elad, H.; Guzner-Gur, H.; Dotan, I. Differential stimulation of peripheral blood mononuclear cells in Crohn’s disease by fungal glycans. J. Gastroenterol. Hepatol. 2014, 29, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 2010, 1, e00164-10. [Google Scholar] [CrossRef] [PubMed]
- Ara, Y.; Saito, T.; Takagi, T.; Hagiwara, E.; Miyagi, Y.; Sugiyama, M.; Kawamoto, S.; Ishii, N.; Yoshida, T.; Hanashi, D.; et al. Zymosan enhances the immune response to DNA vaccine for human immunodeficiency virus type-1 through the activation of complement system. Immunology 2001, 103, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Bønløkke, J.H.; Stridh, G.; Sigsgaard, T.; Kjaergaard, S.K.; Löfstedt, H.; Andersson, K.; Bonefeld-Jørgensen, E.C.; Jayatissa, M.N.; Bodin, L.; Juto, J.E.; et al. Upper-airway inflammation in relation to dust spiked with aldehydes or glucan. Scand. J. Work Environ. Health 2006, 32, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Javmen, A.; Nemeikaitė-Čėnienė, A.; Bratchikov, M.; Grigiškis, S.; Grigas, F.; Jonauskienė, I.; Zabulytė, D.; Mauricas, M. β-Glucan from Saccharomyces cerevisiae Induces IFN-γ Production In Vivo in BALB/c Mice. In Vivo 2015, 29, 359–363. [Google Scholar] [PubMed]
- Johnson, E.; Førland, D.T.; Saetre, L.; Bernardshaw, S.V.; Lyberg, T.; Hetland, G. Effect of an extract based on the medicinal mushroom Agaricus blazei murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scand. J. Immunol. 2009, 69, 242–250. [Google Scholar] [CrossRef]
- Barton, C.; Vigor, K.; Scott, R.; Jones, P.; Lentfer, H.; Bax, H.J.; Josephs, D.H.; Karagiannis, S.N.; Spicer, J.F. Beta-glucan contamination of pharmaceutical products: How much should we accept? Cancer immunol. Immunother. CII 2016, 65, 1289–1301. [Google Scholar] [CrossRef]
- Ławniczek-Wałczyk, A.; Górny, R.L. Endotoxins and β-glucans as markers of microbiological contamination--characteristics, detection, and environmental exposure. Ann. Agric. Environ. Med. 2010, 17, 193–208. [Google Scholar]
- Liss, B.; Cornely, O.A.; Hoffmann, D.; Dimitriou, V.; Wisplinghoff, H. 1,3-β-D-Glucan contamination of common antimicrobials. J. Antimicrob. Chemother. 2016, 71, 913–915. [Google Scholar] [CrossRef]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Luther, K.; Torosantucci, A.; Brakhage, A.A.; Heesemann, J.; Ebel, F. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell. Microbiol. 2007, 9, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Kirschning, C.J.; Nikolaus, T.; Wagner, H.; Heesemann, J.; Ebel, F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell. Microbiol. 2003, 5, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef]
- Steele, C.; Rapaka, R.R.; Metz, A.; Pop, S.M.; Williams, D.L.; Gordon, S.; Kolls, J.K.; Brown, G.D. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 2005, 1, e42. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Pang, L.; Yao, L.; Shang, Z.; Pan, Y. Agaricus bisporus-derived β-glucan enter macrophages and adipocytes by CD36 receptor. Nat. Prod. Res. 2019, 1–4. [Google Scholar] [CrossRef]
- Rice, P.J.; Kelley, J.L.; Kogan, G.; Ensley, H.E.; Kalbfleisch, J.H.; Browder, I.W.; Williams, D.L. Human monocyte scavenger receptors are pattern recognition receptors for (1-->3)-beta-D-glucans. J. Leukoc. Biol. 2002, 72, 140–146. [Google Scholar]
- Czop, J.K.; Austen, K.F. Generation of leukotrienes by human monocytes upon stimulation of their beta-glucan receptor during phagocytosis. Proc. Natl. Acad. Sci. USA 1985, 82, 2751–2755. [Google Scholar] [CrossRef]
- Ross, G.D.; Cain, J.A.; Myones, B.L.; Newman, S.L.; Lachmann, P.J. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement 1987, 4, 61–74. [Google Scholar] [CrossRef]
- Thornton, B.P.; Vĕtvicka, V.; Pitman, M.; Goldman, R.C.; Ross, G.D. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. (Baltimore Md. 1950) 1996, 156, 1235–1246. [Google Scholar]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2020, e1901071. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, L.; Milling, S. Recovery training or over-training? The contribution of TLR10 to monocyte fitness. Immunology 2020, 159, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Associates of Cape Cod. Fungitell: Product Brochure; Associates of Cape Cod: East Falmouth, MA, USA, 2017; pp. 1–7. [Google Scholar]
- Ellis, M.; Al-Ramadi, B.; Finkelman, M.; Hedstrom, U.; Kristensen, J.; Ali-Zadeh, H.; Klingspor, L. Assessment of the clinical utility of serial beta-D-glucan concentrations in patients with persistent neutropenic fever. J. Med. Microbiol. 2008, 57 Pt 3, 287–295. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Alexander, B.D.; Kett, D.H.; Vazquez, J.; Pappas, P.G.; Saeki, F.; Ketchum, P.A.; Wingard, J.; Schiff, R.; Tamura, H.; et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 2005, 41, 654–659. [Google Scholar] [CrossRef]
- Pazos, C.; Pontón, J.; Del Palacio, A. Contribution of (1->3)-beta-D-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: A comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 2005, 43, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sima, P.; Richter, J.; Vetvicka, V. Glucans as New Anticancer Agents. Anticancer Res. 2019, 39, 3373–3378. [Google Scholar] [CrossRef]
- Barthow, C.; Hood, F.; McKinlay, E.; Hilder, J.; Cleghorn, C.; Huthwaite, M.; Weatherall, M.; Parry-Strong, A.; Pullon, S.; Gray, B.; et al. Food 4 Health–He Oranga Kai: Assessing the efficacy, acceptability and economic implications of Lactobacillus rhamnosus HN001 and β-glucan to improve glycated haemoglobin, metabolic health, and general well-being in adults with pre-diabetes: Study protocol for a 2 × 2 factorial design, parallel group, placebo-controlled randomized controlled trial, with embedded qualitative study and economic analysis. Trials 2019, 20, 464. [Google Scholar]
- Bordoni, A.; Boesch, C.; Malpuech-Brugère, C.; Orfila, C.; Tomás-Cobos, L. The role of bioactives in energy metabolism and metabolic syndrome. Proc. Nutr. Soc. 2019, 78, 340–350. [Google Scholar] [CrossRef]
- Bose, N.; Ottoson, N.R.; Qiu, X.; Harrison, B.; Lowe, J.R.; Uhlik, M.T.; Rathmann, B.T.; Kangas, T.O.; Jordan, L.R.; Ertelt, K.E.; et al. Immune Pharmacodynamic Responses of the Novel Cancer Immunotherapeutic Imprime PGG in Healthy Volunteers. J. Immunol. (Baltimore Md. 1950) 2019, 202, 2945–2956. [Google Scholar] [CrossRef]
- Dicks, L.; Ellinger, S. Effect of the Intake of Oyster Mushrooms (Pleurotus ostreatus) on Cardiometabolic Parameters-A Systematic Review of Clinical Trials. Nutrients 2020, 12, 1134. [Google Scholar] [CrossRef]
- Rahmani, J.; Miri, A.; Černevičiūtė, R.; Thompson, J.; de Souza, N.N.; Sultana, R.; Kord Varkaneh, H.; Mousavi, S.M.; Hekmatdoost, A. Effects of cereal beta-glucan consumption on body weight, body mass index, waist circumference and total energy intake: A meta-analysis of randomized controlled trials. Complement Ther. Med. 2019, 43, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rieder, A.; Knutsen, S.H.; Sainz Fernandez, A.; Ballance, S. At a high dose even partially degraded beta-glucan with decreased solubility significantly reduced the glycaemic response to bread. Food Funct. 2019, 10, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Tosh, S.M.; Spruill, S.E.; Jenkins, A.L.; Ezatagha, A.; Duss, R.; Johnson, J.; Chu, Y.; Steinert, R.E. Increasing oat β-glucan viscosity in a breakfast meal slows gastric emptying and reduces glycemic and insulinemic responses but has no effect on appetite, food intake, or plasma ghrelin and PYY responses in healthy humans: A randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 319–328. [Google Scholar] [PubMed]
- Gefroh, E.; Hewig, A.; Vedantham, G.; McClure, M.; Krivosheyeva, A.; Lajmi, A.; Lu, Y. Multipronged approach to managing beta-glucan contaminants in the downstream process: Control of raw materials and filtration with charge-modified nylon 6,6 membrane filters. Biotechnol. Prog. 2013, 29, 672–680. [Google Scholar] [CrossRef]
- US FDA. Guidance for industry Immunogenicity Assessment for Therapeutic Protein Products; US FDA: Montgomery, MD, USA, 2014.
- Dobrovolskaia, M.A.; McNeil, S.E. Endotoxin and engineered nanomaterials. In Handbook of Immunological Properties of Engineered Nanomaterials; Dobrovolskaia, M.A., McNeil, S.E., Eds.; World Scientific Publishing Ltd.: Singapore, 2013. [Google Scholar]
- Szebeni, J.; Simberg, D.; González-Fernández, Á.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef]
- Gordon, M.; Bihari, B.; Goosby, E.; Gorter, R.; Greco, M.; Guralnik, M.; Mimura, T.; Rudinicki, V.; Wong, R.; Kaneko, Y. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: A phase I/II trial. J. Med. 1998, 29, 305–330. [Google Scholar]
- Wada, T.; Nishide, T.; Hatayama, K.; Chang, S.W.; Tatsuta, M.; Yasutomi, M. [A comparative clinical trial with tegafur plus lentinan treatment at two different doses in advanced cancer]. Gan Kagaku Ryoho 1987, 14, 2509–2512. [Google Scholar]
- Roslansky, P.F.; Novitsky, T.J. Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans. J. Clin. Microbiol. 1991, 29, 2477–2483. [Google Scholar] [CrossRef]
- Neun, B.W.; Dobrovolskaia, M.A. Considerations and Some Practical Solutions to Overcome Nanoparticle Interference with LAL Assays and to Avoid Endotoxin Contamination in Nanoformulations. Methods Mol. Biol. 2018, 1682, 23–33. [Google Scholar]
- Dobrovolskaia, M.A.; Neun, B.W.; Clogston, J.D.; Ding, H.; Ljubimova, J.; McNeil, S.E. Ambiguities in applying traditional Limulus amebocyte lysate tests to quantify endotoxin in nanoparticle formulations. Nanomedicine 2010, 5, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Neun, B.W.; Clogston, J.D.; Grossman, J.H.; McNeil, S.E. Choice of method for endotoxin detection depends on nanoformulation. Nanomedicine 2014, 9, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Beal, S.G. Application of the 1,3-beta-D-Glucan (Fungitell) Assay in the Diagnosis of Invasive Fungal Infections. Arch. Pathol. Lab. Med. 2016, 140, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Associates of Cape Cod 1,3-beta-D-glucan Detection Reagent Kit. Available online: https://www.acciusa.com/pdfs/accProduct/inserts/Glucatell_Kit.pdf (accessed on 23 July 2020).
- Associates of Cape Cod Glucashield: Glucan Inhibiting Buffer. Available online: https://www.acciusa.com/pdfs/accProduct/inserts/Glucashield_13DGlucan_Inhibiting_Buffer.pdf (accessed on 23 July 2020).
- Fuji Film β-Glucan Test. Available online: https://www.wako-chemicals.de/en/product/v-glucan-test (accessed on 23 July 2020).
- Biovision QuickDetectTM 1-3-β-d-glucan (Human) ELISA Kit. Available online: https://www.biovision.com/documentation/datasheets/E4446.pdf (accessed on 23 July 2020).
- Megazyme β-Glucan Assay Kit (Yeast & Mushroom). Available online: https://www.megazyme.com/beta-glucan-assay-kit-yeast-mushroom (accessed on 23 July 2020).
- Megazyme Enzymatic Yeast β-Glucan Assay Kit. Available online: https://www.megazyme.com/yeast-beta-glucan-assay-kit (accessed on 23 July 2020).
- Megazyme β-Glucan Assay Kit (Mixed Linkage). Available online: https://www.megazyme.com/beta-glucan-assay-kit (accessed on 23 July 2020).
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef]
- Charles River Endosafe Nexgen-PTS. Available online: https://www.criver.com/sites/default/files/resource-files/Endosafe-nexgen-PTS-Sheet.pdf (accessed on 23 July 2020).
- Neun, B.W.; Dobrovolskaia, M.A. Understanding endotoxin and beta-glucan contamination in nanotechnology-based drug products. In Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems; Williams, K.L., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 481–496. Available online: https://link.springer.com/chapter/10.1007/978-3-030-17148-3_12 (accessed on 23 July 2020).
- Neun, B.W.; Dobrovolskaia, M.A. Detection and quantitative evaluation of endotoxin contamination in nanoparticle formulations by LAL-based assays. Methods Mol. Biol. 2011, 697, 121–130. [Google Scholar]
- Schubert, C.; Moudgal, C. Parenteral Safety of Beta Glucans. In Proceedings of the Presentation at the PDA Endotoxins Workshop, Washington, DC, USA, 18–19 October 2017. [Google Scholar]
- Finkelman, M.A. (1″3)-β-D-Glucan: Pharmaceutical Contaminant and Biological Response Modifier. In American Pharmaceutical Review. Endotoxin Supplement; Associates of Cape Cod: East Falmouth, MA, USA, 2016; pp. 16–19. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/190861-1-3-D-Glucan-Pharmaceutical-Contaminant-and-Biological-Response-Modifier/ (accessed on 23 July 2020).
- ICH. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Impurities: Guideline for Residual Solvents q3c(R6). 2016. Available online: https://database.ich.org/sites/default/files/Q3C-R6_Guideline_ErrorCorrection_2019_0410_0.pdf (accessed on 23 July 2020).
Sample Availability: Protocol for the assessment of beta-glucans that was used to generate the data summarized in Table 4 along with other protocols for nanoparticle characterization are available for public use at https://ncl.cancer.gov/resources/assay-cascade-protocols. |

| Type of β-glucan Molecule * | Source | On Beads (B), Particulate (P) or Soluble (S) | Immunological Response | In Vitro or In Vivo | Reference |
|---|---|---|---|---|---|
| β-(1,3)-d-glucan | N.S. | N.S. | IL-6, TNFα, IL-1RA production by human monocytes | In vitro | [22] |
| β-(1,6)-d-glucan (pustulan) | Yeast | B | Activation of phagocytic function, production of ROS and high levels of HSP by human neutrophils | In vitro | [23] |
| β-(1,3)-d-glucan (laminarin) | Yeast | B | Low levels of HSP production by human neutrophils | In vitro | [23] |
| β-(1,4)-d-glucan | Yeast | B | Not immunostimulatory in human neutrophils as indicated by ROS and HSP levels | In vitro | [23] |
| β-(1,3)-d-glucan with some β-(1,6) branching at 30:1 ratio (laminarin) | Algae | S | Lymphocyte proliferation in porcine PBMC | In vitro | [24] |
| β-(1,3)-d-glucan with some β-(1,6) branching at 6:1 ratio (scleroglucan) | Fungi | S | Lymphocyte proliferation; TNFα and IL-10 secretion by porcine PBMC | In vitro | [24] |
| β-(1,3)-d-glucan unbranched (curdlan) | Gm-Bacteria | P | Lymphocyte proliferation; ROS production by monocytes and neutrophils; TNFα and IL-10 secretion by PBMC of porcine origin | In vitro | [24] |
| β-(1,3)-d-glucan unbranched | Algae | P | Lymphocyte proliferation; ROS production by monocytes and neutrophils; TNFα and IL-10 secretion by PBMC of porcine origin | In vitro | [24] |
| β-(1,3)-d-glucan with some β-(1,6) branching at 30:1 ratio | Yeast | P | Lymphocyte proliferation; ROS production by monocytes and neutrophils; TNFα and IL-10 secretion by PBMC of porcine origin | In vitro | [24] |
| β-(1,3), β-(1,6) branched with 10:1 or 20:1 ratio (macrogard) | Yeast | P | Lymphocyte proliferation; ROS production by monocytes and neutrophils; TNFα and IL-10 secretion by PBMC of porcine origin | In vitro | [24] |
| β-(1,3), β-(1,6)-d-glucan uniformly branched (Zymozan) | Yeast | P | Lymphocyte proliferation; ROS production by monocytes and neutrophils; TNFα and IL-10 secretion by PBMC of porcine origin | In vitro | [24] |
| β-(1,3)-d-glucan unbranched (curdlan) | Gm- Bacteria | P | Maturation of human monocyte-derived DC; Th17 differentiation and stimulation of mixed leukocyte reaction | In vitro | [25] |
| β-(1,3)-d-glucan unbranched (curdlan) | Gm- Bacteria | P | Secretion of IL-1β, IL-6, IL-23, IL-10, and TNFα by human PBMC | In vitro | [26] |
| β-(1,3), β-(1,6)-d-glucan uniformly branched (Zymozan) | Yeast | P | Secretion of IL-1β, IL-6, IL-23, IL-10, and TNFα by human PBMC | In vitro | [26] |
| β-(1,3)-d-glucan | Yeast | P | Antigen-specific IgG2c and potent CD4+ T-cell activation in mice after s.c. injection | In vivo | [27] |
| β-(1,3), β-(1,6)-d-glucan uniformly branched (Zymozan) | Yeast | P | Complement activation; foot swelling; CTL activation; antigen-specific IgG2a antibody response and potent CD4+ Th1 response in mice after i.m. injection | In vivo | [28] |
| β-(1,3)-d-glucan unbranched (curdlan) | N.S. | N.S. | Elevated IL-8 levels in nasal secretions of human subjects exposed via the inhalation route | In vivo | [29] |
| β-(1,3)-d-glucan | Yeast | N.S. | Induction of IFNγ responses in mice after oral administration | In vivo | [30] |
| β-(1,6)-backbone and β-(1-3)-side branches | Mushrooms | N.S. | Elevation of L-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, MCP-1, MIP-1β and TNF-α levels after oral administration in healthy human donors, and in human blood cultures | In vivo and in vitro | [31] |
| Source of Contamination | Solution |
|---|---|
| Cellulose-fiber containing PPE suits | Use PPE suits made of other polymers (e.g., high- density polyethylene fibers like those in DuPontTM Tyvek® suits) |
| Cotton-containing plugs in serological pipettes and tips | Use pipettes and tips with synthetic polymer-based aerosol control barriers |
| Cellulose-based filters | Replace cellulose-acetate filters with other filter types; prime the filter to reduce levels of eluted glucans (the number of priming cycles may vary for different filtration units and should be determined empirically). |
| Sucrose and sucrose-containing buffers | Screen multiple batches and select that with minimal to no contamination; Establish a reliable supply chain |
| Starting materials (especially of plant origin) | Screen all starting materials and use those free of contamination; Establish a reliable supply chain |
| Water | Screen water and use batches free of contamination; Establish a reliable water source |
| Fungal contamination in the laboratory environment | Perform regular microbiological monitoring of the laboratory equipment and environment to detect and eliminate fungal contamination |
| Assay Type/Name | Manufacturer | Detection Range | Diagnostic (D), R&D (R), or Food (F) | Type of Assay | Reference |
|---|---|---|---|---|---|
| Biochemical/Fungitell | Associates of Cape Cod | 31.25–500 pg/mL | D | Modified LAL assay based on the measurement of optical density at 405 nm | [48] |
| Biochemical/Glucatell | Associates of Cape Cod | 5–40 pg/mL | R | Modified LAL assay based on the measurement of optical density 405 nm (kinetic) or 540 nm (end-point) | [72] |
| ELISA/QuickDetect™ | Biovision | 0.8–50 pg/mL | D&R | Sandwich ELISA detecting absorbance at 450 nm | [75] |
| Biochemical/ Toxinometer MT-6500 | Fuji Film | 6–600 pg/mL | D | Modified turbidimetic LAL assay | [74] |
| Biochemical/Endosafe Nexgen-PTS | Charles River | 10–1000pg/mL | R | Modified LAL, cartridge-based dedicated spectrophotometric assay | [80] |
| Chemical&Enzymatic/β-glucan yeast & mushroom | Megazyme | 1 g/100 g | F | Acid-based hydrolysis of beta-glucans, followed by enzymatic degradation and measurement of absorbance at 510 nm | [76] |
| Enzymatic/yeast β-glucan | Megazyme | 1 g/100 g | F | Enzymatic degradation assay measuring absorbance at 510 nm | [77] |
| Enzymatic/ β-glucan (mixed linkage) | Megazyme | 0.5 g/100 g | F | Enzymatic degradation assay measuring absorbance at 510 nm | [78] |
| Platform | API or * Active Component | β-Glucan Conc., pg/mg API (Spike Recovery, %) | Lowest Dilution with Acceptable Spike Recovery |
|---|---|---|---|
| Nano-albumin | Paclitaxel | 5.84 (123) | 5 |
| Liposome | Amphotericin | 21.3 (142) | 5 |
| PEG-liposome | Doxorubicin | 154 (120) | 50 |
| SPIO | Iron | 10.2 (133) | 50 |
| Nanorods | * Gold | 38.5 (70) | 50 |
| Polymer-Antibody-Drug Conjugate | Cisplatin | 181,000 (168) | 50 |
| Polysaccharide Nanoparticles | Paclitaxel | BLOQ (104) | 500 |
| Nanogel | Nanogel | 109 (56) | 50 |
| Polymeric Nanoparticle | Iodine | 21.9 (59) | 50 |
| Polymeric Nanoemulsion | Propofol | 117 (111) | 500 |
| Nanocrystal | Docetaxel | 129 (64) | 50 |
| Polymeric Nanoparticle | miRNA | 3128 (81) | 50 |
| Polymeric Micelle | Paclitaxel | 1179 (62) | 500 |
| PEG-oligo(FdUMP) | FdUMP | 4.5 (93) | 5 |
| Polymeric Micelle | Neoantigen | BLOQ (64) | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neun, B.W.; Cedrone, E.; Potter, T.M.; Crist, R.M.; Dobrovolskaia, M.A. Detection of Beta-Glucan Contamination in Nanotechnology-Based Formulations. Molecules 2020, 25, 3367. https://doi.org/10.3390/molecules25153367
Neun BW, Cedrone E, Potter TM, Crist RM, Dobrovolskaia MA. Detection of Beta-Glucan Contamination in Nanotechnology-Based Formulations. Molecules. 2020; 25(15):3367. https://doi.org/10.3390/molecules25153367
Chicago/Turabian StyleNeun, Barry W., Edward Cedrone, Timothy M. Potter, Rachael M. Crist, and Marina A. Dobrovolskaia. 2020. "Detection of Beta-Glucan Contamination in Nanotechnology-Based Formulations" Molecules 25, no. 15: 3367. https://doi.org/10.3390/molecules25153367
APA StyleNeun, B. W., Cedrone, E., Potter, T. M., Crist, R. M., & Dobrovolskaia, M. A. (2020). Detection of Beta-Glucan Contamination in Nanotechnology-Based Formulations. Molecules, 25(15), 3367. https://doi.org/10.3390/molecules25153367







